Back to Journals » Therapeutics and Clinical Risk Management » Volume 17
High Pretreatment Platelet-to-Albumin Ratio Predicts Poor Survival Results in Locally Advanced Nasopharyngeal Cancers Treated with Chemoradiotherapy
Authors Haksoyler V, Topkan E
Received 13 May 2021
Accepted for publication 28 June 2021
Published 5 July 2021 Volume 2021:17 Pages 691—700
DOI https://doi.org/10.2147/TCRM.S320145
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Veysel Haksoyler,1 Erkan Topkan2
1Medline Hospital, Department of Medical Oncology, Adana, Turkey; 2Baskent University Medical Faculty, Department of Radiation Oncology, Adana, Turkey
Correspondence: Erkan Topkan
Baskent University Medical Faculty, Department of Radiation Oncology, Adana, 01120, Turkey
Tel +90-533-7381069
Fax +90-322-3444452
Email [email protected]
Purpose: In a lack of similar research, we assessed the prognostic utility of pretreatment platelet-to-albumin ratio (PAR) in locally advanced nasopharyngeal carcinoma (LANPC) patients managed with concurrent chemoradiotherapy (CCRT).
Patients and Methods: Present retrospective analysis included a sum of 128 consecutively treated LANPC patients who underwent cisplatinum-based radical CCRT. Availability of an ideal pretreatment PAR cutoff that may stratify the study population into two cohorts with significantly distinct survival outcomes was sought by utilizing the receiver operating characteristic (ROC) curve analysis. The primary and secondary endpoints were overall survival (OS) and progression-free survival (PFS), respectively.
Results: A rounded 5.2 [area under the curve (AUC): 68.9%; sensitivity: 67.4%; and specificity: 65.2%] value was identified as the ideal PAR cutoff that grouped patients into two gatherings [PAR≥ 5.2 (N=60) versus < 5.2 (N=68)]. The median follow-up duration was 86.4 months (range: 9– 147). Kaplan–Meier comparisons between the two PAR groups revealed significantly diminished median PFS (69.4 versus 106.8 months for PAR< 5.2; P< 0.012) and OS (88.3 versus not reached yet for PAR< 5.2; P= 0.023) for the PAR ≥ 5.2 group. The results of multivariate analyses affirmed the pretreatment PAR≥ 5.2 as an independent prognostic factor that indicates diminished PFS (P= 0.016) and OS (P= 0.019) together with the respective N2-3 nodal stage (versus N0-1; P< 0.05 for PFS and OS, respectively) and weight loss > 5% at past six months (≤ 5%; P< 0.05 for PFS and OS, respectively).
Conclusion: The results of the current retrospective analysis provided a robust and independent adverse prognostic value for pretreatment PAR ≥ 5.2 in terms of median and long-term PFS and OS outcomes in LA-NPC patients this patient group treated with conclusive CCRT.
Keywords: concurrent chemoradiotherapy nasopharyngeal cancer, platelet-to-albumin ratio, prognostic worth, survival results
Plain Language Summary
We aimed to assess the prognostic utility of pretreatment platelet-to-albumin ratio (PAR) in locally advanced nasopharyngeal carcinoma (LANPC) patients managed with concurrent chemoradiotherapy (CCRT). A sum of 128 LANPC patients was included. Receiver operating characteristic (ROC) curve analysis was used to search for an ideal PAR cutoff that may stratify patients into two groups. The cutoff was identified at 5.2, with PAR ≥ 5.2 group demonstrating significantly shorter PFS and OS results compared to the PAR < 5.2 group. The results of the current retrospective analysis provided a robust and independent adverse prognostic value for pretreatment PAR ≥ 5.2 in terms of median and long-term PFS and OS outcomes in LA-NPC patients this patient group treated with conclusive CCRT.
Introduction
Nasopharyngeal cancers (NPCs) are highly aggressive malignant tumors of the nasopharyngeal epithelium, wherein the radical concurrent chemoradiotherapy (CCRT) with intensity-modulated radiotherapy (IMRT) remains the current highest quality level therapy for medically fit locally advanced NPCs (LANPC) patients.1–3 Although the marked improvements in diagnostic and staging tools, collectively with the successful implementation of IMRT, had positively enhanced the loco-regional tumor control rates of LANPCs,2–4 yet, up to 20% of all LANPCs experience distant metastasis (DM) during their disease course.5 Currently, the TNM (tumor-node-metastasis) staging system is globally noticed to embody the most reliable framework with regard to the patient stratification, selecting the fittest treatment, and accurate prediction of the clinical outcomes in these patients’ groups. Nevertheless, it is not uncommon to observe notably divergent clinical results between the LANPC patients with identical TNM stages after interchangeable treatments.6,7 These significant outcome contrasts are mainly associated with the truth that the TNM framework depends solely upon the local and regional tumor extent without esteeming the broad biological and functional differences among the tumor- and host-related response factors.6,8 Such biological and functional diversities among the equivalent stage tumors render the gold standard TNM framework flawed in terms of accurate patient stratification and prediction of the clinical outcomes, which emphasize the pressing necessity for the discovery of novel biomarkers for more refined prognostic stratification of LA-NPC patients and confident prediction of their ultimate outcomes following various treatments.
Diminished anti-tumoral immunity, systemic inflammation, and malnutrition represent the three key factors that invariably enhance all phases of the carcinogenesis and cancer progression from the first initiation to the last fatal widespread distant metastases steps.9–11 In this regard, the acute phase reactants albumin (ALB) and C-reactive protein (CRP), and peripheral neutrophils, monocytes, lymphocytes, and platelets (PLTs), and their various two or three cell combinations, specifically the neutrophil-to-lymphocyte ratio (NLR), PLT-to-lymphocyte ratio (PLR), and lymphocyte-to-monocyte ratio (LMR), systemic inflammatory response index (SIRI), and systemic immune-inflammation index (SII) have shown to be independent prognosticators for many cancers including the NPCs.12–17 In like manner, the platelet-to-albumin ratio (PAR) has been recently proposed as a novel index that may dependably reflect the actual systemic inflammation and immune-nutritional status of the cancer patients.18 Past investigations on the prognostic worth of PAR showed that the preoperative PAR was robustly associated with the patients’ prognoses in pancreatic adenocarcinomas, cholangiocarcinomas, hepatocellular carcinomas, and non-small cell lung carcinomas, as well as the malignant melanomas undergoing 1 surgery.18–21 To our best data, though the amassed proof proposes a respectable independent prognostic power for PAR in prediction of the outcomes of patients with various cancers, to date, no study has explored the prognostic worth of pre-CCRT PAR in LANPC patients treated with definitive CCRT. Therefore, the present retrospective cohort investigation was planned to uncover the plausible prognostic usefulness of pre-CCRT PAR measures in LANPC patients treated with radical CCRT.
Patients and Methods
Data Collection
We assembled all data by performing a retrospective review of the medical records of LANPC patients treated with radical CCRT between January 2007 and December 2017 at Baskent University Medical Faculty, Department of Radiation Oncology. The inclusion criteria were: age 18–80 years, Eastern Cooperative Oncology Group (ECOG) performance of 0–1, proven type 2–3 squamous cell carcinoma (SCC), clinical/radiological proof of T1-2N2-3M0 or T3‐4N0‐3M0 NPC stage per AJCC 8th ed., body mass index ≥20.0 kg/m2, available baseline complete blood count and biochemistry tests, no chemotherapy/radiotherapy (RT) history, received at least one cycle of platinum-based chemotherapy concurrent with RT, available baseline head and neck clinical examinations, chest computerized tomography (CT), brain magnetic resonance imaging (MRI), and fluorodeoxyglucose-positron emission CT (PET-CT) scans, available RT and chemotherapy charts, and available records of follow-up and survival data. Because the PAR mirrors the actual inflammatory, nutritional, and immune status of cancer patients during the measurement instance, the patients with the following conditions were excluded from the analyses to prevent the unpredictable alterations on the PLT and/or ALB measures, and therefore, the clinical outcomes: diseases or medications causing thrombocytopenia or thrombocytosis, steroid treatment, acute or chronic infectious diseases, dehydration, and complete blood or platelet transfusions at the preceding three months.
This retrospective study protocol was carried out following the official guidelines stipulated in the Declaration of Helsinki and was approved by the Baskent University Ethics and Scientific Committee before the procuring of any patient data. All patients provided written informed consent before the commencement of CCRT, either themselves or legitimately sanctioned representatives for assortment and analysis of blood samples, pathologic specimens, and academic presentation and publication of outcomes in an anonymized fashion.
Chemoradiotherapy Protocol
Each eligible patient underwent definitive CCRT with the RT and chemotherapy schemes as detailed elsewhere.22 In brief, the RT technique was 3-dimensional conformal RT (3D-CRT) between January 2007 to June 2011 and intensity-modulated RT (IMRT) from that point, administered in a daily fractionation premise: 5 days/week, for seven weeks.
Platelet-to-Albumin Ratio Calculations
We calculated the PAR by utilizing the PLT and serum ALB measures procured from the total blood count and biochemistry tests obtained on the first CCRT day: PAR= PLT (109)/serum ALB (g/dL).
Evaluation of Adverse Events and Response
Acute adverse events and general health status were evaluated at once per week intervals, or more frequently, throughout the CCRT course. While following the completion of the CCRT, patients were surveyed 3- and 6-monthly intervals for the first two and successive three years, and yearly intervals the fifth follow-up year, if not demanded otherwise. The Common Terminology Criteria for Adverse Events v3 scoring criteria were used to objectively assess the acute and chronic adverse events, with prerecorded scores being the worst grade observed.
Despite representing a retrospectively designed study protocol, treatment response was assessed prospectively within the above-mentioned visit intervals for chronic toxicity evaluations according to our institutional follow-up standards for NPC patients. All patients were assessed with detailed endoscopic head and neck examinations at each follow-up, with first imaging evaluations being performed via restaging PET-CT scans at the 90-days follow-up visit. Treatment response was scored per the EORTC-1999 guidelines until 2009 and per the PET Response Criteria in Solid Tumors (PERCIST) afterward. Head and neck MRI and/or CT replaced the PET-CT scans whenever the complete metabolic response was affirmed. Other restaging tools were additionally utilized only if locoregional relapses or DM were suspected. Appropriate single or combined modality salvage or palliative interventions were administered to the patients with proven local and/or regional relapses or DM.
Statistics
The association between the pre-CCRT PAR levels and the OS (interval between the first day of CCRT and death/last visit) was the primary objective of our present analysis. While the progression-free survival (PFS: interval between the first day of CCRT and the date of any type of disease progression/death/the last follow-up) comprised the secondary objective.
Continuous variables were described by using means, medians, and ranges, while categorical variables were described by frequency distributions. The frequency distributions and their correlations among different groups were compared by Chi-square tests, Student’s t-tests, Fisher's exact test, or Spearman correlations as appropriate. Comparisons between the frequency distributions were performed by utilizing Chi-square test, Student’s t-test, Fisher's exact test or Spearman correlation estimates, as fitted. The ability of pre-CCRT PAR levels in discriminating the groups with distinctive PFS and OS at a certain cutoff(s) was tested by using the receiver operating characteristic (ROC) curve analysis. However, we utilized the well-recognized 70 years of age cutoff of the International Society of Geriatric Oncology and the critical weight loss cutoff of >5% at past 6 months recommended by the Delphi consensus on cancer cachexia, respectively.23,24 Kaplan–Meier estimates and Log rank tests were utilized to compare the OS and PFS results between the risk groups, while the Cox proportional hazards model was employed for multivariate analysis. Any two-sided P <0.05 value was considered significant.
Results
Medical records of a sum of 216 NPC patients were assessed, and 128 consecutive patients who met the prespecified eligibility criteria and underwent definitive CCRT for LANPC between January 2007 and December 2017 at our institution were eligible for the present analysis. The remaining 88 patients were excluded from the study: due to presentation with stage IVB (N=52) and III (N=36) NPCs, respectively. Pretreatment patient and disease characteristics are as depicted in Table 1. Of note, 95 (74.2%), 28 (21.9%), and 5 (3.9%) patients were able to receive prescribed 3, 2, and 1 cycle(s) of chemotherapy concurrent with RT, while 73 (57.0) of them were able to receive 2 (N=57; 44.5%) or 1 (N=16; 12.5%) additional adjuvant chemotherapy cycles after the CCRT. Only 1 (0.8%) case of treatment-related death, ascribed to tracheoesophageal fistula-related aspiration pneumonia that occurred in the 16th months of follow-up was reported.
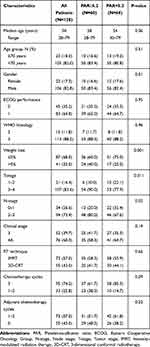 |
Table 1 Baseline and Treatment Characteristics |
Median follow-up was 86.4 months (range: 9–147), with 78 (60.9%) and 63 (49.2%) patients being still alive and free of disease progression during the final analysis. Although the median OS was not reached yet for the entire study population, the median PFS was 89.1 months [95% confidence interval (CI) = 71.8–106.4]. The respective 10-year PFS and OS rates were 34.3% and 54.7%.
We explored the convenience of relevant PAR cutoffs that may dichotomize patients into two cohorts with significantly distinct PFS and OS outcomes by using the well-recognized ROC curve analysis method. The ROC curve analyses exhibited significance at a rounded PAR value of 5.2 [cutoff: 5.24; area under the curve (AUC): 77.2%; sensitivity: 74.3%; and specificity: 72.6% for PFS, and cutoff: 5.17; AUC: 68.9%; sensitivity: 67.4%; and specificity: 65.2% for OS, individually] (Figure 1). Hence, the patients were stratified into two groups per this cutoff value for further analyses: Group 1: PAR ≥ 5.2 and Group 2: PAR < 5.2, separately.
 |
Figure 1 Results of receiver operating characteristic curve analyses: (A) progression-free survival, and (B) overall survival. |
Although the majority of the baseline patient and disease characteristics were equally distributed among the two PAR groups, yet, presenting with higher N2-3 status (80.0% versus 67.6%; P= 0.02) and weight loss WL>5% over past six months (40.0% versus 25.0%; P= 0.001) were significantly more prevalent in the PAR ≥ 5.2 group (Table 1). There was no statistically significant difference between the RT techniques (P= 0.66) or the cycles of concurrent chemotherapy received during the RT course (P= 0.09) among the two PAR cohorts. As depicted in Figure 2, comparative Kaplan–Meier survival estimates showed that the median PFS (69.4 versus 106.8 months; P=0.012) and OS (88.3 months versus not reached yet; P=0.023) lengths were significantly shorter in the PAR ≥ 5.2 group (Figure 2). Likewise, the respective 10-year PFS (14.5% versus 45.1%; P<0.001) and OS (31.6% versus 74.1%; P=0.001) rates were also inferior in the PAR ≥ 5.2 group (Figure 2).
 |
Figure 2 Survival outcomes according to pre-chemoradiotherapy platelet-to-albumin ratio (PAR) groups: (A) progression-free survival, and (B) overall survival. |
In univariate analyses (Table 2), we discovered the advanced N-stage (2–3 versus 0–1), more profound WL over past six months (>5% versus ≤5%), and higher PAR values (≥5.2 versus <5.2) were the factors demonstrating significant associations with unfavorable PFS (P < 0.05, for each variable) and OS (P < 0.05, for each variable) outcomes. The multivariate analyses results indicated all three variables to retain their independent significant adverse influence on both of the PFS (P < 0.05, for each variable) and OS (P < 0.05, for each variable) results (Tables 2 and 3).
 |
Table 2 Outcomes of Univariate and Multivariate Analyses |
 |
Table 3 Median Progression-Free- and Overall Survival Results per Factors with Independent Multivariate Significance |
Discussion
In lack of comparable studies, we investigated the influence of pre-CCRT PAR values on the survival outcomes of a sum of 128 LA-NPC consecutive patients treated with radical CCRT. Our retrospective, but first, study results in LA-NPC patients assigned a strong and independent adverse prognostic worth to the pretreatment PAR≥5.2 in terms of median and 10-year PFS and OS results for these patients group treated with conclusive CCRT.
The present results verified the independent prognostic significance of the well-recognized N-stage and weight loss status (Tables 2 and 3). The N-stage, which remains the indispensable parameter of the current staging system, appears to reflect the locoregional aggressiveness and tendency for the development of DMs, and therefore, dismal prognosis in such patients.25 The degree of weight loss in the past 6-months has been specified as one of the key measures to define cancer cachexia in both the Washington and Delphi consensus, respectively.24,26 Additionally, our results confirm the recently published findings of a comprehensive study by Ou et al, who proposed the pretreatment percent weight loss as a vigorous prognostic marker after treatment in a group of 681 NPC patients.27
The most remarkable finding of this current study was the successful demonstration of the significant prognostic influence of the pretreatment PAR values on the survival outcomes of LANPC patients treated with radical CCRT. Such that, a pretreatment PAR cutoff of 5.2 was able to stratify the study cohort into two gatherings with significantly different median and long-term PFS and OS results (Table 3). It is not desirable to remark robustly on the true worth of a suggestion of a robust and independent relationship between the diminished PFS (P= 0.012) and OS (P= 0.001) results and a PAR≥5.2 esteem observed here in the absence of similarly designed LANPC research. Yet, present outcomes seem, by all accounts, to be in good congruency with those previously reported for pancreatic adenocarcinoma, cholangiocarcinoma, hepatocellular carcinoma, and non-small cell lung carcinoma patients undergoing surgery.18–21 Guo et al21 in a group of 198 stage I–IV non-small cell lung cancer patients who underwent thoracic surgery found that the preoperative PAR>8.8 was an independent predictor of significantly worse median OS times (P<0.001). Likewise, in the largest ever PAR study, Li et al20 reported that patients with high preoperative PAR (>4.8) had a higher recurrence risk and lower long-term survival chance than those with low PAR in a total of 628 hepatocellular carcinoma patients treated with liver resection. The remaining two studies in pancreatic cancer and cholangiocarcinoma patients further confirmed the poor prognostic characteristic of the high preoperative PAR values in these tumor primaries, respectively.18,19 As apparent, all four studies were conducted in patients treated with surgery, which is currently not accepted as an appropriate treatment for newly diagnosed LANPCs. Therefore, our study appears to be the first for both the LANPC and CCRT literature. Nevertheless, all five studies invariably suggest a poor prognostic worth for high PAR values regardless of the tumor primary, including the one presented here. Studies on the individual components of the PAR formula have also demonstrated that the pretreatment high PLT and low ALB levels were associated with significantly more dismal prognoses in NPC patients.28–31 Although subsequent corroborative investigations are required, yet, we believe the PAR is conceivably a more powerful index than the individual PLT and ALB counts in the prediction of outcomes in cancer patients. This belief is mainly based on two facts: First, the PAR is a more reputable biological marker, as the chance of being affected by various physiological and/or disease conditions is less likely for the composite PAR than its PLTs and ALB components individually. And second, the PAR has the ability to reflect the patient’s actual immune, systemic inflammation, and nutritional status simultaneously considering the individual functions of PLTs and ALB.
Albeit the previous studies meticulously examined and proved the prognostic worth of PLT and ALB individually or as an essential component of various blend index scores, yet, the exact mechanisms underlying the intricate connection between a high PAR measure and reduced survival times have not been clarified to date. In any case, it is possible to speculate reasonably by directly assessing the association between the PLT and ALB functions in cancer and their probable influence on survival results of NPC patients. First, striking proof from recent clinical and experimental studies exhibited that the PLTs play crucial roles in the multistep process of tumor invasion and metastasis.32 Once activated, PLTs stimulate cell-to-cell communication through the secretion of various key factors and microvesicles.33 PLTs may not only provoke the progression of aggressive tumor behaviors by protecting the tumor cells from immune elimination by aggregation and thrombus formation around the tumor cells and adhesion to the vascular endothelium,33 but may further promote tumor growth, invasion, transendothelial migration, and metastasis processes via the secretion of a variety of growth factors, as well.33–36 PLTs can likewise instigate epithelial-mesenchymal transition (EMT) and incite a more malignant phenotype in cancer cells characterized by enhanced migratory and early metastatic capabilities.37,38 More recently, animal studies in mice have proposed that PLT signals may recruit granulocytes to the tumor site that stimulates the formation of a profoundly inflammatory and immune depressed early prometastatic microenvironment.39 The second component of the PAR formula is the ALB which is broadly perceived as a reliable marker of nutritional status, immune functions, and the seventh hallmark of cancer: systemic inflammation.40 Reduced levels of ALB are commonly detected in many cancer primaries and universally esteemed as an indicator of poor prognosis, including the LANPCs.41 Any reduction in Alb measures indicates the presence of an aggravated systemic inflammatory response as it is almost invariably associated with elevated C-RP measures.42,43 In addition, similar to the many other stress factors, the frequently encountered Epstein-Barr virus (EBV) infection may stimulate the production of inflammatory cytokines, like interleukin (IL)-1, IL-6, IL-8, vascular endothelial growth factor (VEGF) and tumor necrosis factor (TNF)-α in LANPCs.44,45 Consequently, this incited state may stimulate the rapid CRP synthesis and resultant ALB catabolism in the liver. Confirming the adverse prognostic worth of reduced ALB levels, Tao et al demonstrated that the increased levels of C-RP/ALB ratio were significantly associated with OS in patients with NPC who received IMRT, which represents an after effect of the depressed ALB and elevated C-RP measures, or both.46 Albumin, besides, represents an essential component of the cancer cachexia definition of the Washington Consensus, where decreased ALB levels indicate malnutritional body status, which inevitably ends up with death in the absence of effective anti-cachectic treatments.47 In support, depressed ALB levels were shown to be connected with dismal prognoses in many cancers including the NPCs.22,26,48–50 Moreover, as aforementioned, a recently published meta-analysis comprising 7339 NPCs from 10 studies showed that lower pre-treatment serum ALB levels were associated with significantly worse OS (HR= 1.32, 95% CI 1.17–1.48) and DM-free survival (HR= 1.40, 95% CI 1.08–1.80) outcomes, and the results were relevant for all disease stages.31 Therefore, although further research may provide valuable insights into the exact relationship between a high PAR and diminished survival results, the present and the above-mentioned results altogether suggest convenient usefulness for the novel PAR in the prognostic stratification of LANPC patients treated with definitive CCRT.
Chief strengths of our present investigation are as follows: First, head and neck MRI and PET-CT constituted the standard initial staging procedures for each qualified patient, as both were found to increase the staging accuracy of NPCs in terms of exclusion of second primary cancers; T, N, and M staging; target volume delineation; response, and toxicity evaluations; as well as the prognostic stratification of patients.51,52 Second, to avoid time-dependent potential measurement biases, the PAR was calculated by utilizing the PLT and ALB counts obtained on the first day of the CCRT in all patients. And lastly, the RT and concurrent chemotherapy regimens were standard for the whole study cohort. Conversely, the present study also has some certain drawbacks: First, the results introduced here must be granted as just hypothesis-generating, as they would have been unintentionally altered in favor of one group by some unforeseeable factors, which represent a familiar problem for all retrospectively designed single-institutional studies with relatively small cohort sizes. Second, absence of internal and/or external validation cohort may have undervalued some prognostically important factors, such as the superiority of IMRT technique over the 3D-CRT in LA-NPC patients. Third, the PAR cutoff used here and its effect on the results reflected only a solitary time-point estimation and related PFS and OS results that neglect the fluctuating nature of the PLT and ALB measures, as we did not perform multiple measurements during the CCRT course and subsequent period. Therefore, forthcoming large-scale studies explicitly focusing on the PAR dynamics all through the disease course may provide more reliable PAR cutoff(s) with more robust usefulness for the precise prognostic stratification of such patients. Fourth, we may have forfeited the opportunity to evaluate the potential associations between the PAR and other nutritional and immune-inflammatory factors in the lack of such analyses. And fifth, likely variations among the salvage interventions may have unintentionally favored one group in an unpredictable manner. Acknowledging these impediments, we recommend that the present results should be granted just as hypothesis-generating until the confirmatory results become available from appropriately designed ensuing investigations explicitly addressing these issues.
Conclusions
The results of the current retrospective analysis provided a robust and independent adverse prognostic value for pretreatment PAR ≥ 5.2 in terms of median and long-term PFS and OS outcomes in LA-NPC patients this patient group treated with conclusive CCRT.
Data Sharing Statement
Data cannot be shared publicly because the data is owned and saved by Baskent University Medical Faculty. Data are available from the Baskent University Radiation Oncology Institutional Data Access/Ethics Committee (contact via Baskent University Ethics Committee) for researchers who meet the criteria for access to confidential data: contact address, [email protected].
Disclosure
The authors report no competing interests in this work.
References
1. Baujat B, Audry H, Bourhis J, et al. Chemotherapy in locally advanced nasopharyngeal carcinoma: an individual patient data meta-analysis of eight randomized trials and 1753 patients. Int J Radiat Oncol Biol Phys. 2006;64(1):47–56. doi:10.1016/j.ijrobp.2005.06.037
2. Lee AW, Ma BB, Ng WT, Chan AT. Management of nasopharyngeal carcinoma: current practice and future perspective. J Clin Oncol. 2015;33(29):
3. Lin JC, Jan JS, Hsu CY, Liang WM, Jiang RS, Wang WY. Phase III study of concurrent chemoradiotherapy versus radiotherapy alone for advanced nasopharyngeal carcinoma: positive effect on overall and progression-free survival. J Clin Oncol. 2003;21:631–637. doi:10.1200/JCO.2003.06.158
4. Gordin A, Golz A, Daitzchman M, et al. Fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography imaging in patients with carcinoma of the nasopharynx: diagnostic accuracy and impact on clinical management. Int J Radiat Oncol Biol Phys. 2007;68(2):370–376. doi:10.1016/j.ijrobp.2006.12.028
5. Yang Q, Cao S, Guo L, et al. Induction chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: long-term results of a phase III multicentre randomised controlled trial. Eur J Cancer. 2019;119:87–96. doi:10.1016/j.ejca.2019.07.007
6. Wei WI, Sham JS. Nasopharyngeal carcinoma. Lancet. 2005;365(9476):
7. Chen YP, Wang YQ, Lv JW, et al. Identification and validation of novel microenvironment‐based immune molecular subgroups of head and neck squamous cell carcinoma: implications for immunotherapy. Ann Oncol. 2019;30(1):
8. Fernandes JV, Cobucci RN, Jatobá CA, et al. The role of the mediators of inflammation in cancer development. Pathol Oncol Res. 2015;21:527–534. doi:10.1007/s12253-015-9913-z
9. Liebowitz D. Nasopharyngeal carcinoma: the Epstein-Barr virus association. Semin Oncol. 1994;21(3):376–381.
10. Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi:10.1038/nature01322
11. Elinav E, Nowarski R, Thaiss CA, et al. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13(11):759–771. doi:10.1038/nrc3611
12. Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer. 2016;16(7):
13. Feng PH, Lee KY, Chang YL, et al. CD14(+)S100A9(+) monocytic myeloid‐derived suppressor cells and their clinical relevance in non‐small cell lung cancer. Am J Resp Crit Care Med. 2012;186(10):
14. Bozan N, Kocak OF, Dinc ME, et al. Mean platelet volume, red cell distribution width, and neutrophil‐to‐lymphocyte ratio before and after surgery in patients with carotid body tumors. J Craniofac Surg. 2017;28(7):
15. Yang J, Guo X, Wang M, et al. Pre‐treatment inflammatory indexes as predictors of survival and cetuximab efficacy in metastatic colorectal cancer patients with wild‐type RAS. Sci Rep. 2017;7(1):17166. doi:10.1038/s41598-017-17130-6
16. Li S, Lan X, Gao H, et al. Systemic Inflammation Response Index (SIRI), cancer stem cells and survival of localised gastric adenocarcinoma after curative resection. J Cancer Res Clin Oncol. 2017;143(12):
17. Tong YS, Tan J, Zhou XL, Song YQ, Song YJ. Systemic immune‐inflammation index predicting chemoradiation resistance and poor outcome in patients with stage III non‐small cell lung cancer. J Transl Med. 2017;15(1):221. doi:10.1186/s12967-017-1326-1
18. Shirai Y, Shiba H, Haruki K, et al. Preoperative platelet-to-albumin ratio predicts prognosis of patients with pancreatic ductal adenocarcinoma after pancreatic resection. Anticancer Res. 2017;37(2):787–793. doi:10.21873/anticanres.11378
19. Saito N, Shirai Y, Horiuchi T, et al. Preoperative platelet to albumin ratio predicts outcome of patients with cholangiocarcinoma. Anticancer Res. 2018;38(2):987–992.
20. Li C, Peng W, Zhang XY, Wen TF, Chen LP. The preoperative platelet to albumin ratio predicts the prognosis of hepatocellular carcinoma patients without portal hypertension after liver resection. Medicine (Baltimore). 2019;98(45):e17920. doi:10.1097/MD.0000000000017920
21. Guo M, Sun T, Zhao Z, Ming L. Preoperative platelet to albumin ratio predicts outcome of patients with non-small-cell lung cancer. Ann Thorac Cardiovasc Surg. 2020. doi:10.5761/atcs.oa.20-00090
22. Topkan E, Ekici NY, Ozdemir Y, et al. Baseline hemoglobin <11.0 g/dL has stronger prognostic value than anemia status in nasopharynx cancers treated with chemoradiotherapy. Int J Biol Markers. 2019;34(2):139–147.
23. Decoster L, van Puyvelde SK, Mohile U, et al. Screening tools for multidimensional health problems warranting a geriatric assessment in older cancer patients: an update on SIOG recommendations. Ann Oncol. 2015;26:288–300. doi:10.1093/annonc/mdu210
24. Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12(5):489–495. doi:10.1016/S1470-2045(10)70218-7
25. Qu W, Li S, Zhang M, Qiao Q. Pattern and prognosis of distant metastases in nasopharyngeal carcinoma: a large-population retrospective analysis. Cancer Med. 2020;9(17):6147–6158. doi:10.1002/cam4.3301
26. Evans WJ, Morley JE, Argilés J, et al. Cachexia: a new definition. Clin Nutr. 2008;27(6):793–799. doi:10.1016/j.clnu.2008.06.013
27. Ou Q, Cui C, Zeng X, et al. Grading and prognosis of weight loss before and after treatment with optimal cutoff values in nasopharyngeal carcinoma. Nutrition. 2020;78:110943. doi:10.1016/j.nut.2020.110943
28. Chen YP, Bing-cheng Zhao BC, Chen C, et al. Pretreatment platelet count improves the prognostic performance of the TNM staging system and aids in planning therapeutic regimens for nasopharyngeal carcinoma: a single-institutional study of 2626 patients. Chin J Cancer. 2015;34(3):137–146. doi:10.1186/s40880-015-0006-x
29. Chang H, Gao J, Xu BQ, et al. Haemoglobin, neutrophil to lymphocyte ratio and platelet count improve prognosis prediction of the TNM staging system in nasopharyngeal carcinoma: development and validation in 3237 patients from a single institution. Clin Oncol (R Coll Radiol). 2013;25(11):639–646. doi:10.1016/j.clon.2013.07.004
30. Gao J, Zhang HY, Xia YF, et al. Increased platelet count is an indicator of metastasis in patients with nasopharyngeal carcinoma. Tumour Biol. 2013;34(1):39–45. doi:10.1007/s13277-012-0508-y
31. Yang H, Wang K, Liang Z, et al. Prognostic role of pre-treatment serum albumin in patients with nasopharyngeal carcinoma: a meta-analysis and systematic review. Clin Otolaryngol. 2020;45(2):167–176. doi:10.1111/coa.13454
32. Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. 2011;11(2):123–134. doi:10.1038/nrc3004
33. Dovizio M, Alberti S, Guillem-Llobat P, Patrignani P. Role of platelets in inflammation and cancer: novel therapeutic strategies. Basic Clin Pharmacol Toxicol. 2011;14(1):118–127.
34. Schumacher D, Strilic B, Sivaraj KK, Wettschureck N, Offermanns S. Platelet derived nucleotides promote tumor-cell transendothelial migration and metastasis via P2Y2 receptor. Cancer Cell. 2013;24(1):130–137. doi:10.1016/j.ccr.2013.05.008
35. Ariad S, Seymour L, Bezwoda WR. Platelet-derived growth factor (PDGF) in plasma of breast cancer patients: correlation with stage and rate of progression, Breast Cancer Res. Treat. 1991;20(1):11–17.
36. Goubran HA, Burnouf T, Radosevic M, El-Ekiaby M. The platelet-cancer loop, Eur. J Intern Med. 2013;24(5):393–400.
37. Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial–mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20(5):576–590. doi:10.1016/j.ccr.2011.09.009
38. Dovizio M, Maier TJ, Alberti S, et al. Pharmacological inhibition of platelet–tumor cell cross-talk prevents platelet-induced overexpression of cyclooxygenase-2 in HT29 human colon carcinoma cells. Mol Pharmacol. 2013;84(1):25–40. doi:10.1124/mol.113.084988
39. Labelle M, Begum S, Hynes RO. Platelets guide the formation of early metastatic niches. Proc Natl Acad Sci U S A. 2014;111:E3053–61. doi:10.1073/pnas.1411082111
40. Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30(7):1073–1081. doi:10.1093/carcin/bgp127
41. Gao N, Yang RN, Meng Z, Wang WH. The prognostic value of C-reactive protein/albumin ratio in nasopharyngeal carcinoma: a meta-analysis. Biosci Rep. 2018;38(6):BSR20180686. doi:10.1042/BSR20180686
42. Vigushin DM K, Pepys MB, Hawkins PN. Metabolic and scintigraphic studies of radioiodinated human C-reactive protein in health and disease. J Clin Invest. 1993;91(4):1351–1357. doi:10.1172/JCI116336
43. Chojkier M. Inhibition of albumin synthesis in chronic diseases: molecular mechanisms. J Clin Gastroenterol. 2005;39(4 Suppl 2):S143–146. doi:10.1097/01.mcg.0000155514.17715.39
44. Eliopoulos AG, Stack M, Dawson CW, et al. Epstein-Barr virus-encoded LMP1 and CD40 mediate IL-6 production in epithelial cells via an NF-kappaB pathway involving TNF receptor-associated factors. Oncogene. 1997;14:2899–2916. doi:10.1038/sj.onc.1201258
45. Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111:1805–1812. doi:10.1172/JCI200318921
46. Tao CJ, Chen YY, Jiang F, et al. The C-reactive protein/albumin ratio is an independent prognostic factor for overall survival in patients with nasopharyngeal carcinoma receiving intensity-modulated radiotherapy. J Cancer. 2016;7(14):2005–2011. doi:10.7150/jca.16210
47. Lambert JW, Ingham M, Gibbs BB, et al. Using preoperative albumin levels as a surrogate marker for outcomes after radical cystectomy for bladder cancer. Urology. 2013;81:587–592. doi:10.1016/j.urology.2012.10.055
48. Espinosa E, Feliu J, Zamora P, et al. Serum albumin and other prognostic factors related to response and survival in patients with advanced non-small cell lung cancer. Lung Cancer. 1995;12:67–76. doi:10.1016/0169-5002(95)00407-R
49. Bizzo SM, Meira DD, Lima JM, et al. Serum albumin and vascular endothelial growth factor in epithelial ovarian cancer: looking at adnexal tumor drainage. Arch Gynecol Obstet. 2011;283:855–859. doi:10.1007/s00404-010-1491-4
50. Lis CG, Grutsch JF, Vashi PG, et al. Is serum albumin an independent predictor of survival in patients with breast cancer? JPEN J Parenter Enteral Nutr. 2003;27:10–15. doi:10.1177/014860710302700110
51. Mohandas A, Marcus C, Kang H, Truong MT, Subramaniam RM. FDG PET/CT in the management of nasopharyngeal carcinoma. AJR Am J Roentgenol. 2014;203(2):146–157. doi:10.2214/AJR.13.12420
52. Lai V, Khong PL. Updates on MR imaging and 18F-FDG PET/CT imaging in nasopharyngeal carcinoma. Oral Oncol. 2014;50(6):539–548. doi:10.1016/j.oraloncology.2013.05.005
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
