Back to Journals » Journal of Inflammation Research » Volume 15
Histopathological Features of Helicobacter pylori Infection in Gastric Mucosa
Authors Wang YK, Li C, Zhou YM, Zeng L, Li YY, Huang SL, Zhu CY, Wang Y, Wang SN, Chen XD
Received 20 July 2022
Accepted for publication 23 September 2022
Published 10 November 2022 Volume 2022:15 Pages 6231—6243
DOI https://doi.org/10.2147/JIR.S383075
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Yang-Kun Wang,1,* Chun Li,2,* Yong-Mei Zhou,1 Lei Zeng,1 Ying-Ying Li,3 Si-Lin Huang,4 Chao-Ya Zhu,5 Yue Wang,6 Su-Nan Wang,3 Xiao-Dong Chen1
1Department of Pathology, Foresea Life Insurance Guangzhou General Hospital, Guangzhou, 511300, People’s Republic of China; 2The First Affiliated Hospital of Henan University of Science and Technology, Luoyang, 471000, People’s Republic of China; 3Shenzhen Polytechnic, Shenzhen, 518055, People’s Republic of China; 4Department of Gastroenterology, South China Hospital Affiliated to Shenzhen University, Shenzhen, 518111, People’s Republic of China; 5Department of Pathology, the Third Affiliated Hospital of Zhengzhou University, Zhengzhou, 450052, People’s Republic of China; 6Shenzhen Hezheng Hospital, Shenzhen, 518053, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Su-Nan Wang; Xiao-Dong Chen, Email [email protected]; [email protected]
Objective: To investigate the histopathological characteristics of Helicobacter pylori (Hp) infection in the gastric mucosa in the process from occurrence to intraepithelial neoplasia.
Methods: Specimens obtained from the endoscopic biopsy and endoscopic submucosal dissection of 2457 cases of gastric Hp infection were observed and assessed in detail using histology and immunohistochemistry techniques. The condition was divided according to the histopathological characteristics of gastric mucosal damage caused by Hp infection. The histopathological characteristics and immunophenotype of each stage were subsequently elucidated.
Results: Helicobacter pylori is initially implanted in the mucus layer covered by the epithelium on the surface of the gastric mucosa. It then selectively adheres to the cytoplasm of the surface mucus cells, which makes the oval and spherical particles containing mucus that is wrapped by the bounded membrane in the cytoplasm on the nucleus of the surface mucus cells disappear, while the cytoplasm undergoes spiderweb-like vacuolar degeneration. This leads to the proliferation and transformation of the surface mucous cells before developing into intraepithelial neoplasia. In the process of histomorphology, mucosal ulcers, mucosal lymphoid tissue proliferation, gland atrophy, intestinal epithelial metaplasia, mucosa-associated lymphoid tissue lymphoma, and adenocarcinoma may occur. In this study, the condition was divided into five stages according to the histopathological characteristics of gastric mucosal damage caused by Hp infection, as well as the degree of gastric mucosal damage and involvement depth as follows: the mucus infection stage, the surface epithelial cell infection stage, the lamina propria lesion stage, the mucosal atrophy stage, and the intraepithelial neoplasia stage.
Conclusion: Understanding the histopathological characteristics of gastric Hp infection in terms of its occurrence and development into intraepithelial neoplasia is conducive to the precise treatment and tracking of malignant cell transformation, and is of great significance in controlling the occurrence and development of gastric cancer.
Keywords: Helicobacter pylori in the stomach, cell morphology, intraepithelial neoplasia, histopathological staging, immunohistochemistry
Introduction
Helicobacter pylori (H. pylori; hereafter referred to as “Hp”) is recognized as the main cause of chronic gastritis, gastric ulcers, gastric adenocarcinoma, and gastric mucosa-associated lymphoid tissue (MALT) lymphoma.1–3 Presently, various methods are used to detect Hp infection in the gastric mucosa, including carbon-13 urea breath testing, fecal H. pylori antigen detection, serological detection, polymerase chain reaction (PCR) analysis, rapid urease testing, bacteriological culture analysis, endoscopy, fluorescent quantitative PCR, and various methods adopting in-situ hybridization technology.4–7 Each of these methods has relative advantages and disadvantages. However, the pathological detection of endoscopic biopsy tissue cannot be replaced.
With the development of endoscopic technology, histopathological diagnosis, based on endoscopic biopsy, is both extremely common and important. Endoscopic biopsy is employed to perform a diagnostic examination of Hp infection8–10 related to both tumor and non-tumor lesions of the gastric mucosa. However, the pathological examination of Hp infection via gastric mucosal biopsy includes several flaws and shortcomings. Specifically, cases of Hp infection in the gastric mucosa cannot be simply classified in terms of mild, moderate, or severe, while descriptions in pathological reports cannot be expressed in terms of +, ++, or +++. As such, the method cannot reflect the histopathological characteristics, nor can it provide any information about the relationship between clinical Hp infection and gastric cancer, and is not conducive to evaluating the relationship between Hp infection and carcinogenesis. The authors’ previous study found that Hp infection caused a papilloma-like proliferation of glandular epithelial cells11 and also led to extensive segmental atrophy of the glands in the lamina propria of the gastric mucosa.12 The World Health Organization classified Hp infection as a carcinogen of gastric cancer, accordingly, giving rise to a trend to eliminate Hp to prevent gastric cancer through the detection and treatment of the bacteria.13,14 Several studies have investigated the incidence of Hp infection (detected via pathological biopsy) and its relationship with chronic gastritis, chronic atrophy, and intestinal metaplasia, and compared the prevalence of atrophy and intestinal metaplasia with the incidence of gastric cancer in different countries. Research involving a total of 1906 patients infected with Hp across seven different countries showed the incidence of gastric cancer in different geographic regions.15–19 Helicobacter pylori infection is considered the most important risk factor for gastric cancer, and elimination of the bacteria reduces the attendant risk of developing this disease.20–22
The present study aimed to further explore the histomorphological changes arising from the occurrence of Hp infection to its development into intraepithelial neoplasia. A total of 2457 cases of Hp infection were collected, and the histological characteristics, pathological stages, and histopathological diagnostic criteria of each stage were subsequently studied to provide a clinical reference for the precise treatment of Hp infection.
Data and Methods
Materials
A total of 2457 cases of Hp infection were collected from the Foresea Life Insurance Guangzhou General Hospital, the First Affiliated Hospital of Henan University of Science and Technology, the Affiliated South China Hospital of Shenzhen University, and the Third Affiliated Hospital of Zhengzhou University from December 2019 to December 2021. Among the cases, 62 patients with high-grade intraepithelial neoplasia underwent endoscopic submucosal dissection (ESD), while the remaining 2395 were biopsy cases. The sample included 1515 male patients and 942 female patients, whose ages ranged from 13 to 82 years (average, 48.7 years). In terms of lesions site, 1763 cases involved the antrum, and 263 cases involved the body of the stomach, while 431 cases involved infection in both the antrum and the body of the stomach. A minimum of three tissue samples were obtained from each of the above areas via clamping. The specimens were then fixed with 10% neutral formalin before being routinely dehydrated, paraffin-embedded, sliced into 4-μm-thick sections, stained with hematoxylin and eosin, and observed in terms of tissue structure and cell morphology under a light microscope. The pathological stage is based on histological and morphological changes, according to the degree of injury and depth of involvement.12
Immunohistochemical Staining
The EnVisionTM two-step method was adopted for immunohistochemical staining. The paraffin was removed from the tissue sections, which were then hydrated and rinsed with distilled water before being placed in Tris-buffered saline (TBS) for 10 min. The endogenous peroxidase was then blocked for 5 min and the sections were treated with TBS for 10 min. Each antibody (HP, MUC5AC, MUC6, MUC2, CEA, villin, CDX2, p53, and Ki-67) was incubated with the sections at room temperature for 30 min. After cleaning in TBS for 10 min, the sections were incubated in the EnVision incubator before being washed with TBS for a further 10 min and were thereafter incubated with the second antibody for 10 min. The sections were then incubated for 10 min using a chromogenic substrate solution and rinsed with distilled water before being developed with diaminobenzidine and counterstained with hematoxylin. Any sections determined to be gastric mucosal segments were used as the positive controls, while phosphate buffered saline was used as the negative control in place of the primary antibody. The working fluids were all purchased from Fuzhou Maxim Biotechnology Co., Ltd. (China); all procedures were carried out strictly in accordance with the manufacturer’s instructions. The results of immunohistochemical staining are the marking results based on the morphological characteristics of each stage.12
Statistical Analysis
The data were analyzed using the SPSS Statistics 22.0 software program; comparisons were made in terms of gender and age using a chi-square test. A P-value of <0.05 was regarded as statistically significant.
Results
Clinical Characteristics
The enrolled patients included 1515 males (61.6%) and 942 females (38.3%), and the Hp infection rate was clearly higher in males than in females. The age at the onset of Hp infection was <60 years old in 1502 cases (61.1%) and >60 years old in 955 cases (38.9%), with the proportion of patients with Hp infection aged over 60 significantly higher. Details of the relationships between Hp-induced gastric mucosal intraepithelial neoplasia, age, and gender are presented in Table 1.
 |
Table 1 Correlation of Age and Gender with H. pylori Infection Developing to Intraepithelial Neoplasia |
The Occurrence and Development of Gastric Mucosal Tumorigenesis Due to Helicobacter pylori Infection
Helicobacter pylori initially colonizes in the mucous layer covered by the epithelium on the surface of the gastric mucosa before penetrating the mucus layer and specifically adhering to the surface mucous cytoplasm. The bacteria then selectively destroys the cytoplasm of the surface mucous cells and can also affect the proliferating neck mucous cells, which subsequently leads to lamina propria lesions, gastric mucosal atrophy, and intraepithelial neoplasia. Cytologically, the oval and spherical particles containing mucus that is wrapped by the bounded membrane in the cytoplasm on the nucleus of the surface mucus cells disappear, and the cytoplasm exhibits spiderweb-like vacuolar degeneration. The various stages of the occurrence and development of gastric mucosal Hp infection are presented in Figure 1.
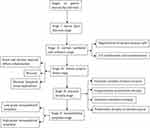 |
Figure 1 Pathological stages of the occurrence and development of H. pylori infection in gastric mucosa. |
Pathological Staging and Characteristics of Gastric Mucosal Damage Caused by Helicobacter pylori Infection
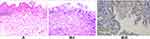
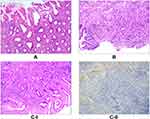
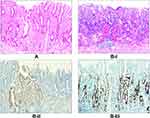
Stage I, which involves Hp mucous layer infection, is regarded as the initial stage of Hp infection. As noted, the Hp colonizes in the mucous layer covered by the epithelium on the surface of the gastric mucosa (Figure 2A) and is subsequently expressed (Figure 2B). Stage II involves infection of the surface epithelial cells, with stage IIA characterized by the degeneration of these cells. Here, the Hp adheres to the cytoplasm of the surface mucous cells and selectively disrupts the cytoplasm of the cells, leading to cytoplasmic swelling and vacuolar degeneration (Figure 3A). Stage IIB is characterized by cell proliferation and transformation, wherein a large number of Hp bacteria adhere in the cytoplasm of the surface epithelial cells, resulting in massive proliferation and transformation of cells in the depth of the gastric pit and the isthmus of the gastric gland, as well as in the proliferative area in the upper part of the gland neck (Figure 3B–I). The Hp is then positively expressed (Figure 3B–II). Stage III is the lamina propria lesion stage, with stage IIIA characterized by diffuse acute and chronic inflammation that emerge throughout the lamina propria (Figure 4A). Stage IIIB involves mucosal ulcers, with cell necrosis and tissue debris, as well as fibrous materials, small blood vessels, and inflammatory cells constituting the ulcers (Figure 4B). Stage IIIC involves mucosal lymphoid hyperplasia, with diffuse small lymphocytic hyperplasia dominant alongside a minimal proliferation of lymphoid follicles and glands (Figure 4C–I). The Hp bacteria is then positively expressed (Figure 4C–II). In stage IV, the Hp mucosal atrophy stage, mucosal thinning and glandular atrophy are the dominant features, with stage IVA characterized by glandular atrophy of the lamina propria, wherein its glands become significantly smaller and reduced, and the interstitial fibrous tissue and smooth muscle tissue proliferate and become intertwined with infiltrating lymphocytes and proliferating lymphoid follicles (Figure 5A). Stage IVB involves compensated proliferative atrophy, with the compensatory hyperplasia of the surface epithelial cells typically being between 0.8 and 1.5 mm in height (Figure 5B). Stage IVC involves intestinal metaplastic atrophy, wherein intrinsic gland atrophy coexists with intestinal metaplasia (Figure 5C). Stage IVD involves smooth muscle proliferative atrophy from the muscularis mucosa to the mucosal proliferative zone, with the smooth muscle forming a muscle fiber plate with characteristic fibrous tissue and smooth muscle together with the muscularis mucosa (Figure 5D–I). Here, MUC6 is positively expressed, leading to reduced fundic glands (Figure 5D–II). Stage V is the intraepithelial neoplasia stage, with stage VA characterized by low-grade intraepithelial neoplasia, wherein the glands are irregularly branched with enhanced basophilic cytoplasm and elongated nuclei located at the base of the glandular epithelium (Figure 6A). Stage VB involves high-grade intraepithelial neoplasia, wherein the irregular branching of the glands is obvious, with large nuclei, an increased nuclear plasma ratio, and obvious nucleoli, where mitoses increases and pathological mitoses can be observed (Figure 6B–I). Here, CDX2 is positively expressed (Figure 6B–II), while 30%–60% of the cells are Ki-67-positive (Figure 6B–III). The histomorphological characteristics, immunophenotype, and pathological staging of gastric mucosal Hp infection in terms of the process from occurrence to development into intraepithelial neoplasia are shown in Table 2.
 |
Table 2 The Histomorphological Characteristics, Immunophenotype, and Pathological Stages of Helicobacter pylori Infection in the Gastric Mucosa from Occurrence to Intraepithelial Neoplasia |
Immunohistochemical Staining Results
In the different stages of Hp infection, Hp markers were found to be positively expressed to varying degrees. The level of injury and the depth of involvement of the gastric mucosa ranged from mild to severe, with the positive expression levels of MUC5AC and MUC6 exhibiting a decreased or enhanced trend; there were different degrees of the positive expression of CEA, MUC2, CDX2, villin and p53, and the proliferative index, Ki-67, differed in each stage (Table 2).
Discussion
The present study found that patients with gastric mucosal Hp infection not only presented differences in terms of the abundance of the bacteria but also concerning the degree of gastric mucosal damage and the histopathological types of Hp infection in the gastric mucosa that developed into intraepithelial neoplasia. A further conclusion was that, for gastric mucosal Hp infection biopsy cases, the infection cannot simply be classified as mild, moderate, or severe in the pathological test report, nor can it be expressed in terms of +, ++, and +++. As such, the cell morphology and histopathological characteristics cannot be reflected, nor can any information about the relationship between clinical Hp infection and gastric cancer be provided. The method is thus not conducive to evaluating the relationship between Hp infection and carcinogenesis.
The mucous layer of the gastric mucosa is an insoluble mucus with a high concentration of bicarbonate, which coats the epithelial surface and plays an important protective role. The pathogenicity of Hp located in the mucous layer cannot be determined because the bacteria become pathogenic only when entering the surface epithelial cells, causing damage to the gastric mucosa. It was also found that Hp specifically and selectively adhered to the surface mucous cytoplasm but can also affect the cervical mucous glands. Cytologically, the oval and spherical particles containing mucus that is wrapped by the bounded membrane in the cytoplasm on the nucleus of the surface mucus cells disappear, and the cytoplasm exhibits spiderweb-like vacuolar degeneration before giving rise to the proliferation and transformation of the surface mucous cells, which develops into intraepithelial neoplasia. During the histomorphological process, mucosal ulcers, mucosal lymphoid tissue proliferation, gland atrophy, intestinal epithelial metaplasia, MALT lymphoma, and adenocarcinoma may occur.
In this study, the histopathological staging and histopathological characteristics of each stage were proposed, based on the degree of gastric mucosal damage and the degree of involvement of Hp infection. The results could potentially serve as a reference for the precise treatment and tracking of malignant cell transformation and are of great significance for controlling the occurrence and development of gastric cancer.
Helicobacter pylori are the most common type of bacteria with recorded infection rates worldwide. It is essentially a flagellated pathogen, ie, a unipolar, polyflagellar, blunt-ended, spirally curved Gram-negative bacterium, with a length of 2.5–4.0 μm and a width of 0.5–1.0 μm, and a high level of genetic diversity to adapt to the properties of the host’s gastric juice. The bacterium can deform spherically in adverse environments or following drug treatment; spherical Hp has an intact membranous structure with flagella and vitality for the potential to lead to superinfection.23,24
Infection with Hp can easily be identified via immunohistochemically-stained sections. Numerous reports have been published on how Hp colonizes the gastric mucosa of humans, and it has also been reported to colonize the duodenal mucosa.25–27 The present study found that pathogenic Hp only settled in the cytoplasm of the mucous cells on the surface of the human gastric mucosa; when the surface epithelial cells degenerated and fell off, the cells proliferated and transformed or developed into intraepithelial neoplasia, at which point the Hp bacteria would continue to decrease and disappear. The present study also revealed that intestinal metaplastic cells were not infected by Hp; conversely, following Hp infection, malignant transformation of the gastric glandular epithelial cells occurred, and the epithelial cells exhibited characteristic hazy basophilic changes in the cytoplasm and nucleus. Furthermore, vacuolar degeneration of the cytoplasm occurred, and milky white spherical bodies were observed in the nucleus. Since Hp infection is specific and selectively adheres to the cytoplasm of the surface epithelial cells, in patients with Hp infection developing into intraepithelial neoplasia, and, ultimately, gastric adenocarcinoma, the disappearance of the surface epithelial cells renders determining the cause of the infection in the gastric adenocarcinoma tissue problematic, which is also why Hp infection leads to a low rate of gastric cancer.
Helicobacter pylori have unique virulence factors that enable the bacteria to colonize the cytoplasm of epithelial cells on the surface of the gastric mucosa; the structures to which the bacteria attach include urease, spiral-shaped, flagella, and adhesion molecules.28–30 Therefore, Hp infection in the gastric mucosa should be controlled as soon as possible using measures such as endoscopic biopsy and pathological detection, with timely treatment and regular biopsy reexaminations. The present study concluded that Hp infection in the gastric mucosa must be determined via gastric mucosal biopsy, using no less than three samples of endoscopic biopsy tissue for the examination. More importantly, the pathological diagnosis report should mark the colonization site of the Hp infection, while the histomorphological characteristics caused by the infection, the immunophenotype, and the pathological stages should be recorded in detail. If early control is provided, most cases of Hp infection in the gastric mucosa at the mucous layer infection stage, surface epithelial cell infection stage, and lamina propria lesion stage can be improved, with full recovery frequently achieved. However, curing and improvement rates among patients at the mucosal atrophy stage are low, and the severity of the disease is increased at this stage; for these patients, a biopsy reexamination will also be required every six months or once a year.31 Intraepithelial neoplasia requires a three or six-month period of close follow-up or ESD.32
This study found that atrophy of the gastric mucosa caused by Hp infection could be divided into five stages as follows: glandular atrophy of the lamina propria, compensatory proliferative atrophy, intestinal metaplasia atrophy, smooth muscle proliferative atrophy, and proliferative zone atrophy. Accordingly, the histopathological characteristics of atrophy at each stage were studied along with the immunophenotypic characteristics. The study found that the mechanism of Hp infection leading to gastric mucosal atrophy involved the inability of stem cells in the proliferating area of the gastric mucosa to differentiate and migrate normally. When Hp infection led to a proliferation disorder among stem cells in the proliferative area, on the one hand, there was insufficient downward migration, which morphologically manifested as atrophy of the lamina propria glands. On the other hand, the increase in proliferating regions was excessive, leading to the transformation of downward migrating cells, resulting in morphological intraepithelial neoplasia. In this study, this process was described as the course of tumorigenesis of the gastric mucosa, caused by glandular atrophy of the latter. The proposed histomorphological characteristics, immunophenotype, and pathological stages of Hp infection in the gastric mucosa, from occurrence to development into intraepithelial neoplasia, provide a novel reference for the study of the formation of early gastric cancer. However, this study only used the methods of histomorphology and immunohistochemistry, which is very limited or not enough in terms of technical methods and research means. Molecular biological techniques are still needed to study the pathogenic mechanism.
In summary, this study determined the histomorphological characteristics, immunophenotype, and pathological stages of gastric mucosal Hp infection. These aspects were studied in terms of the process that began with the occurrence of a bacterial infection to development into intraepithelial neoplasia, based on the degree and depth of the gastric mucosal damage caused by Hp infection, as well as the regularity of the occurrence and the development of lesions. Tracking the precise treatment prescribed by clinicians for malignant cell transformation is of great significance for controlling the occurrence and development of gastric cancer. The complex pathogenesis of Hp-induced gastric cancer must be studied in future research that includes a large number of cases.
Data Sharing Statement
All data generated or analysed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Ethics Approval
The study was conducted in accordance with the Declaration of Helsinki (as was revised in 2013). The study was approved by Ethics Committee of the Foresea Life Insurance Guangzhou General Hospital.
Consent to Participate
All participants signed a document of informed consent. Legal guardian of patients under 16 years of age provided informed consent.
Acknowledgments
We are particularly grateful to all the people who have given us help on our article.
Funding
This study was funded by the Key scientific and technological research projects in Henan Province (132102310008).
Disclosure
The authors declare that they have no competing interests.
References
1. Bomme M, Hansen JM, Wildner-Christensen M, Hallas J, Schaffalitzky de Muckadell OB. Effects of Community Screening for Helicobacter pylori: 13-Year Follow-Up Evaluation of a Randomized Controlled Trial. Clin Gastroenterol Hepatol. 2017;15(11):1715–1723.e7. doi:10.1016/j.cgh.2017.06.006
2. Mitsui Y, Miyoshi A, Okamoto K, et al. Different phenotypes of gastric fundic gland polyposis and cancer in patients with familial adenomatous polyposis depending on Helicobacter pylori infection. Gastric Cancer. 2019;22(6):1294–1300. doi:10.1007/s10120-019-01005-y
3. Zullo A, Hassan C, Cristofari F, et al. Effects of Helicobacter pylori eradication on early stage gastric mucosa-associated lymphoid tissue lymphoma. Clin Gastroenterol Hepatol. 2010;8(2):105–110. doi:10.1016/j.cgh.2009.07.017
4. Huh CW, Kim BW. [Diagnosis of Helicobacter pylori Infection]. Korean J Gastroenterol. 2018 Nov 25;72(5):229236. Korean. PMID: 30642138. doi:10.4166/kjg.2018.72.5.229
5. Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection-The Maastricht V/Florence Consensus Report. Gut. 2017;66(1):6–30. doi:10.1136/gutjnl-2016-312288
6. Sabbagh P, Mohammadnia-Afrouzi M, Javanian M, et al. Diagnostic methods for Helicobacter pylori infection: ideals, options, and limitations. Eur J Clin Microbiol Infect Dis. 2019;38(1):55–66. doi:10.1007/s10096-018-3414-4
7. Lauwers GY, Fujita H, Nagata K, Shimizu M. Pathology of non-Helicobacter pylori gastritis: extending the histopathologic horizons. J Gastroenterol. 2010;45(2):131–145. doi:10.1007/s00535-009-0146-3
8. Chatrangsun B, Vilaichone RK. Endoscopic Diagnosis for H. pylori Infection: white Light Imaging (WLI) vs. Image-Enhanced Endoscopy (IEE). Asian Pac J Cancer Prev. 2021;22(9):3031–3038. doi:10.31557/APJCP.2021.22.9.3031
9. Watanabe T, Nadatani Y, Suda W, et al. Long-term persistence of gastric dysbiosis after eradication of Helicobacter pylori in patients who underwent endoscopic submucosal dissection for early gastric cancer. Gastric Cancer. 2021;24(3):710–720. doi:10.1007/s10120-020-01141-w
10. Tiwari A, Rai R, Dahal P, Regmi S. Prevalence of Helicobacter Pylori in Endoscopic Gastric Biopsies of Chronic Gastritis Patients at A Tertiary Care Centre. JNMA J Nepal Med Assoc. 2020;58(228):564–568. doi:10.31729/jnma.5210
11. Wang Y, Shen L, Zhao G, et al. Histomorphological Characteristics and Pathological Types of Hyperproliferation of Gastric Surface Epithelial Cells. Gastroenterol Res Pract. 2021;2021:8828326. doi:10.1155/2021/8828326
12. Wang YK, Zhou JL, Meng NL, Zhu CY, Wang SN, Chen XD. How Does Helicobacter pylori Infection Cause Gastric Mucosal Atrophy. Infect Drug Resist. 2022;15:3619–3629. doi:10.2147/IDR.S355981
13. Suzuki H, Mori H. World trends for H. pylori eradication therapy and gastric cancer prevention strategy by H. pylori test-and-treat. J Gastroenterol. 2018;53(3):354–361. doi:10.1007/s00535-017-1407-1
14. Khatoon J, Prasad KN, Prakash Rai R, Ghoshal UC, Krishnani N. Association of heterogenicity of Helicobacter pylori cag pathogenicity island with peptic ulcer diseases and gastric cancer. Br J Biomed Sci. 2017;74(3):121–126. doi:10.1080/09674845.2017.1278887
15. Salar A. Gastric MALT lymphoma and Helicobacter pylori. Linfoma MALT gástrico y Helicobacter pylori. Med Clin (Barc). 2019;152(2):65–71. doi:10.1016/j.medcli.2018.09.006
16. Marques MS, Melo J, Cavadas B, et al. Afadin Downregulation by Helicobacter pylori Induces Epithelial to Mesenchymal Transition in Gastric Cells. Front Microbiol. 2018;9:2712. doi:10.3389/fmicb.2018.02712
17. Quach DT, Vilaichone RK, Vu KV, Yamaoka Y, Sugano K, Mahachai V. Helicobacter pylori Infection and Related Gastrointestinal Diseases in Southeast Asian Countries: an Expert Opinion Survey. Asian Pac J Cancer Prev. 2018;19(12):3565–3569. doi:10.31557/APJCP.2018.19.12.3565
18. El-Zimaity HM, Graham DY. Evaluation of gastric mucosal biopsy site and number for identification of Helicobacter pylori or intestinal metaplasia: role of the Sydney System. Hum Pathol. 1999;30(1):72–77. doi:10.1016/S0046-8177(99)90303-9
19. Udoh MO, Obaseki DE. Histopathological Evaluation Of H. Pylori Associated Gastric Lesions In Benin City, Nigeria. East Afr Med J. 2012;89(12):408–413.
20. El-Serag HB, Kao JY, Kanwal F, et al. Houston Consensus Conference on Testing for Helicobacter pylori Infection in the United States. Clin Gastroenterol Hepatol. 2018;16(7):992–1002.e6. doi:10.1016/j.cgh.2018.03.013
21. Horiuchi Y, Fujisaki J, Yamamoto N, et al. Biological behavior of the intramucosal Helicobacter pylori-negative undifferentiated-type early gastric cancer: comparison with Helicobacter pylori-positive early gastric cancer. Gastric Cancer. 2016;19(1):160–165. doi:10.1007/s10120-014-0452-1
22. Take S, Mizuno M, Ishiki K, et al. Low Incidence of Esophageal Adenocarcinoma After Eradication of Helicobacter pylori in Japan. Clin Gastroenterol Hepatol. 2018;16(12):1995–1996. doi:10.1016/j.cgh.2018.03.030
23. Gu H. Role of Flagella in the Pathogenesis of Helicobacter pylori. Curr Microbiol. 2017;74(7):863–869. doi:10.1007/s00284-017-1256-4
24. Koido S, Odahara S, Mitsunaga M, Aizawa M, Itoh S, Uchiyama K, Komita H, Satoh K, Kuniyasu Y, Yamane T, Ohkusa T. [Diagnosis of Helicobacter pylori infection: comparison with gold standard]. Rinsho Byori. 2008 Nov;56(11):100713. Available from: https://pubmed.ncbi.nlm.nih.gov/19086456/. PMID: 19086456.
25. Sgambato D, Miranda A, Romano L, Romano M. Gut microbiota and gastric disease. Minerva Gastroenterol Dietol. 2017;63(4):345–354. doi:10.23736/S1121-421X.17.02380-7
26. Roesler BM, Rabelo-Gonçalves EM, Zeitune JM. Virulence Factors of Helicobacter pylori: a Review. Clin Med Insights Gastroenterol. 2014;7:9–17. doi:10.4137/CGast.S13760
27. Allen LA. Phagocytosis and persistence of Helicobacter pylori. Cell Microbiol. 2007;9(4):817–828. doi:10.1111/j.1462-5822.2007.00906.x
28. Marques MS, Costa AC, Osório H, et al. Helicobacter pylori PqqE is a new virulence factor that cleaves junctional adhesion molecule A and disrupts gastric epithelial integrity. Gut Microbes. 2021;13(1):1–21. doi:10.1080/19490976.2021.1921928
29. Šterbenc A, Jarc E, Poljak M, Homan M. Helicobacter pylori virulence genes. World J Gastroenterol. 2019;25(33):4870–4884. doi:10.3748/wjg.v25.i33.4870
30. Sharndama HC, Mba IE. Helicobacter pylori: an up-to-date overview on the virulence and pathogenesis mechanisms. Braz J Microbiol. 2022;53(1):33–50. doi:10.1007/s42770-021-00675-0
31. Zhao G, Zhang Z, Li B, et al. Follow-up analysis and histopathological study of gastric mucosa in patients with Helicobacter pylori infection. J Int Med Res. 2021;49(12):3000605211055397. doi:10.1177/03000605211055397
32. Toracchio S, Caruso RA, Perconti S, et al. Evolutionarily-Related Helicobacter pylori Genotypes and Gastric Intraepithelial Neoplasia in a High-Risk Area of Northern Italy. Microorganisms. 2020;8(3):324. doi:10.3390/microorganisms8030324
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.