Back to Journals » OncoTargets and Therapy » Volume 13
High Soluble Programmed Death-Ligand 1 Predicts Poor Prognosis in Patients with Nasopharyngeal Carcinoma
Authors Lu T , Chen Y, Li J, Guo Q , Lin W, Zheng Y, Su Y, Zong J, Lin S , Ye Y, Pan J
Received 16 December 2019
Accepted for publication 18 February 2020
Published 26 February 2020 Volume 2020:13 Pages 1757—1765
DOI https://doi.org/10.2147/OTT.S242517
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Nicola Silvestris
Tianzhu Lu,1,* Yiping Chen,2,* Jieyu Li,3 Qiaojuan Guo,2 Wansong Lin,3 Yuhong Zheng,4 Ying Su,5 Jingfeng Zong,2 Shaojun Lin,1,2 Yunbin Ye,3,6 Jianji Pan1,2
1The School of Clinical Medicine, Fujian Medical University, Fuzhou, People’s Republic of China; 2Department of Radiation Oncology, Fujian Cancer Hospital, Fujian Medical University, Fuzhou, People’s Republic of China; 3Laboratory of Immuno-Oncology, Fujian Cancer Hospital, Fuzhou, People’s Republic of China; 4Department of Clinical Laboratory, Fujian Cancer Hospital, Fujian Medical University, Fuzhou, People’s Republic of China; 5Department of Radiation Biology, Fujian Cancer Hospital, Fujian Medical University, Fuzhou, People’s Republic of China; 6The School of Basic Medical Sciences, Fujian Medical University, Fuzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jianji Pan
The School of Clinical Medicine, Fujian Medical University, Fuzhou, People’s Republic of China
Tel +86 591-83638732
Fax +86 591-83928767
Email [email protected]
Yunbin Ye
The School of Basic Medical Sciences, Fujian Medical University, Fuzhou 350014, People’s Republic of China
Tel +86 591-83638732
Fax +86 591-83928767
Email [email protected]
Purpose: Immune checkpoint proteins in the tumor microenvironment can enter the blood circulation and are potential markers for liquid biopsy. The aims of this study were to explore differences in immune checkpoint protein expression between patients with nasopharyngeal carcinoma (NPC) and healthy controls and to investigate the prognostic value of the soluble form of programmed death-ligand 1 (sPD-L1) in NPC.
Methods: In total, 242 patients were included in the disease group. Plasma samples from 23 NPC patients and 15 healthy control were used for immune checkpoint protein panel assays. Samples from 219 patients with NPC including 30 paired pre-treatment and post-radiotherapy samples were evaluated by enzyme-linked immunosorbent assay to determine sPD-L1 levels.
Results: A total of 14 immune checkpoint proteins, including sPD-L1were upregulated in 23 patients with NPC (all p< 0.001) compared with 15 healthy controls. Among 219 patients, the median follow-up time was 50 months (7– 82 months). Based on the optimal cutoff value of 93.7 pg/mL, patients with high expression of sPD-L1 had worse distant metastasis-free survival (87.5% vs 74.0%, p=0.006) than those of patients with low expression. Multivariate analysis showed that sPD-L1 (HR=1.99, p=0.048) and EBV-DNA (HR=2.51, p=0.030) were poor prognostic factors for DMFS. In the group with high EBV-DNA expression, DMFS was worse for patients with high sPD-L1 expression than those with low sPD-L1 expression (56.4% vs 82.6%, p=0.002).
Conclusion: Plasma immune checkpoint protein expression differed significantly between patients with NPC and healthy donors. Plasma sPD-L1 levels are a candidate prognostic biomarker, especially when combined with EBV-DNA.
Keywords: nasopharyngeal carcinoma, programmed death-ligand 1, Epstein-Barr virus, immune checkpoint protein
Introduction
Nasopharyngeal carcinoma (NPC) has a high incidence in South China and Southeast Asia1 and is closely associated with Epstein–Barr virus (EBV) infection, which promotes a classic “inflamed-tumor” environment with abundant T-lymphocyte infiltration.2,3 In the era of intensity-modulated radiotherapy combined with chemotherapy, clinical outcomes among patients with NPC have improved, but metastasis is still a tough challenge, with an incidence of 20–30%.4,5
Immune escape is an important feature of tumors with essential roles in tumorigenesis and tumor development.6 Targeting immune escape with immune checkpoint inhibitors (ICIs), particularly inhibitors of programmed cell death 1 (PD-1)/programmed death-ligand 1 (PD-L1), has beneficial effects in various tumors, including NPC.7–9 Immune checkpoint proteins in the tumor microenvironment can enter the blood circulation through tumor cell apoptosis or exosomes and are potential markers for liquid biopsy.10–13 Soluble immune checkpoint proteins, especially soluble PD-L1 (sPD-L1), have potential prognostic value14,15 but are not well characterized in NPC.
Therefore, the aims of this study were (1) to explore the difference in the expression profile of immune checkpoint proteins between patients with NPC and healthy donors and (2) to investigate the prognostic value of sPD-L1 in NPC.
Materials and Methods
Patients
This study was conducted according to the guidelines of the reporting recommendations for tumor marker prognostic studies (REMARK). After obtaining Hospital Review Board approval, this retrospective study was conducted at Fujian Cancer Hospital. The patients and healthy donors provided written informed consent, in accordance with the Declaration of Helsinki. From July 2012 to March 2015, plasma samples from in our blood sample bank were included in the disease group. Of these, 23 were used for panel detection, and 219 were used for enzyme-linked immunosorbent assays (ELISA). Additionally, 15 plasma samples were collected from healthy donors as a control group. All patients with NPC were confirmed by histopathology and re-classified according to the TNM-8.16 Age, gender, and clinical stage (stage I–IVB) at diagnosis were recorded (Tables 1 and 2).
 |
Table 1 The Characteristics of Healthy Controls and Nasopharyngeal Carcinoma |
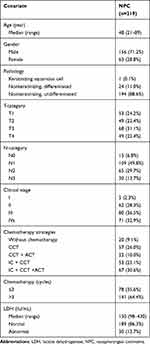 |
Table 2 The Characteristic of 219 Nasopharyngeal Carcinoma Patients |
Immuno-Oncology Checkpoint Protein Panels
A total of 23 plasma samples from patients with NPC and 15 samples from healthy controls were evaluated by the Human Immuno-Oncology Checkpoint Protein Magnetic Bead Panel (Cat. # HCKPMAG-11K; EMD Millipore Corporation, Billerica MA, USA). Samples were preserved in our blood sample bank at −80°C and thawed to room temperature (20°C) before detection. Plasma levels of BTLA, CD27, CD28, TIM-3, HVEM, CD40, GITR, GITRL, LAG3, TLR-2, PD-1, PD-L1, CTLA4, CD80/B7-1, CD86/B7-2, and ICOS were analyzed. The panel assays were performed according to the manufacturer’s instructions.
Enzyme-Linked Immunosorbent Assay
Plasma sPD-L1 concentrations were measured by ELISA using the Human PD-L1 [28–8] SimpleStep ELISA Kit (ab214565; Abcam, Cambridge, UK). A total of 249 plasma samples from 219 patients with NPC, including 30 paired pre-treatment and post-radiotherapy samples, 189 pretreatment samples, were included. Post-radiotherapy was defined as the time of the complement of radiation ± 3 days. ELISAs were conducted following the manufacturer’s instructions. All samples, standards and negative controls were tested in duplicate. The results were obtained using a spectrophotometer (reading at 450nm), and concentrations were calculated according to the standard curves.
Plasma EBV DNA Measurement
Plasma EBV DNA concentrations were measured by quantitative-polymerase chain reaction (q-PCR), as described in a previous publication. In brief, plasma samples were subjected to DNA extraction using a commercial magnetic beads kit (PerkinElmer EA20160201; Waltham, MA, USA) and analyzed using the Automated Nucleic Acid Extraction Workstation (Pre-NAT, PerkinElmer). A total of 450 μL of each plasma sample was used for DNA extraction per column. The exact amount was documented for the calculation of the target DNA concentration. A final volume of 60 μL was used to elute the DNA from the extraction column. Circulating EBV DNA concentrations were measured using a real-time q-PCR system to amplify a DNA segment in the BamHI-W fragment region of the EBV genome. The sequences of the forward and reverse primers were: 5ʹ-TGCCAAAGAGCCAGATCTAAGG-3ʹ and 5ʹ-AAAGTGTCAGATTTTGGGTCCAA-3ʹ respectively. A dual fluorescently-labelled oligomer, 5ʹ-FAM-CAGCCCCAAAGCGGGTGCAGTAAC-BHQ1-3ʹ served as the probe. Data were collected using an ABI Prism 7500 Sequence Detector and analyzed using Sequence Detection System (version 1.6.3; Applied Biosystems, Foster City, CA, USA). Results are expressed as copies of EBV genomes per milliliter of plasma. Multiple negative water blanks were included in every analysis.
Treatments and Follow-Ups
All patients received intensity-modulated radiation therapy according to our institutional protocols, as described previously. Generally, stage I disease was treated by radiation alone, while stage Ⅱ to Ⅳ diseases were treated with chemo-radiotherapy. The main chemotherapy strategies are induction chemotherapy (IC) + concurrent chemotherapy (CCT), IC + CCT + adjuvant chemotherapy (ACT), CCT, and CCT+ACT. The most commonly used chemotherapy regimen for IC and ACT was platinum (cisplatin 80mg/m2, or nedaplatin 80mg/m2 intravenously in three daily doses), plus paclitaxel (135 mg/m2 intravenously on Day 1) or gemcitabine (1000 mg/m2 intravenously on Days 1 and 8). The CCT regimen was cisplatin (80mg/m2 intravenously in three daily doses) or nedaplatin (80mg/m2 intravenously in three daily doses). Once the treatments were completed, follow-up intervals were 3 months within the first 2 years, 3–6 months for the next 3–5 years, and annually thereafter.
Statistical Analyses
The Mann–Whitney U-test was used to detect the differential expression of the plasma immune checkpoint proteins between NPC patients and healthy controls. The association between plasma sPD-L1 levels and the tumor burden was evaluated using Spearman’s rank correlation test. The training cohort was evaluated using X-Tile (version 3.6.1; Yale University, New Haven, CT, USA) to find the optimal cutoff value. The distant metastasis-free survival (DMFS), locoregional recurrence-free survival (RFS), and overall survival (OS) were defined as the time from the day of diagnosis to the date of the first distant metastasis, the first relapse, and the death from any cause or the last follow-up, respectively. Kaplan–Meier survival analyses were used to estimate DMFS, OS, and RFS and the Log rank test was used to compare survival curves. Multivariate analyses (MVA) with Cox proportional hazard methods were used to estimate the risk of sPD-L1. All statistical tests were two-sided; p < 0.05 was considered statistically significant. Statistical analyses were performed using GraphPad Prism (version 8.0.2), SPSS (IBM version 18.0), and R version 3.6.1.
Results
Immune Checkpoint Protein Expression Profiles in NPC and Healthy Controls
Immune checkpoint protein panels were used for detection in 23 patients with NPC and 15 healthy donors. Among patients with NPC, 18 (78.3%) were men and 5 (21.7%) were women (male: female ratio, 3.6:1), with a median age of 48 years (range, 23–74 years). Among 15 healthy controls, 9 were men and 6 were women (male: female ratio, 1.5:1). Other clinical characteristics of patients with NPC and healthy controls are listed in Table 1. As shown in Figure 1A, the costimulatory immune checkpoint proteins TLR2 CD28, GITR, GITRL, CD80, CD86, CD40, and ICOS were substantially higher in NPC plasma samples (all p < 0.001) than in healthy controls, and there was no difference in CD27 between groups (p = 0.129). The coinhibitory immune checkpoint proteins PD1, PD-L1, CTLA4, LAG3, BTLA, and HVEM differed significantly between groups (all p < 0.001), with no difference in TIM-3 (p = 0.813), showed in Figure 1B.
Association Between Circulating sPD-L1 and the NPC Burden
Soluble PD-L1 was abnormally highly expressed in the plasma of patients with NPC. Accordingly, we further expanded the sample to detect the expression of PD-L1 in the plasma of patients with NPC by ELISA. Among 219 patients with NPC, 156 men, and 63 were women (male: female ratio, 2.48:1), with a median age of 48 years (21–89 years). Other clinical characteristics of patients are summarized in Table 2. The median level of sPD-L1 was 81.7 pg/mL (35.8–479.1 pg/mL). The median sPD-L1 levels for patients with stage I, stage II, stage II, and stage IV disease were 58.7 pg/mL (IQR =52.9–89.1 pg/mL), 75.7 pg/mL (IQR = 56.3–93.7 pg/mL), 80.7 pg/mL (IQR = 64.0–105.0 pg/mL), and 85.4 pg/mL (IQR = 67.4–111.3 pg/mL), respectively. Patients with advanced stage NPC tended to have higher plasma levels of sPD-L1 in (R = 0.204, p = 0.002) [Figure 2A]. Similar trends were observed with respect to the T-category (R = 0.172, p = 0.011) and N-category (R = 0.184, p = 0.006) [Figure 2B–C]. We then analyzed paired samples obtained at diagnosis and the end of radiotherapy for a subset of 30 patients with NPC. The levels of post-treatment sPD-L1 were significantly lower than pre-treatment levels in most patients (p < 0.001) (Figure 2D). The level of sPD-L1 increased significantly at the end of radiotherapy in only three cases. Metastasis was verified in these three patients at 4-, 10-, and 11-month follow-ups.
Association of Treatment-Naïve Plasma PD-L1 Levels with Survival in NPC
Among the 219 patients, the median follow-up time was 50 months (7–82 months). The median plasma concentration of sPD-L1 was 81.7 pg/mL (35.8–479.1 pg/mL). The median plasma EBV-DNA level was 11,400 copies/mL. The cut-off value for sPD-L1, as analyzed using X-Tile, was 93.7 pg/mL. According to the cut-off value, 66 patients were assigned to the high-expression group and 153 patients had low expression. Kaplan–Meier survival analyses showed that patients with high expression of sPD-L1 had a worse 4-year DMFS (87.5% vs 74.0%, p = 0.006, Figure 3A), RFS (93.9% vs 86.3%, p = 0.033, Figure 3B), and OS (90.1% vs 81.2%, p=0.018, Figure 3C) than those of patients with low expression. When compared with patients with low levels of EBV DNA (below the median), patients with high EBV DNA levels (above the median) had a significantly worse 5-year DMFS (75.3% vs 93.4%, p < 0.001, Figure D) and OS (92.9% vs 81.7%, p = 0.013). RFS was similar in the groups with low and high levels of EBV DNA (89.4% vs 94.6%, p = 0.120).
Based on a multivariate analysis, high sPD-L1 expression was poor prognostic factor for DMFS (HR = 1.99, 95% CI: 1.01–3.93, p = 0.048) after adjustment for gender, age, clinical T stage, clinical N stage, chemotherapy cycles, lactate dehydrogenase, and EBV-DNA. High sPD-L1 expression was not associated with a low RFS (HR = 2.39, 95% CI: 0.90–6.37, p = 0.081) or OS (HR = 1.71, 95% CI: 0.81–3.62, p = 0.162) (Table 3). Additionally, EBV DNA was an adverse prognostic factor for DMFS (HR = 2.51, 95% CI: 1.09–5.73, p = 0.030) but not for RFS (HR = 0.36, 95% CI: 0.13–1.01, p = 0.053) or OS (HR = 1.54, 95% CI: 0.65–3.68, p = 0.329) (Table 3).
 |
Table 3 Multivariate Analysis of DMFS by Soluble PD-L1 Adjusting for Other Potential Predictors in 219 Nasopharyngeal Carcinoma Patients |
Prognostic Value of the Combination of sPD-L1 and EBV-DNA
As plasma EBV-DNA is a widely accepted NPC biomarker, the prognostic value of pretreatment sPD-L1 combined with EBV-DNA was explored. Among patients with low EBV DNA, 4-year DMFS (92.3% vs 88.0%, p = 0.785) was similar in the low and high sPD-L1 expression groups (Figure 3E). In the group with high EBV DNA expression, DMFS was shorter for patients with high SPD-L1 expression than with low sPD-L1 expression (56.4% vs 82.6%, p = 0.002; Figure 3F).
Discussion
ICIs are a broad-spectrum and long-lasting anti-tumor treatment strategy and are changing the war against cancer.8,17 Immune checkpoint proteins in the bloodstream are potential markers for cancer diagnosis, prognosis assessment, and guiding immunotherapy.8,10–12,14,15,18,19 Our results indicated that the expression profiles of immune checkpoint proteins differ significantly between the plasma of patients with NPC and healthy volunteers, and sPD-L1 is highly expressed in the plasma of patients with NPC. Furthermore, high levels of sPD-L1 were associated with the tumor burden and levels decreased significantly after treatment. High expression of sPD-L1 before treatment suggested a higher risk of metastasis. Based on these findings, pre-treatment sPD-L1 is a candidate prognostic biomarker for NPC.
In this study, 14 immune checkpoint proteins were more highly expressed in the plasma of patients with NPC than in healthy controls. Other than CD27, eight circulating immune costimulatory proteins were upregulated in patients with NPC (ie, TLR2 CD28, GITR, GITRL, CD80, CD86, CD40, ICOS, and GITR). NPC is a classic virus-associated cancer characterized by abundant immune cells, especially T-lymphocytes.2 The abnormally high expression of immune co-stimulatory proteins may be explained by abundant immune cells. EBV infection results in an inflamed tumor microenvironment and chronic inflammation.2,20,21 This inflammatory state contributes to a suppressive immune environment, consistent with the high levels of plasma PD-1/PD-L1, CTLA4, and so on, and is well recognized as a hallmark of cancer.6 Various costimulatory and coinhibitory molecules were up-regulated in the plasma of patients with NPC.
PD-L1 is expressed on tumor cells or tumor-associated cells, such as macrophages or fibroblasts.22 PD-L1 is constitutively expressed on tumor cells with oncogenic pathway or is induced by interferon-γ secreted from infiltrating T cells in tumor sites.23 It is speculated that plasma sPD-L1 originates from the membrane-bound form or from vesicles that are actively excreted from PD-L1-expressing cells.6 Our study showed that plasma sPD-L1 was a positive correlation with the tumor load, which also was consistent with another study conducted by Jia Yang et al.15 Besides, our study showed that sPD-L1 significantly decreased at post-treatment. These results reflect that sPD-L1 is mainly derived from NPC cell, and has the potential to be a biomarker for NPC.
More importantly, sPD-L1 is a strong prognostic factor for distant metastasis and local-regional recurrence in our study. As a pan-tumor immune-suppressive molecule, the prognostic value of the sPD-L1 had been confirmed in multiple tumors, including pancreatic cancer,24 advanced lung cancer,25 hematological malignancies,26,27 and so on.28 Distant metastasis is the most failure pattern in clinical practice for NPC patients.5 MAV showed that high level of sPD-L1 was an inferior prognostic factor of DMFS. sPD-L1 did not have prognostic value for OS and LRFS; this can be explained by the short follow-up time and relatively small sample size. Theodoraki et al demonstrated that plasma exosome PD-L1 is responsible for the biological functions of sPD-L1.29 However, it is important to mention that the source of sPD-L1 could not be determined in our study. Compared with exosome PD-L1, sPD-L1 may be a more effective prognostic biomarker for clinical testing. The detection of exosome PD-L1 is more difficult and costly than sPD-L1 detection. Furthermore, EBV-DNA has been established as a prognostic biomarker of NPC,30 and this was confirmed in our study. However, this is the first analysis of the prognostic values of the combination of sPD-L1 and EBV-DNA. In patients with high EBV DNA levels in the plasma, the level of sPD-L1 can indicate the risk of distant metastasis. Accordingly, the combination could better assess the risk of distant metastasis. It will be necessary to perform clinical trials to explore the treatment value of adjuvant chemotherapy, maintenance chemotherapy, and immunotherapy for patients at high risk of distant metastasis.
Although the levels of 14 soluble immune checkpoint proteins were significantly higher in NPC samples than in controls, we only used ELISA to explore the prognostic value of sPD-L1. The prognostic value of other soluble immune checkpoint proteins should be evaluated in future studies. Additionally, it is not clear whether the expression of sPD-L1 could guide the treatment of PD-1/PD-L1 inhibitors in NPC. If the expression of sPD-L1 can be used as a biomarker of patients expected to benefit from treatment with PD-1/PD-L1 inhibitors, it will have significant clinical implications and is worthy of further analysis.
In our study, the immune status of patients with NPC differs from that of healthy donors and was characterized by the up-regulation of costimulatory and coinhibitory checkpoint molecules in the plasma. In particular, plasma sPD-L1 was positively associated with the tumor burden and was identified as a prognostic factor for DMFS and RFS. The combination of sPD-L1 with EBV-DNA had particularly high prognostic value for DMFS.
Acknowledgment
We thank editage for editing this manuscript.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This work was sponsored by National Clinical Key Specialty Construction Program and Key Clinical Specialty Discipline Construction Program of Fujian, China. This research is also supported by grants from the Fujian Provincial Natural Science Foundation of China (Grant Nos. 2019J01194, 2019J01197, and 2019Y0061), and Joint Funds for the innovation of Science and Technology and Fujian province Grant Nos. 2018Y9109 and 2018Y9114, and was also supported by Science and Technology Program of Fujian Province, China (No. 2018Y2003), and National Natural Science Foundation of China (No. 81972717) and Startup Fund for scientific research, Fujian Medical University, Grant/Award Number: 2017XQ1213.
Disclosure
All authors declare that they have no conflicts of interest in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Gourzones C, Barjon C, Busson P. Host-tumor interactions in nasopharyngeal carcinomas. Semin Cancer Biol. 2012;22(2):127–136. doi:10.1016/j.semcancer.2012.01.002
3. Le QT, Colevas AD, O’Sullivan B, et al. Current treatment landscape of nasopharyngeal carcinoma and potential trials evaluating the value of immunotherapy. J Natl Cancer Inst. 2019;111:655–663. doi:10.1093/jnci/djz044
4. Agulnik M, Epstein JB. Nasopharyngeal carcinoma: current management, future directions and dental implications. Oral Oncol. 2008;44(7):617–627. doi:10.1016/j.oraloncology.2007.08.003
5. Lee AW, Ma BB, Ng WT, Chan AT. Management of nasopharyngeal carcinoma: current practice and future perspective. J Clin Oncol. 2015;33(29):3356–3364. doi:10.1200/JCO.2015.60.9347
6. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi:10.1016/j.cell.2011.02.013
7. Yao S, Zhu Y, Chen L. Advances in targeting cell surface signalling molecules for immune modulation. Nat Rev Drug Discov. 2013;12(2):130–146. doi:10.1038/nrd3877
8. Akinleye A, Rasool Z. Immune checkpoint inhibitors of PD-L1 as cancer therapeutics. J Hematol Oncol. 2019;12(1):92. doi:10.1186/s13045-019-0779-5
9. Fang W, Yang Y, Ma Y, et al. Camrelizumab (SHR-1210) alone or in combination with gemcitabine plus cisplatin for nasopharyngeal carcinoma: results from two single-arm, Phase 1 trials. Lancet Oncol. 2018;19(10):1338–1350. doi:10.1016/S1470-2045(18)30495-9
10. Zhou J, Mahoney KM, Giobbie-Hurder A, et al. Soluble PD-L1 as a biomarker in malignant melanoma treated with checkpoint blockade. Cancer Immunol Res. 2017;5(6):480–492. doi:10.1158/2326-6066.CIR-16-0329
11. Chen G, Huang AC, Zhang W, et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018;560(7718):382–386. doi:10.1038/s41586-018-0392-8
12. Li C, Li C, Zhi C, et al. Clinical significance of PD-L1 expression in serum-derived exosomes in NSCLC patients. J Transl Med. 2019;17(1):355. doi:10.1186/s12967-019-2101-2
13. Wang Q, Zhang J, Tu H, et al. Soluble immune checkpoint-related proteins as predictors of tumor recurrence, survival, and T cell phenotypes in clear cell renal cell carcinoma patients. J Immunother Cancer. 2019;7(1):334. doi:10.1186/s40425-019-0810-y
14. Buderath P, Schwich E, Jensen C, et al. Soluble programmed death receptor ligands sPD-L1 and sPD-L2 as liquid biopsy markers for prognosis and platinum response in epithelial ovarian cancer. Front Oncol. 2019;9:1015. doi:10.3389/fonc.2019.01015
15. Yang J, Hu M, Bai X, et al. Plasma levels of soluble programmed death ligand 1 (sPD-L1) in WHO II/III nasopharyngeal carcinoma (NPC): a preliminary study. Medicine. 2019;98(39):e17231. doi:10.1097/MD.0000000000017231
16. Pan JJ, Ng WT, Zong JF, et al. Proposal for the 8th edition of the AJCC/UICC staging system for nasopharyngeal cancer in the era of intensity-modulated radiotherapy. Cancer. 2016;122(4):546–558. doi:10.1002/cncr.v122.4
17. Darvin P, Toor SM, Sasidharan Nair V, Elkord E. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med. 2018;50(12):165. doi:10.1038/s12276-018-0191-1
18. Costantini A, Julie C, Dumenil C, et al. Predictive role of plasmatic biomarkers in advanced non-small cell lung cancer treated by nivolumab. Oncoimmunology. 2018;7(8):e1452581. doi:10.1080/2162402X.2018.1452581
19. Strati A, Koutsodontis G, Papaxoinis G, et al. Prognostic significance of PD-L1 expression on circulating tumor cells in patients with head and neck squamous cell carcinoma. Ann Oncol. 2017;28(8):1923–1933. doi:10.1093/annonc/mdx206
20. Tsao SW, Tsang CM, Lo KW. Epstein-Barr virus infection and nasopharyngeal carcinoma. Philos T R Soc Lond Ser B Biol Sci. 2017;372:1732.
21. Tan GW, Visser L, Tan LP, van den Berg A, Diepstra A. The microenvironment in Epstein-Barr virus-associated malignancies. Pathogens (Basel, Switzerland). 2018;7:2.
22. Havel JJ, Chowell D, Chan TA. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat Rev Cancer. 2019;19(3):133–150. doi:10.1038/s41568-019-0116-x
23. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi:10.1038/nrc3239
24. Park H, Bang JH, Nam AR, et al. Prognostic implications of soluble programmed death-ligand 1 and its dynamics during chemotherapy in unresectable pancreatic cancer. Sci Rep. 2019;9(1):11131. doi:10.1038/s41598-019-47330-1
25. Okuma Y, Hosomi Y, Nakahara Y, Watanabe K, Sagawa Y, Homma S. High plasma levels of soluble programmed cell death ligand 1 are prognostic for reduced survival in advanced lung cancer. Lung Cancer. 2017;104:1–6. doi:10.1016/j.lungcan.2016.11.023
26. Rossille D, Gressier M, Damotte D, et al. High level of soluble programmed cell death ligand 1 in blood impacts overall survival in aggressive diffuse large B-Cell lymphoma: results from a French multicenter clinical trial. Leukemia. 2014;28(12):2367–2375. doi:10.1038/leu.2014.137
27. Zhang X, Liu L, Zhou S, et al. Plasma soluble programmed death ligand 1 levels predict clinical response in peripheral T-cell lymphomas. Hematol Oncol. 2019;37(3):270–276. doi:10.1002/hon.v37.3
28. Wei W, Xu B, Wang Y, Wu C, Jiang J, Wu C. Prognostic significance of circulating soluble programmed death ligand-1 in patients with solid tumors: a meta-analysis. Medicine. 2018;97(3):e9617. doi:10.1097/MD.0000000000009617
29. Theodoraki MN, Yerneni SS, Hoffmann TK, Gooding WE, Whiteside TL. Clinical significance of PD-L1(+) exosomes in plasma of head and neck cancer patients. Clin Cancer Res. 2018;24(4):896–905. doi:10.1158/1078-0432.CCR-17-2664
30. Lam WKJ, Chan KCA, Lo YMD. Plasma Epstein-Barr virus DNA as an archetypal circulating tumour DNA marker. J Pathol. 2019;247(5):641–649. doi:10.1002/path.5249
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



