Back to Journals » Cancer Management and Research » Volume 12
High Preoperative Controlling Nutritional Status Score Predicts a Poor Prognosis in Patients with Localized Upper Tract Urothelial Cancer: A Propensity Score Matching Study in a Large Chinese Center
Authors Bao Z , Li Y, Guan B, Xiong G , Zhang L, Tang Q, Wang T, Li X, Fang D , Zhou L
Received 1 August 2019
Accepted for publication 28 November 2019
Published 15 January 2020 Volume 2020:12 Pages 323—335
DOI https://doi.org/10.2147/CMAR.S225711
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Harikrishna Nakshatri
Zhengqing Bao, 1–3,* Yifan Li, 4,* Bao Guan, 1–3,* Gengyan Xiong, 1–3 Lei Zhang, 1–3 Qi Tang, 1–3 Tianyu Wang, 1–3 Xuesong Li, 1–3 Dong Fang, 1–3, 5 Liqun Zhou 1–3
1Department of Urology, Peking University First Hospital, Beijing 100034, People’s Republic of China; 2Institute of Urology, Peking University, Beijing 100034, People’s Republic of China; 3National Urological Cancer Center, Beijing 100034, People’s Republic of China; 4Department of Urology, Affiliated Hospital of Yangzhou University, Yangzhou University, Yangzhou, Jiangsu 225000, People’s Republic of China; 5Andrology Center, Peking University First Hospital, Beijing 100034, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Dong Fang; Liqun Zhou
Department of Urology, Peking University First Hospital, Institute of Urology, Peking University, National Urological Cancer Centre, No. 8 Xishiku StXicheng District, Beijing 100034, People’s Republic of China
Tel +86-10-83575006
Fax +86-10-66551726
Email [email protected]; [email protected]
Purpose: The aim of this study was to elucidate the prognostic value of the preoperative controlling nutritional status (CONUT) score, a new index based on the total lymphocyte count, serum albumin concentration and total cholesterol concentration, in patients with localized upper tract urothelial cancer (UTUC) after radical nephroureterectomy (RNU) using propensity score matching (PSM) analysis.
Methods: We retrospectively reviewed 908 consecutive patients with localized UTUC who underwent RNU between 1999 and 2015. Patients were divided into two groups according to the optimal cutoff value of the preoperative CONUT score. Relationships between the CONUT score with clinicopathological characteristics, overall survival (OS), cancer-specific survival (CSS), and disease-free survival (DFS) were analyzed before and after 1:1 PSM.
Results: A high preoperative CONUT score was significantly correlated with older age, low body mass index (BMI), poor American Statistical Association (ASA) score, advanced pathological T stage, and tumor squamous or glandular differentiation (all p< 0.05). Kaplan-Meier curves showed poor OS, CSS, and DFS for patients with a high CONUT score before and after PSM (all p< 0.001). Furthermore, multivariate analyses revealed that a high preoperative CONUT score was an independent risk factor for poor DFS (hazard ratio [HR] 1.418, 95% confidence interval [CI] 1.132– 1.776, p=0.002) before PSM and an independent risk factor for poor DFS (HR 1.333, 95% CI 1.010– 1.760, p=0.042) and OS (HR 1.459, 95% CI 1.010– 2.107, p=0.044) after PSM.
Conclusion: A high preoperative CONUT score is an independent prognostic factor for poor outcomes in patients with localized UTUC after RNU.
Keywords: controlling nutritional status score, upper tract urothelial carcinoma, propensity score matching, prognosis
Introduction
Upper tract urothelial carcinoma (UTUC) is relatively rare, with an approximate annual incidence of almost 2 cases per 100,000 individuals in Western countries, and accounts for only 5–10% of all urothelial carcinomas (UCs).1,2 Radical nephroureterectomy (RNU) with excision of the bladder cuff is the standard treatment for high-risk UTUCs,3,4 and kidney-sparing surgery can be the primary option for patients with low-risk tumors.1,5 Although the diagnosis and treatment for UTUC have significantly improved, the clinical outcome remains poor, as 5-year specific survival is less than 50% for patients with pT2/pT3 and less than 10% for patients with pT4.6–8 Therefore, it is necessary to identify biomarkers that have the potential to predict prognosis and individualize adjuvant therapy based on the stratification of risks.
There is accumulating evidence that the preoperative immune-nutritional status plays crucial roles in human cancer development and progression.9,10 Recently, several biomarkers, such as serum sodium, hemoglobin, C-reactive protein (CRP), albumin, albumin to globulin ratio (AGR), plasma fibrinogen, neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR), lymphocyte to monocyte ratio (LMR), body mass index (BMI), sarcopenia and prognostic nutritional index (PNI), have been reported to be independent prognostic factors in many human malignancies, including UTUC.11,12
The controlling nutritional status (CONUT) score, a new index calculated from three peripheral blood parameters (total lymphocyte count [TLC], serum albumin concentration and total cholesterol concentration [TCC]), was originally reported as an early screening tool to detect a poor nutritional status in 2005 (Table 1).13 The CONUT score was proposed as a comprehensive index that could assess the immune response status and long-term nutritional effect of the host. Recently, the prognostic value of the preoperative CONUT score has been reported in many types of malignancies, such as colorectal cancer, esophageal cancer, gastric cancer, and renal cell carcinoma.14–17 However, there are few studies on UTUC. Shihara and Xu reported that the CONUT score is an independent prognostic factor in UTUC patients treated with surgery.18,19 However, their studies were limited by the presence of selection bias, a short follow-up interval, a small sample size and limited covariates.
The aim of this study was to elucidate the prognostic value of the preoperative CONUT score in patients with localized UTUC treated with RNU using propensity score matching (PSM) analysis based on a nationwide high-volume cohort in China.
Materials and Methods
Patient Enrollment and Evaluation
After obtaining approval by the internal ethics review board of Peking University First Hospital (approval no. 2015[977]), we retrospectively collected the records of 908 consecutive patients with localized UTUC who received surgical treatment at Peking University First Hospital between 1999 and 2015. A total of 154 patients were excluded from this study because of accompanying colorectal cancer, breast cancer or other malignancies (n=16), receiving adjuvant chemotherapy (n=44), missing clinicopathological data or follow-up data (n=90), or hepatitis (n=4). Ultimately, 754 patients were enrolled (Figure 1). All patients were treated with standard RNU with bladder cuff resection without any preoperative treatment. Routine lymph node dissection was performed in selected patients with enlarged lymph nodes that were found intraoperatively or by preoperative imaging.
 |
Figure 1 Workflow of this study. |
The clinicopathological and follow-up data were collected from a database containing the comprehensive medical records of UTUC patients. Staging was assessed according to the 2002 Union for International Cancer Control (UICC) TNM classification guidelines. Patients were graded based on the World Health Organization (WHO) 1973 grading system. Blood samples were obtained and measured within one week before the operation as regular preoperative laboratory tests. Exposure to aristolochic acids (AAs) was defined as a history of long-term exposure (>3 months) of intermittent intake of regular doses of AA-containing traditional Chinese medicine. All procedures performed in this study were in accordance with the 1964 Declaration of Helsinki and its later amendments, and written informed consent was obtained from all patients.
For patients who were followed at our institute, their follow-up regimen included cystoscopy every 3 months for the first 3 years. The cystoscopy interval was extended to 1 year thereafter. Chest X-ray, urine cytology, serum creatinine tests, and abdominal ultrasound or CT/MRI evaluation were performed at the same time. Overall survival (OS) was determined by a review of patient medical records or data obtained from the Chinese National Statistical Office database. Cancer-specific survival (CSS) and disease-free survival (DFS) were determined at the last follow-up based on examination results.
As a study in a single center, bias is inevitable. Therefore, all patient variables were adjusted using 1:1 PSM analysis to minimize selection bias.
Statistical Analysis
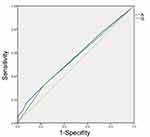
All statistical tests were performed with SPSS 22.0 (IBM Corp., Armonk, NY, USA) or SAS 9.4 (SAS institute Inc., USA), and statistical significance was set at p<0.05. To determine the optimum cutoff value for the preoperative CONT score, the receiver operating characteristic (ROC) curve of the CONUT score for DFS was analyzed, and the cutoff value for the preoperative CONUT score was defined by the maximum Youden index value. Patients with a preoperative CONUT score over the cutoff value were as assigned to the high CONUT score group, while the remaining patients (CONUT score less than the cutoff value) were assigned to the low CONUT score group.
Several preoperative characteristics, such as age, BMI, American Statistical Association (ASA) score and hydronephrosis, were notably different between the low and high CONUT score patient cohorts (all p<0.1). These preoperative characteristics might have an important impact on the CONUT score. To minimize bias, we performed PSM. The propensity scores were evaluated by nonparsimonious multivariate logistic regression according to the preoperative characteristics. In total, 204 patients with a high CONUT score were matched to 204 patients with a low CONUT score at a ratio of 1:1 using the nearest-neighbor method of PSM with a caliber of 0.1 (Figure 1). After PSM, the differences in preoperative characteristics between the low and high CONUT score groups were acceptable (Figure 2 and Table 3).
 |
Table 1 CONUT Score System |
 |
Figure 2 Line plot of individual differences (A) and dot-plot of standardized mean differences (B) in propensity score matching. |
Pearson’s test and the chi-square test were used to determine the distribution of categorical variables, and the Mann–Whitney U-test was used for continuous variables. OS, CSS and DFS curves were plotted using the Kaplan-Meier method and analyzed by the Log rank test. The univariate analysis was assessed by the Log rank test, and the multivariate analysis was evaluated using the Cox proportional hazard regression model. Only those variables that were identified as significant (p<0.05) in the univariate analysis were included in the multivariate analysis. Sensitivity analyses were also used to measure the potential effect of an unmeasured confounder on the relationship between preoperative CONUT score and the oncological outcomes.
Results
Patient Characteristics
Given that a CONUT score of 3 had a maximum Youden index value, the cutoff value for the CONUT score was set as 3 (Figure 3). Patients with a CONUT score ≥3 were assigned to the high CONUT score group, and those with a CONUT score<3 were assigned to the low CONUT score group. Of the 754 UTUC patients, 204 (27.1%) had a high CONUT score. The patient clinicopathological characteristics before and after PSM are described in Tables 2 and 3, respectively. The median patient age was 69 years (interquartile range [IQR] 61–74 years), and the median follow-up duration was 61 months (IQR 45–105 months). Patients in the high CONUT score group were significantly older (p=0.018), had a lower BMI (p=0.005), had a high ASA score (p<0.001) and were more likely to have hydronephrosis (p=0.055, 53.5% vs 61.3%). These patients were also more likely to have advanced pathological T stage (p=0.001), squamous differentiation (p=0.013) and glandular differentiation (p=0.043) compared with the low CONUT score group. These patients also tended to have an advanced cellular grade (≥G3, p=0.098, 40.4% vs 47.1%) and sessile tumors (p=0.090, 18.9% vs 24.5%) compared with the low CONUT score group.
Survival Outcomes
Before PSM
After a median (IQR) of 32 (19–56) months postoperatively, 234 (31.0%) patients died from any cause. Postoperatively, 165 (21.5%) patients experienced cancer-specific mortality after a median (IQR) of 26 (16–44) months, and 362 (48.0%) patients experienced disease progression after a median (IQR) of 21 (11–39) months. The Kaplan-Meier curves showed significantly poor OS (p=0.021), CSS (p=0.028), and DFS (p<0.001) for patients with a high preoperative CONUT score (Figure 4A, C and E). A high preoperative CONUT score was found to be an independent risk factor for DFS (hazard ratio [HR] 1.418, 95% confidence interval [CI] 1.132–1.776, p=0.002) but not OS (HR 1.273, 95% CI 0.960–1.686, p=0.093) or CSS (HR 1.328, 95% CI 0.954–1.847, p=0.092) based on multivariate Cox regression analyses when adjusted for clinicopathological parameters, as shown in Table 4. For OS, patient sex, hypertension, ASA score ≥3, pathological T and N stages, tumor size, and glandular differentiation were significant co-predictors (all p<0.05). Moreover, patient sex, hypertension, pathologic T and N stages, and tumor size were proved to be independent co-predictors of postoperative CSS and DFS (all p<0.05). Tumor glandular differentiation (p=0.011) significantly predicted DFS but not CSS (Table 4).
 |
Table 4 Univariate and Multivariate Analyses of the Relationship Between Preoperative CONUT# Score and Oncological Outcome in Patients with Localized UTUC Before Propensity Score Matching |
After PSM
After a median (IQR) of 30 (18–53) months postoperatively, 127 (31.1%) patients died from any cause. Preoperatively, 90 (22.1%) patients experienced cancer-specific mortality after a median (IQR) of 24 (15–41) months, and 213 (52.2%) patients experienced disease progression after a median (IQR) of 19 (10–39) months. The Kaplan-Meier curves showed significantly inferior OS (p=0.017), CSS (p=0.020), and DFS (p=0.004) for patients with a high preoperative CONUT score (Figure 4B, D and F). Multivariate Cox regression analyses demonstrated that a high CONUT score was an independent risk factor for OS (HR 1.459, 95% CI 1.010–2.107, p=0.044) and DFS (HR 1.333, 95% CI 1.010–1.760, p=0.042) but not CSS (HR 1.336, 95% CI 0.863–2.067, p=0.194), as shown in Table 5. For OS, patient sex, hypertension, ASA score ≥3, pathological T and N stages and glandular differentiation were significant co-predictors. Moreover, patient sex, pathological N stage and tumor glandular differentiation were revealed as independent co-predictors of postoperative CSS and DFS (all p<0.05). Pathological T stage (p<0.001) significantly predicted CSS but not DFS in the propensity score-matched cohort (Table 5).
 |
Table 5 Univariate and Multivariate Analyses of the Relationship Between Preoperative CONUT# Score and Oncological Outcome in Patients with Localized UTUC# After Propensity Score Matching |
Sensitivity analyses revealed that the relationship between preoperative CONUT score and the oncological outcomes was maintained (data not shown).
Discussion
Based on patients with localized UTUC treated with RNU from a nationwide high-volume cohort in China, we found that a high preoperative CONUT score was significantly related to certain adverse clinical and pathological characteristics, and a high CONUT score was associated with poor oncological outcomes before and after PSM. These results suggest that a high preoperative CONUT score is a robust independent predictor for localized UTUC.
The optimum cutoff value of the CONUT score vary from study to study. In our study, it was 3 according to the ROC curve, which was in accordance with previous studies.19–21 PSM analysis has been used in many studies to balance subgroups across selected factors.22 In our study, to balance the baseline characteristics of preoperative covariates between the two groups, we used the PSM method. The differences in preoperative covariates between the low and high CONUT score groups were acceptable.
Ideal cancer predictors should be objective, inexpensive, quickly measured, simple to use, and available before surgery. Similar to objective data assessment (ODA) and subjective global assessment (SGA), the CONUT score has been reported to be a simple and cost-effective method of comprehensively and objectively detecting and controlling hospital malnutrition.13 It is comprised of the TLC, serum albumin concentration and TCC, reflecting immune function, host protein metabolism, and lipid metabolism, respectively.13 Thus, the CONUT score reflects not only the host status of nutrition but also systemic inflammation and the immune response. The combination of these three parameters can conceal their individual weaknesses and emphasize the advantage of each parameter. The mechanism underlying the association between a high CONUT score and a poor prognosis in human cancer remains unclear. The prognostic value of the preoperative CONUT score in UTUC patients might be explained by the functions of these three parameters.
The TLC, an indicator of the immunological status,11 plays important roles in inhibiting tumor cell malignant phenotypes, such as cell proliferation, invasion, and migration, by initiating a cytotoxic immune response.10 The number of tumor infiltrating lymphocytes (TILs) in the tumor microenvironment has a crucial impact on the prognosis and immunotherapy outcome of various human tumors.23,24 A previous study indicated that TILs might affect the host response to neoadjuvant chemotherapy in bladder cancer.23 The TLC in the peripheral blood may indicate the number of TILs. Several biomarkers based on the TLC, such as NLR and PLR, were proven to be indexes for the immune status and serve as prognostic predictors for UTUC.11,25,26
As a reliable indicator of the status of host nutrition and systematic inflammation, serum albumin can be influenced not only by the production of proinflammatory cytokines,27 such as interleukin-6 and tumor necrosis factor-alpha, but also by body fluid retention and damage to hepatocytes.28,29 Previous studies revealed that hypoalbuminemia was associated with immune deficiency. Furthermore, hypoalbuminemia was demonstrated to be associated with poor outcomes in UTUC patients.30
Finally, as an important component of human cell membranes, cholesterol plays an essential role in maintaining the membrane structure, fluidity and membrane protein activity.31,32 Thus, hypocholesterolemia may affect the mobility of cell surface receptors and their value in transmitting transmembrane signals. Increasing evidence has revealed an inverse association between the TCC in the blood and cancer incidence and mortality.33,34 Abnormal lipid metabolism has been demonstrated to be closely related to cancer development. The increased mRNA expression of low-density lipoprotein (LDL) receptor was found in colorectal tumor tissue compared to normal tissue, resulting in a high intake of LDL, which increases tumor growth.35 A previous study revealed that chronic cholesterol depletion promoted tumor cell proliferation by inducing NFkB activation.36 Furthermore, hypocholesterolemia was shown to be associated with a poor prognosis in many cancer patients.37,38
Previous studies indicated that nutritional intervention could reduce postoperative complications and improve the tolerance of patients to anticancer treatment.39,40 However, the effects of nutritional intervention on the long-term oncological outcomes in patients with cancer-related malnutrition have not been confirmed. Our results remind us of the importance of perioperative nutritional support in UTUC patients. In addition, immunotherapy is becoming a promising strategy for the management of UTUC. The CONUT score may be a useful index for the selection of patients who need perioperative nutritional intervention in UTUC treatment.
Although important discoveries were revealed, our study had certain limitations. First, it was a retrospective single-center and single-race study. Therefore, we matched the patient preoperative variables between the high and low CONUT score groups using PSM analysis, which made the differences in preoperative covariates between the two groups acceptable. However, further multicenter interracial prospective studies are needed to clarify the prognostic role of the preoperative CONUT score in UTUC patients. Second, the CONUT score might have been biased by medicine or the presence of undetected conditions that affect the TLC, serum albumin concentration or TCC. Third, we did not obtain information on the CONUT score or nutritional support after surgery, which might influence our results. Finally, the mechanism by which the CONUT score affects the survival of patients with UTUC should be further explored using basic studies. Despite these limitations, we revealed that the preoperative CONUT score was correlated with oncological outcome in patients with UTUC.
Conclusion
Our study revealed that the preoperative CONUT score is a convenient and effective factor for predicting prognosis in patients with localized UTUC after RNU. Perioperative nutritional intervention should be emphasized in UTUC patients.
Abbreviations
CONUT, controlling nutritional status; UTUC, upper tract urothelial cancer; RNU, radical nephroureterectomy; PSM, propensity score matching; OS: overall survival; CSS, cancer-specific survival; DFS, disease-free survival; ASA, American Statistical Association; HR, hazard ratio; CI, confidence interval; AGR, albumin to globulin ratio; NLR, neutrophil to lymphocyte ratio; PLR, platelet to lymphocyte ratio; LMR, lymphocyte to monocyte ratio; BMI, body mass index; PNI, prognostic nutritional index; TLC, total lymphocyte count; TCC, total cholesterol concentration; UICC, Union for International Cancer Control; WHO, World Health Organization; AA, aristolochic acid; ROC, receiver operating characteristic; IQR, interquartile range; ODA, objective data assessment; SGA, subjective global assessment; TILs, tumor infiltrating lymphocytes; LDL, low-density lipoprotein.
Data Sharing Statment
Supporting data are available from the first author, Zhengqing Bao E-mail: [email protected].
Acknowledgments
This study was funded by the Peking University Medicine Fund of Fostering Young Scholars’ Scientific & Technological Innovation (Fundamental Research Funds for the Central Universities, BMU2017PY009), the National Natural Science Foundation of China (81672546, 81872083, 81772703, 81602253) and the Beijing Natural Science Foundation (L182004, 7172219, 7152146).
Author Contributions
Zhengqing Bao, Yifan Li and Bao Guan carried out the study, participated in the data analysis, and drafted the manuscript. Gengyan Xiong, Lei Zhang, Qi Tang and Tianyu Wang collected the data. Zhengqing Bao and Xuesong Li conceived and designed the study. Dong Fang and Liqun Zhou initiated this study and participated in revising the manuscript critically for important intellectual content. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors have stated that they have no conflicts of interest.
References
1. Roupret M, Babjuk M, Comperat E, et al. European association of urology guidelines on upper urinary tract urothelial carcinoma: 2017 update. Eur Urol. 2018;73(1):111–122. doi:10.1016/j.eururo.2017.07.036
2. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. Ca Cancer J Clin. 2015;60(5):277–300.
3. Li CC, Chang TH, Wu WJ, et al. Significant predictive factors for prognosis of primary upper urinary tract cancer after radical nephroureterectomy in Taiwanese patients. Eur Urol. 2008;54(5):1127–1134. doi:10.1016/j.eururo.2008.01.054
4. Vitaly M, Shariat SF, Matin SF, et al. Outcomes of radical nephroureterectomy: a series from the Upper Tract Urothelial Carcinoma Collaboration. Cancer. 2010;115(6):1224–1233.
5. Seisen T, Peyronnet B, Dominguez-Escrig JL, et al. Oncologic outcomes of kidney-sparing surgery versus radical nephroureterectomy for upper tract urothelial carcinoma: a systematic review by the EAU non-muscle invasive bladder cancer guidelines panel. Eur Urol. 2016;70(6):1052–1068. doi:10.1016/j.eururo.2016.07.014
6. Abouassaly R, Alibhai SM, Shah N, Timilshina N, Fleshner N, Finelli A. Troubling outcomes from population-level analysis of surgery for upper tract urothelial carcinoma. Urology. 2010;76(4):895–901. doi:10.1016/j.urology.2010.04.020
7. Jeldres C, Sun M, Isbarn H, et al. A population-based assessment of perioperative mortality after nephroureterectomy for upper-tract urothelial carcinoma. Urology. 2010;75(2):315–320. doi:10.1016/j.urology.2009.10.004
8. Lughezzani G, Burger M, Margulis V, et al. Prognostic factors in upper urinary tract urothelial carcinomas: a comprehensive review of the current literature. Eur Urol. 2012;62(1):100–114. doi:10.1016/j.eururo.2012.02.030
9. Von Meyenfeldt M. Cancer-associated malnutrition: an introduction. Eur j Oncol Nurs. 2005;9(Suppl 2):S35–38. doi:10.1016/j.ejon.2005.09.001
10. Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13(11):759–771. doi:10.1038/nrc3611
11. Itami Y, Miyake M, Tatsumi Y, et al. Preoperative predictive factors focused on inflammation-, nutrition-, and muscle-status in patients with upper urinary tract urothelial carcinoma undergoing nephroureterectomy. Int J Clin Oncol. 2019;24(5):533–545. doi:10.1007/s10147-018-01381-y
12. Xue W, Tan P, Xu H, Yang L, Wei Q. Impact of the preoperative prognostic nutritional index on survival outcomes in upper tract urothelial carcinomas. Cancer Med. 2019;8(6):2971–2978. doi:10.1002/cam4.2161
13. De Ulibarri JI, Gonzalez-Madrono A, De Villar NG, et al. CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp. 2005;20(1):38–45.
14. Daitoku N, Miyamoto Y, Tokunaga R, et al. Controlling nutritional status (CONUT) score is a prognostic marker in metastatic colorectal cancer patients receiving first-line chemotherapy. Anticancer Res. 2018;38(8):4883–4888. doi:10.21873/anticanres.12802
15. Yoshida N, Harada K, Baba Y, et al. Preoperative controlling nutritional status (CONUT) is useful to estimate the prognosis after esophagectomy for esophageal cancer. Langenbeck’s Archiv Surg. 2017;402(2):333–341. doi:10.1007/s00423-017-1553-1
16. Ryo S, Kanda M, Ito S, et al. The controlling nutritional status score serves as a predictor of short- and long-term outcomes for patients with stage 2 or 3 gastric cancer: analysis of a multi-institutional data set. Ann Surg Oncol. 2019;26(2):456–464. doi:10.1245/s10434-018-07121-w
17. Elghiaty A, Kim J, Jang WS, et al. Preoperative controlling nutritional status (CONUT) score as a novel immune-nutritional predictor of survival in non-metastatic clear cell renal cell carcinoma of</= 7 cm on preoperative imaging. J Cancer Res Clin Oncol. 2019;145(4):957–965. doi:10.1007/s00432-019-02846-x
18. Xu H, Tan P, Jin X, et al. Validation of the preoperative controlling nutritional status score as an independent predictor in a large Chinese cohort of patients with upper tract urothelial carcinoma. Cancer Med. 2018;7(12):6112–6123. doi:10.1002/cam4.1902
19. Ishihara H, Kondo T, Yoshida K, et al. Preoperative controlling nutritional status (CONUT) score as a novel predictive biomarker of survival in patients with localized urothelial carcinoma of the upper urinary tract treated with radical nephroureterectomy. Urol Oncol. 2017;35(9):
20. Iseki Y, Shibutani M, Maeda K, et al. Impact of the preoperative controlling nutritional status (CONUT) score on the survival after curative surgery for colorectal cancer. PLoS One. 2015;10(7):e0132488. doi:10.1371/journal.pone.0132488
21. Liu X, Zhang D, Lin E, et al. Preoperative controlling nutritional status (CONUT) score as a predictor of long-term outcome after curative resection followed by adjuvant chemotherapy in stage II-III gastric Cancer. BMC Cancer. 2018;18(1):699. doi:10.1186/s12885-018-4616-y
22. Harimoto N, Yoshizumi T, Inokuchi S, et al. Prognostic significance of preoperative controlling nutritional status (CONUT) score in patients undergoing hepatic resection for hepatocellular carcinoma: a multi-institutional study. Ann Surg Oncol. 2018;25(11):3316–3323. doi:10.1245/s10434-018-6672-6
23. Badalamenti G, Fanale D, Incorvaia L, et al. Role of tumor-infiltrating lymphocytes in patients with solid tumors: can a drop dig a stone? Cell Immunol. 2018. doi:10.1016/j.cellimm.2018.01.013
24. Yao W, He JC, Yang Y, et al. The prognostic value of tumor-infiltrating lymphocytes in hepatocellular carcinoma: a systematic review and meta-analysis. Sci Rep. 2017;7(1):7525. doi:10.1038/s41598-017-08128-1
25. Tanaka N, Kikuchi E, Kanao K, et al. Impact of combined use of blood-based inflammatory markers on patients with upper tract urothelial carcinoma following radical nephroureterectomy: proposal of a cumulative marker score as a novel predictive tool for prognosis. Eur Urol Focus. 2015;1(1):54–63. doi:10.1016/j.euf.2015.02.001
26. Son S, Hwang EC, Jung SI, et al. Prognostic value of preoperative systemic inflammation markers in localized upper tract urothelial cell carcinoma: a large, multicenter cohort analysis. Minerva Urol Nefrol. 2018;70(3):300–309. doi:10.23736/s0393-2249.18.02914-4
27. Deehan DJ, Heys SD, Simpson W, Herriot R, Broom J, Eremin O. Correlation of serum cytokine and acute phase reactant levels with alterations in weight and serum albumin in patients receiving immunotherapy with recombinant IL-2. Clin Exp Immunol. 1994;95(3):366–372. doi:10.1111/j.1365-2249.1994.tb07005.x
28. Tanriverdi O. A discussion of serum albumin level in advanced-stage hepatocellular carcinoma: a medical oncologist’s perspective. Med Oncol. 2014;31(11):282. doi:10.1007/s12032-014-0282-3
29. Yeun JY, Kaysen GA. Factors influencing serum albumin in dialysis patients. Am J Kidney Dis. 1998;32(6):118–125. doi:10.1016/S0272-6386(98)70174-X
30. Fukushima H, Kobayashi M, Kawano K, Morimoto S. Prognostic value of albumin/globulin ratio in patients with upper tract urothelial carcinoma patients treated with radical nephroureterectomy. Anticancer Res. 2018;38(4):2329–2334. doi:10.21873/anticanres.12478
31. Harikumar KG, Potter RM, Patil A, Echeveste V, Miller LJ. Membrane cholesterol affects stimulus-activity coupling in type 1, but not type 2, CCK receptors: use of cell lines with elevated cholesterol. Lipids. 2013;48(3):231–244. doi:10.1007/s11745-012-3744-4
32. Carsten S, Nölke T, Kruppke AS, Schubert DM, Lang AE, Aktories K. Cholesterol- and sphingolipid-rich microdomains are essential for microtubule-based membrane protrusions induced by Clostridium difficile transferase (CDT). J Biol Chem. 2011;286(33):29356–29365. doi:10.1074/jbc.M111.261925
33. Kitahara CM, De González AB, Freedman ND, et al. Total cholesterol and cancer risk in a large prospective study in Korea. J Clin Oncol. 2011;29(12):1592–1598. doi:10.1200/JCO.2010.31.5200
34. Bing W, Teng L, Du H, Dong-Dong Y, Jiang F. Dose-response relation between serum total cholesterol levels and overall cancer risk: evidence from 12 prospective studies involving 1,926,275 participants. Int J Food Sci Nutr. 2019. 70;1–10.
35. Niendorf A, N?Gele H, Gerding D, Meyer-Pannwitt U, Gebhardt A. Increased LDL receptor mRNA expression in colon cancer is correlated with a rise in plasma cholesterol levels after curative surgery. Int j Cancer. 2010;61(4):461–464. doi:10.1002/ijc.2910610405
36. Calleros L, Lasa M, Toro MJ, Chiloeches A. Low cell cholesterol levels increase NFkappaB activity through a p38 MAPK-dependent mechanism. Cell Signal. 2006;18(12):2292–2301. doi:10.1016/j.cellsig.2006.05.012
37. Notarnicola M, Altomare DF, Correale M, et al. Serum lipid profile in colorectal cancer patients with and without synchronous distant metastases. Oncology. 2016;68(4–6):371–374. doi:10.1159/000086977
38. Ko K, Park YH, Lee JW, Ku JH, Kwak C, Kim HH. Influence of nutritional deficiency on prognosis of renal cell carcinoma (RCC). BJU Int. 2014;112(6):775–780. doi:10.1111/bju.12275
39. Kubota K, Kuroda J, Yoshida M, Okada A, Deguchi T, Kitajima M. Preoperative oral supplementation support in patients with esophageal cancer. J Nutr Health Aging. 2014;18(4):437–440. doi:10.1007/s12603-014-0018-2
40. Agostino P, Ildamaria M, Giovanni R. Nutritional intervention for improving treatment tolerance in cancer patients. Curr Opin Oncol. 2011;23(4):322. doi:10.1097/CCO.0b013e3283479c66
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.