Back to Journals » OncoTargets and Therapy » Volume 13
High PD-L1 Expression is Associated with Unfavorable Clinical Outcome in EGFR-Mutated Lung Adenocarcinomas Treated with Targeted Therapy
Authors Yoon BW , Chang B , Lee SH
Received 10 July 2020
Accepted for publication 11 August 2020
Published 20 August 2020 Volume 2020:13 Pages 8273—8285
DOI https://doi.org/10.2147/OTT.S271011
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Arseniy Yuzhalin
Byung Woo Yoon,1,2 Boksoon Chang,3 Seung Hyeun Lee3
1Department of Internal Medicine, Seoul Paik Hospital, Seoul, South Korea; 2Department of Internal Medicine, Inje University College of Medicine, Gimhae, South Korea; 3Department of Internal Medicine, Kyung Hee University School of Medicine, Seoul, South Korea
Correspondence: Seung Hyeun Lee
Division of Pulmonary, Allergy, and Critical Care Medicine, Department of Internal Medicine, Kyung Hee University School of Medicine, Kyungheedae-Ro 23, Dongdaemun-Gu, Seoul 02447, South Korea
Tel +82 2 958 8511
Fax +82 2 968 1848
Email [email protected]
Purpose: Although programmed death-ligand 1 (PD-L1) expression is widely accepted as a predictive and prognostic biomarker in immunotherapy, its implications in lung cancer patients with driving mutations are still unclear. The objective of this study is to determine the association between PD-L1 expression and treatment outcome in epidermal growth factor receptor (EGFR)-mutated lung cancer treated with tyrosine kinase inhibitors (TKIs).
Methods: We retrospectively enrolled EGFR-mutant, advanced lung adenocarcinoma patients who received first-line EGFR-TKIs and evaluated the PD-L1 tumor proportion score (TPS) using the 22C3 pharmDx assay. We investigated the distribution of patients with different PD-L1 TPS values, followed by the analysis of response rate (RR), survival rate, and incidence of secondary T790M mutation according to the PD-L1 TPS group.
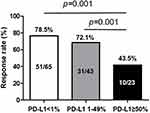
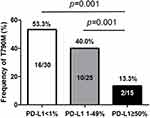
Results: Among the 131 patients analyzed, the proportion of patients with PD-L1 TPS ≥ 50%, 1– 49%, and < 1%, was 17.6%, 32.8%, and 49.6%, respectively. The RR was significantly lower in the group with PD-L1 TPS ≥ 50% than in the other groups (43.5% vs 72.1% vs 78.5%, all p = 0.001). In multivariate analysis, PD-L1 TPS ≥ 50% was independently associated with a significantly shorter PFS in the overall population (hazard ratio [HR] = 2.64, p = 0.004) and associated with shorter OS in patients with exon 19 deletion (HR = 2.55, p = 0.041) compared with PD-L1 TPS < 50%. In addition, the frequency of secondary T790M mutation after TKI failure was significantly lower in the group with PD-L1 TPS ≥ 50% than in the other groups (13.3% vs 40.0% vs 53.3%, all p = 0.001). PD-L1 TPS ≥ 50% was an independent predictor of a lower frequency of this mutation (HR = 0.63, p = 0.043).
Conclusion: High PD-L1 expression was associated with unfavorable clinical outcome and less development of secondary T790M mutation, suggesting a distinct subgroup warranting active surveillance and tailored therapeutic approach.
Keywords: lung cancer, programmed death-ligand 1, epidermal growth factor receptor mutation, tyrosine kinase inhibitor, clinical outcome, prognosis, T790M
Introduction
Lung cancer is the leading cause of cancer-related death worldwide. In Korea, approximately 27,000 new cases and 18,000 lung cancer-related deaths were reported in 2017.1,2 Adenocarcinoma is the major histologic subtype constituting 60% of all cases of non-small cell lung cancers (NSCLC).3 Although new treatment modalities, including molecular-targeted therapy and immune checkpoint inhibitors, have demonstrated remarkable survival benefits in patients with advanced lung adenocarcinoma,3,4 the prognosis is still poor suggesting the need for individualized therapeutic strategies to improve the clinical outcomes.
Immunotherapy based on immune checkpoint inhibitors represents one of the most important breakthroughs in the management of solid tumors, including lung cancers with promising results in numerous clinical trials.5 Programmed death-ligand 1 (PD-L1) expression is consistently associated with clinical efficacy in anti-PD-1/PD-L1 treatment.6–8 The US Food and Drug Administration approved PD-L1 22C3 and 28–8 clones as a companion diagnostic for pembrolizumab and as a complementary diagnostic method for nivolumab, respectively, in 2015. However, the clinical implications of this biomarker in different clinical settings including chemotherapy or targeted therapy are largely unknown. Given that PD-1/PD-L1 interaction is a major immune checkpoint involved in immune escape during cancer development and progression, the upregulated PD-L1 expression may be associated with dismal clinical outcome in lung cancer patients undergoing treatment other than immunotherapy. This concept was partially evident in a previous study demonstrating the poor prognosis of patients with positive PD-L1 expression who received surgical resection for lung cancer.9
Epidermal growth factor receptor (EGFR) mutations are major driver genetic alterations, which are detected in approximately 50% of the population in the Far East diagnosed with lung adenocarcinoma.10,11 EGFR-tyrosine kinase inhibitors (TKIs) have doubled the progression-free survival (PFS) compared with chemotherapy and increased the overall survival (OS) to more than 2 years, and thus recommended as a frontline treatment in patients harboring this mutation.12 However, the response to TKIs is not similar in all EGFR-mutant tumors and resistance inevitably occur after approximately 11 months of treatment in most patients.13–15 The emergence of resistance is the major cause of treatment failure, which is a challenge for the management of those patients.16 The mechanism of primary resistance to EGFR-TKIs is not fully understood, although it is partly explained by De novo T790M mutation, the presence of concurrent genetic alterations, or current smoking.17,18 Thus, identification of mechanisms leading to TKI resistance or determination of predictive and prognostic factors before TKI use is an important step to improve clinical outcomes in this patient population.
To address this issue, we conducted the present study to determine the clinical impact of PD-L1 expression in patients with EGFR-mutant advanced lung adenocarcinoma treated with first-line TKIs. First, we evaluated whether PD-L1 expression was associated with treatment response or survival. We then evaluated its association with the emergence of secondary T790M mutation after TKI failure.
Materials and Methods
Study Subjects and Data Collection
We retrospectively recruited EGFR-mutant patients treated with first-line EGFR-TKIs for histologically confirmed, locally advanced or metastatic lung adenocarcinoma at three referral hospitals in South Korea (Kyung Hee University Medical Center, Kyung Hee University Hospital at Gangdong, and Dongnam Institution of Radiological & Medical Sciences) from January 2014 to November 2019. Patients without follow-up data, a history of other cancers, or other driving genetic alterations including ROS proto-oncogene 1 (ROS1) and anaplastic lymphoma kinase (ALK) fusions, and those with a previous history of chemotherapy or radiotherapy were excluded.
All patients underwent staging workup, including chest computed tomography (CT), brain magnetic resonance imaging, and 18F-fluorodeoxyglucose positron emission tomography-computed tomography. TNM staging was performed according to the 8th edition of the International Association for the Study of Lung Cancer TNM staging system.19 Tumor response was assessed with CT after every two cycles of systemic treatment and evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1.20 We collected demographic information, past medical or social history, and clinical outcome of all participants by reviewing electronic medical records. This study protocol was approved by the Clinical Research Ethics Committee of the Kyung Hee University Medical Center (KHMC 2019–06-030). Written informed consent was obtained from all patients who were alive. All research was carried out in compliance with the Declaration of Helsinki.
PD-L1 Immunohistochemical Staining and Scoring
Immunohistochemical staining for PD-L1 expression was performed using 22C3 pharmDx assay (Agilent, Santa Clara, CA, US) and the Automated Link 48 Platform (Dako, Carpinteria, CA, US) in formalin-fixed tumor samples obtained by surgical resection or small biopsy (percutaneous needle biopsy, bronchoscopic mucosal biopsy, or endobronchial ultrasound-guided transbronchial biopsy) before commencement of first-line TKI treatment. Neoplastic cells were considered positive in the presence of cell membrane staining. PD-L1 expression was determined using the tumor proportion score (TPS), which is defined as the percentage of viable tumor cells showing partial or complete membrane staining. Based on PD-L1 expression, the tumors were categorized into three groups (< 1%, 1–49%, and ≥50%), according to the TPS by counting at least 100 viable cells.21
EGFR Mutation Testing
All the EGFR tests were performed using the tumor tissues. Genomic DNA was extracted from formalin-fixed, paraffin-embedded, 5-µm-thick tissue sections using the High Pure Template Preparation Kit (Roche Applied Science, Mannheim, Germany). The extracted DNA was stored at −20°C until analysis. EGFR Pyro Kit (QIAGEN Korea Ltd., Seoul, Korea) and PyroMark Q24 System (QIAGEN Korea Ltd., Seoul, Korea) were used to detect EGFR mutations via real-time polymerization chain reaction (PCR). The primer sets covered mutations or deletions spanning exons 18 to 21 of the genes encoding the tyrosine kinase domain of EGFR. The results were interpreted according to the manufacturer’s instructions.
Statistical Analyses
Baseline characteristics of different groups were compared using Chi-square test or Fisher’s exact test as appropriate. Clinical outcomes were assessed using response rate (RR), PFS and OS. RR was defined as the percentage of patients who showed complete or partial remission. PFS and OS were defined as the periods from the first day of treatment to disease progression/death and death from any cause, respectively. Data of patients without tumor recurrence or death were censored at the last follow-up. Associations between clinical/pathologic parameters and survival were evaluated by univariate analysis using the Log rank test. Subsequently, the multivariate Cox’s proportional hazard regression analysis was conducted by adjusting parameters with p values < 0.3 from the univariate analysis. Survival curves were generated using the Kaplan–Meier method. P values < 0.05 were considered significant. All analyses were performed using SPSS version 20.0 (IBM Corporation, Armonk, NY, USA) and GraphPad Prism 5.0 (GraphPad Software, San Diego, CA, USA).
Results
Patient Characteristics
During the study period, 684 patients were newly diagnosed with NSCLC in three institutes and 412 patients were diagnosed with advanced disease. Of these patients, 160 underwent first-line treatment with EGFR-TKIs for EGFR-mutant lung adenocarcinoma. Fifteen patients without survival data available, 11 with a history of other cancers, and 3 who received other cancer treatments before the initiation of TKIs were excluded. Finally, a total of 131 patients were eligible for the analysis.
The clinical characteristics of the study population are summarized in Table 1. All subjects were Korean and their median age was 70 years (range, 42–86 years). Sixty-seven (51.1%) patients were aged ≥70 years. Seventy-one (54.2%) patients were female. Forty-seven (35.9%) patients were current or former smokers. One hundred and three (78.6%) patients had an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1. Eight (6.1%) patients had stage III and 123 (93.9%) patients had stage IV disease. Twenty-one (16.0%) patients had metastases involving more than three organs. Thirty-three (25.2%) and 15 (11.5%) patients had brain or liver metastasis, respectively. Seventy-four (56.5%) patients received gefitinib or erlotinib, while 57 (43.5%) received afatinib as a first-line TKI. Sixty-four (48.9%) patients had exon 19 deletion (19del), 52 (39.7%) had L858R point mutations, and 15 (11.5%) had uncommon or compound mutations. The list of uncommon or compound mutations was provided in Table S1. Clinicopathological characteristics did not differ according to EGFR mutational subtypes (Table 1).
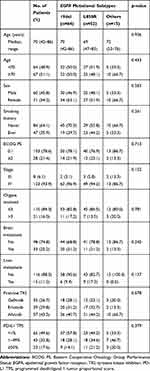 |
Table 1 Characteristics of 131 Study Patients Stratified by EGFR Mutational Subtypes |
Distribution of PD-L1 TPS Expression
The prevalence of different PD-L1 TPS is resented in Table 1. Number of patients with PD-L1 TPS ≥ 50%, 1–49%, and <1% was 23 (17.6%), 43 (32.8%), and 65 (49.6%), respectively. The proportion of patients with PD-L1 TPS ≥ 50% was lower compared with historical NSCLC population without driver mutations22 and was in accordance with previous data evaluating PD-L1 expression in EGFR-mutant lung cancer.23,24 The reduced incidence of high PD-L1 (PD-L1 TPS ≥ 50%) expression was consistent regardless of EGFR mutational subtypes (Table 1).
Response Rate and Progression-Free Survival According to PD-L1 TPS
The median follow-up time for this cohort was 32.8 months (range, 4.2–57.7 months). The RRs according to the PD-L1 TPS and EGFR subtypes are summarized in Table S2. The RRs of patients with PD-L1 TPS ≥ 50%, 1–49%, and <1% were 43.5%, 72.1%, and 78.5%, respectively. The RRs of patients with PD-L1 TPS ≥ 50% were significantly lower compared with those with PD-L1 TPS 1–49% and <1% (both p = 0.001, Figure 1). The lower RR of high PD-L1 TPS group was consistent throughout different EGFR mutational subtypes (Table S2).
The results of PFS analysis according to clinicopathological parameters are summarized in Table 2. The median PFS of all study subjects was 15.1 months (range, 2.2–30.8 months). Based on univariate analysis, metastases involving more than three organs, presence of liver metastasis, and PD-L1 TPS ≥ 50% were significantly associated with shorter PFS (all p < 0.05). Multivariate analysis showed that metastases involving than three organs (HR = 3.20, 95% confidence interval [CI]: 1.01–10.8) and PD-L1 TPS ≥ 50% (HR = 2.64, 95% CI: 1.43–4.80) were independently associated with shorter PFS. Kaplan–Meier survival curves showed that patients with PD-L1 TPS ≥ 50% were likely to have poor PFS compared with other TPS groups (Figure 2A).
 |
Table 2 Progression-Free Survival Analyses Results According to Clinicopathological Parameters of All Study Subjects (n=131) |
To evaluate whether the PD-L1 expression has any different impact on the PFS according to different EGFR mutational subtypes, we performed subgroup analysis. Survival analysis results of patients with 19del and L858R are summarized in Tables S3 and S4, respectively. The univariate analysis of 19del-positive population revealed that male sex, metastases involving more than three organs, and PD-L1 TPS ≥ 50% were significantly associated with shorter PFS (all p < 0.05). Multivariate analysis showed that male sex (HR = 1.91, 95% CI: 1.02–3.71) and PD-L1 TPS ≥ 50% (HR = 3.85, 95% CI: 1.23–11.92) were independently associated with shorter PFS. In case of L858R-positive population, metastases involving more than three organs, and PD-L1 TPS ≥ 50% were significantly associated with shorter PFS (all p < 0.05) in univariate analysis, and only PD-L1 TPS ≥ 50% was an independent predictor of shorter PFS (HR=2.15, 95% CI: 1.12–6.98). Kaplan–Meier survival curves of patients with different mutational subtypes showed that patients with PD-L1 TPS ≥50% were likely to have poor PFS compared with other TPS groups (Figure 2BD).
Overall Survival According to PD-L1 TPS
The results of OS analysis of the overall population are summarized in Table S5. The median OS of all study subjects was 36.8 months (range, 4.2–54.6 months). Univariate analysis showed that male sex, current or former smoking, metastases involving more than three organs, and presence of liver metastasis were significantly associated with shorter OS (all p < 0.05). Multivariate analysis showed that metastases involving more than three organs (HR = 3.05, 95% CI: 1.36–6.72) and presence of liver metastasis (HR = 2.46, 95% CI: 1.10–5.76) were independently associated with shorter OS. PD-L1 TPS ≥ 50% was not associated with shorter OS.
In the subgroup analysis according to different EGFR mutational subtypes, metastases involving more than three organs (HR = 2.90, 95% CI: 1.07–9.23), presence of liver metastasis (HR= 3.70, 95% CI: 1.01–10.86), and PD-L1 TPS ≥ 50% (HR = 2.55, 95% CI: 1.17–6.40) were independently associated with shorter OS in patients with 19del (Table S6). However, PD-L1 TPS ≥ 50% was not associated with shorter OS in those with L858R mutations (Table S7). Kaplan–Meier survival curves for OS of the population and each mutational subtypes are presented in Figure S1.
Frequency of Acquired T790M Mutation According to PD-L1 TPS
To identify the possible association between the emergence of secondary T790M mutation and PD-L1 expression, we evaluated the frequency of the mutation according to different PD-L1 TPS. Among 70 patients who underwent rebiopsy, 28 (40.0%) patients were found to carry T790M mutation. The mutation frequency was 13.3% (2/15), 40.0% (10/25), and 53.3% (16/30) in PD-L1 TPS ≥ 50%, 1–49%, and <1% groups, respectively. Patients with PD-L1 TPS ≥ 50% showed significantly low frequency of T790M mutation compared with the other two groups (all p = 0.001, Figure 3). The analyses results for the factors associated with the emergence of secondary T790M mutation are presented in Table 3. Univariate analysis showed that L858R mutation, TKI use <12 months, and PD-L1 TPS ≥ 50% were significantly associated with lower incidence of T790M (all p < 0.05). Multivariate analysis showed that duration of TKI use <12 months was independently associated with a low emergence of acquired T790M (OR = 0.54, 95% CI: 0.11–0.89). In addition, PD-L1 TPS > 50% was significantly associated with lower frequency of secondary T790M mutation (OR = 0.63, 95% CI: 0.12–0.95).
 |
Table 3 Analysis of the Factors Associated with Emergence of Secondary T790M Mutation |
Discussion
The present study demonstrated that high PD-L1 expression was significantly associated with poor treatment response and shorter PFS regardless of EGFR mutational subtypes in patients who were treated with first-line EGFR-TKIs. In addition, it was associated with less frequent development of secondary T790M mutation after TKI failure. To the best of our knowledge, this is the second study to suggest the possible association between pre-TKI PD-L1 expression and the emergence of T790M mutation. Our data suggest that high PD-L1 expression might confer an aggressive phenotype requiring different therapeutic approaches among EGFR-mutant tumors.
Although components of tumor immune microenvironment (TME) including tumor PD-L1 expression, tumor nonsynonymous mutation burden (TMB), and tumor-infiltrating lymphocytes (TILs) are emerging as predictors of response to immune checkpoint blockade in NSCLC,25,26 their clinical implications in patients with EGFR-mutations remain unclear. Earlier studies demonstrated that EGFR mutations induced PD-L1 expression, suggesting the role of EGFR signaling in remodeling the TME to increase sensitivity to anti-PD-1/PD-L1 treatment.27,28 However, subsequent studies identified that EGFR-mutant lung adenocarcinoma was correlated with an uninflamed phenotype with a high frequency of inactive TIL and low TMB.29,30 Several studies on PD-L1 expression demonstrated a lower proportion of elevated PD-L1 expression among patients with EGFR-mutations (11.8% to 17.5%) compared with EGFR wild-type patients, which are consistent with our results (17.6%).23,24,31 Taken together, our study confirmed the previous findings suggesting that EGFR-mutant NSCLC is characterized by less immunogenic TME in terms of tumor PD-L1 expression, suggesting that immune checkpoint blockade might be of limited benefit in such tumors compared with EGFR-wild type NSCLC.
PD-L1 expression is widely accepted as predictive and prognostic in immunotherapy, however, the implications of this biomarker in EGFR-mutant lung cancer treated with TKIs are still inconclusive. A summary of the published studies and our data is presented in Table 4. Earlier studies showed that positive PD-L1 expression was associated with better RR and longer time to progression or PFS.32,33 However, five recent studies consistently demonstrated that PD-L1 expression was associated with poor response and unfavorable clinical outcomes.24,31,34-36 Yang et al reported that RR and PFS were significantly poor in patients with PD-L1 TPS ≥50%, and PD-L1 TPS<50% was an independent prognostic factor for longer PFS (HR=0.433, 95% CI: 0.250–0.751).24 Our results are in accordance with those recent studies and suggest the predictive and prognostic value of baseline PD-L1 expression in EGFR-mutant tumors. The reason for the inconsistent results is not clear but it can be partly explained by differences in sample size, patients’ ethnicities, antibody clones, and scoring cutoffs among different studies.
 |
Table 4 Summary of Published and Present Data on the Association Between PD-L1 Expression and Clinical Outcomes of EGFR-TKIs |
In addition, male sex was independently associated with shorter PFS in our study. Although the clinical significance of sex in EGFR-mutant NSCLC treated with EGFR-TKIs as first-line treatment is still disputed, many studies have suggested that male sex is an unfavorable predictive factor.31,37,38 Our results are consistent with previous findings. However, further large-scaled studies are needed to validate the impact of sex on survival in this clinical setting.
In the present study, PD-L1 expression was not associated with OS in overall population. To date, three studies have investigated the prognostic value of PD-L1 in EGFR-mutant NSCLC treated with EGFR-TKIs.33,34,39 Although the cutoffs and the antibodies used differed among the studies, no association was observed between PD-L1 positivity and OS, which was in accordance with our finding. However, in the subgroup analysis, we identified that high PD-L1 expression was independently associated with shorter OS among patients with 19del but not among those with L858R. A preclinical study has demonstrated that these two EGFR-mutations show distinct biological properties that may affect the efficacy of EGFR-TKIs.40 In addition, our data showed that compared to other mutational subtypes, patients with 19del are more likely to carry secondary T790M mutation which is associated with longer survival. Although the reason why PD-L1 expression was significant only in 19del-positive patients is not clear, our findings suggest that this immune checkpoint protein may have prognostic value in a certain group of EGFR-mutant patients. This hypothesis should be validated by further studies with long-term follow-up data.
Studies have demonstrated that PD-L1 expression is inducible and can be upregulated by various genomic alterations such as EGFR, ALK, and mitogen-activated protein kinase (MAPK).41–43 Thus high PD-L1 expression may confer to presence of concurrent genetic alterations, resulting in TKI resistance as reported in previous studies.30,44 Recent studies suggest that TME interacts with EGFR-mutant tumors, which affects the efficacy of TKI treatment as shown in the field of immunotherapy.24,45 Matsumoto et al divided the TME into four types based on PD-L1 expression and CD8+ T cells, and demonstrated that PD-L1+/CD8+ tumors exhibited the lowest RR (14.3%) and the shortest median PFS (2.4 months), while PD-L1-/CD8+ patients showed best RR (78.6%) and the longest PFS (17.5 months) in EGFR-mutant patients treated with TKIs.45 In addition, Yang et al evaluated the impact of various immune cells in TME including regulatory T cells and macrophages, and demonstrated the possible association between CD20+ B cell and tumor response in the same clinical setting.24 More interestingly, a recent study suggested that TKI treatment may alter TME in EGFR-mutant NSCLC.46 Using rebiopsy samples after TKI failure, Isomoto et al reported that the proportion of patient with high PD-L1 expression was significantly increased (14% to 28%, p = 0.001) and TMB tended to increase after TKI (3.3 to 4.1 mutations/Mbp, p = 0.0508) and suggested the possible benefit of subsequent anti-PD-1/PD-L1 treatment in those patients.46 Previous studies have demonstrated that patients with driving genetic alterations are characterized by impaired response to immune checkpoint inhibitors.47 However, Sun et al reported that the clinical response to PD-1/PD-L1 blockade reached almost 30% even in EGFR-mutant patients, especially in males or smokers with high PD-L1 expression suggesting the possible clinical benefit of immunotherapy in selected patients harboring the mutation.48 Taken previous and our data together, subsequent immunotherapy after TKI failure in patients with high PD-L1 expression might be a feasible treatment option although further prospective investigations are essential.
In this study, we found that high PD-L1 expression was associated with a low frequency of acquired T790M mutation as a resistance mechanism. The T790M gatekeeper mutation, localized to exon 20 of EGFR has been identified in about half of patients who progressed after first-line EGFR-TKI, which is a major resistance mechanism.16 Because T790M-positive tumors are susceptible to osimertinib, the detection of this mutation is critical for the management of EGFR-mutant patients. Although mutational analysis using tumor tissue or body fluid is the standard method, it is often limited by issues such as invasiveness of the tissue acquisition procedures, inaccessibility or insufficiency of sample tissues. Previous studies demonstrated that 19del and long-term exposure to EGFR-TKIs were associated with more frequent T790M mutation.49–51 Our present data identified that low PD-L1 expression (PD-L1 TPS <50%) can be a predictor for T790M positivity after TKI failure, which is consistent with a recent study.24 The reason why low PD-L1 is associated with frequent T790M emergence is unclear; however, it may be attributed to the longer duration of treatment with EGFR-TKI in patients with low PD-L1 expression as suggested by previous studies.52–54 Chmielecki et al demonstrated that T790M-positive cells were selected after long-term exposure to first-line EGFR-TKIs.52 Other studies have shown that T790M-positive cells undergo selection and enrichment during EGFR-TKI treatment.53,54 T790M-positive tumor is slow-growing with a longer survival compared with T790M-negative counterparts.55 Interestingly, Hata et al reported higher PD-L1 expression in T790M-negative patients compared with T790M-positive patients after EGFR-TKI failure, suggesting a potential benefit of anti-PD-1/PD-L1 treatment in T790M-negative population.56 This hypothesis was partly supported by a subsequent study demonstrating favorable response to nivolumab in such patients.57
Based on our findings, we cautiously suggest that EGFR-mutant tumors should be managed with different treatment strategies according to baseline PD-L1 status. TKI monotherapy would be the standard-of-care for patients with low PD-L1 expression, as indicated by the current guidelines. In contrast, for patients with high PD-L1 expression who show poor response to TKIs, the combination with other agents including chemotherapy or immunotherapy may be a feasible option. Indeed, a very recent NEJ009 trial demonstrated that gefitinib plus carboplatin/pemetrexed combination showed better RR (84% vs 67%, p < 0.001), longer PFS (20.9 vs 11.9 months, HR = 0.49; p < 0.001), and OS (50.9 vs 38.,8 months, HR = 0.722; p = 0.021) in EGFR-mutant NSCLC compared with gefitinib monotherapy.58 Because the elevated PD-L1 expression confers a distinct aggressive phenotype among EGFR-mutant tumors, large-scaled prospective investigations are urgently required to determine the optimal treatment strategy for those patients population.
This study has several limitations. First, it is a retrospective study and selection bias is inevitable. However, we strived to enhance the validity of our data by using a relatively large cohort with long-term follow-up. Second, we did not include clinical characteristics such as underlying medical diseases or nutritional status, which may affect the clinical outcome in our study population. Third, we only used a single antibody (22C3) and did not evaluate other PD-L1 antibodies simultaneously. The recent Blueprint Project has shown that the results of various PD-L1 antibody clones were concordant except SP142.59 Fourth, patients with other genetic alterations such as ROS1 and ALK fusions were excluded because of their rarity among lung adenocarcinoma patients. Fifth, the dynamic changes of PD-L1 expression in patients with progressive disease after EGFR-TKI were not evaluated. As mentioned above, TKI therapy can affect the level of PD-L1 expression and the impact of upregulated or downregulated PD-L1 expression on the subsequent treatment is an interesting topic for future studies. Finally, we did not assess pre-TKI genetic alterations or other immune phenotypes. To address this issue, we are currently working on a comprehensive study investigating the impact of concurrent genetic alterations and immunologic signatures on the clinical course of EGFR-mutant patients.
Conclusion
Our data demonstrate that a high PD-L1 expression is associated with unfavorable clinical outcome not only due to the poor response to frontline TKI treatment but also because of the diminished likelihood of secondary T790M mutation after TKI failure. Although further studies are needed to verify our results, our findings suggest that elevated expression of PD-L1 might confer a distinct aggressive phenotype among EGFR-mutant tumors requiring a tailored therapeutic approach. In addition, future studies should focus on the optimal treatment strategy for populations with high PD-L1 expression to facilitate personalized medicine for patients with driving mutations.
Disclosure
The authors declare that they have no competing interests for this work.
References
1. Park CK, Kim SJ. Trends and updated statistics of lung cancer in Korea. Tuberc Respir Dis. 2019;82(2):175–177. doi:10.4046/trd.2019.0015
2. Park JY, Jang SH. Epidemiology of lung cancer in korea: recent trends. Tuberc Respir Dis. 2016;79(2):58–69. doi:10.4046/trd.2016.79.2.58
3. Kim HC, Jung CY, Cho DG, et al. Clinical characteristics and prognostic factors of lung cancer in korea: a pilot study of data from the korean nationwide lung cancer registry. Tuberc Respir Dis. 2019;82(2):118–125. doi:10.4046/trd.2017.0128
4. Kim HC, Choi CM. Current status of immunotherapy for lung cancer and future perspectives. Tuberc Respir Dis. 2020;83(1):14–19. doi:10.4046/trd.2019.0039
5. Dall’Olio FG, Maggio I, Massucci M, Mollica V, Fragomeno B, Ardizzoni A. ECOG performance status >/=2 as a prognostic factor in patients with advanced non small cell lung cancer treated with immune checkpoint inhibitors-A systematic review and meta-analysis of real world data. Lung Cancer. 2020;145:95–104. doi:10.1016/j.lungcan.2020.04.027
6. Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–1550. doi:10.1016/S0140-6736(15)01281-7
7. Reck M, Rodriguez-Abreu D, Robinson AG, et al. Updated analysis of KEYNOTE-024: pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol. 2019;37(7):537–546. doi:10.1200/JCO.18.00149
8. Rizvi NA, Mazieres J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a Phase 2, single-arm trial. Lancet Oncol. 2015;16(3):257–265. doi:10.1016/S1470-2045(15)70054-9
9. Sun JM, Zhou W, Choi YL, et al. Prognostic significance of PD-L1 in patients with non-small cell lung cancer: a large cohort study of surgically resected cases. J Thorac Oncol. 2016;11(7):1003–1011. doi:10.1016/j.jtho.2016.04.007
10. Jett JR, Carr LL. Targeted therapy for non-small cell lung cancer. Am J Respir Crit Care Med. 2013;188(8):907–912. doi:10.1164/rccm.201301-0189PP
11. Shi Y, Au JS, Thongprasert S, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol. 2014;9(2):154–162. doi:10.1097/JTO.0000000000000033
12. Holleman MS, van Tinteren H, Groen HJ, Al MJ, Uyl-de Groot CA. First-line tyrosine kinase inhibitors in EGFR mutation-positive non-small-cell lung cancer: a network meta-analysis. Onco Targets Ther. 2019;12:1413–1421. doi:10.2147/OTT.S189438
13. Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–957. doi:10.1056/NEJMoa0810699
14. Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised Phase 3 trial. Lancet Oncol. 2012;13(3):239–246. doi:10.1016/S1470-2045(11)70393-X
15. Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31(27):3327–3334. doi:10.1200/JCO.2012.44.2806
16. Chang YS, Choi CM, Lee JC. Mechanisms of epidermal growth factor receptor tyrosine kinase inhibitor resistance and strategies to overcome resistance in lung adenocarcinoma. Tuberc Respir Dis. 2016;79(4):248–256. doi:10.4046/trd.2016.79.4.248
17. Kim IA, Lee JS, Kim HJ, Kim WS, Lee KY. Cumulative smoking dose affects the clinical outcomes of EGFR-mutated lung adenocarcinoma patients treated with EGFR-TKIs: a retrospective study. BMC Cancer. 2018;18(1):768. doi:10.1186/s12885-018-4691-0
18. Nagano T, Tachihara M, Nishimura Y. Mechanism of resistance to epidermal growth factor receptor-tyrosine kinase inhibitors and a potential treatment strategy. Cells. 2018;7:11. doi:10.3390/cells7110212
19. Goldstraw P, Chansky K, Crowley J, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11(1):39–51. doi:10.1016/j.jtho.2015.09.009
20. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. doi:10.1016/j.ejca.2008.10.026
21. Roach C, Zhang N, Corigliano E, et al. Development of a companion diagnostic PD-L1 immunohistochemistry assay for pembrolizumab therapy in non-small-cell lung cancer. Appl Immunohistochem Mol Morphol. 2016;24(6):392–397. doi:10.1097/PAI.0000000000000408
22. Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. doi:10.1056/NEJMoa1606774
23. Takada K, Toyokawa G, Tagawa T, et al. PD-L1 expression according to the EGFR status in primary lung adenocarcinoma. Lung Cancer. 2018;116:1–6. doi:10.1016/j.lungcan.2017.12.003
24. Yang CY, Liao WY, Ho CC, et al. Association between programmed death-ligand 1 expression, immune microenvironments, and clinical outcomes in epidermal growth factor receptor mutant lung adenocarcinoma patients treated with tyrosine kinase inhibitors. Eur J Cancer. 2020;124:110–122. doi:10.1016/j.ejca.2019.10.019
25. Carbone DP, Reck M, Paz-Ares L, et al. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med. 2017;376(25):2415–2426. doi:10.1056/NEJMoa1613493
26. Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124–128. doi:10.1126/science.aaa1348
27. Akbay EA, Koyama S, Carretero J, et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov. 2013;3(12):1355–1363. doi:10.1158/2159-8290.CD-13-0310
28. Azuma K, Ota K, Kawahara A, et al. Association of PD-L1 overexpression with activating EGFR mutations in surgically resected nonsmall-cell lung cancer. Ann Oncol. 2014;25(10):1935–1940. doi:10.1093/annonc/mdu242
29. Dong ZY, Zhang JT, Liu SY, et al. EGFR mutation correlates with uninflamed phenotype and weak immunogenicity, causing impaired response to PD-1 blockade in non-small cell lung cancer. Oncoimmunology. 2017;6(11):e1356145. doi:10.1080/2162402X.2017.1356145
30. Offin M, Rizvi H, Tenet M, et al. Tumor mutation burden and efficacy of EGFR-tyrosine kinase inhibitors in patients with EGFR-mutant lung cancers. Clin Cancer Res. 2019;25(3):1063–1069. doi:10.1158/1078-0432.CCR-18-1102
31. Yoneshima Y, Ijichi K, Anai S, et al. PD-L1 expression in lung adenocarcinoma harboring EGFR mutations or ALK rearrangements. Lung Cancer. 2018;118:36–40. doi:10.1016/j.lungcan.2018.01.024
32. D’Incecco A, Andreozzi M, Ludovini V, et al. PD-1 and PD-L1 expression in molecularly selected non-small-cell lung cancer patients. Br J Cancer. 2015;112(1):95–102. doi:10.1038/bjc.2014.555
33. Lin C, Chen X, Li M, et al. Programmed death-ligand 1 expression predicts tyrosine kinase inhibitor response and better prognosis in a cohort of patients with epidermal growth factor receptor mutation-positive lung adenocarcinoma. Clin Lung Cancer. 2015;16(5):e2535. doi:10.1016/j.cllc.2015.02.002
34. Soo RA, Kim HR, Asuncion BR, et al. Significance of immune checkpoint proteins in EGFR-mutant non-small cell lung cancer. Lung Cancer. 2017;105:17–22. doi:10.1016/j.lungcan.2017.01.008
35. Su S, Dong ZY, Xie Z, et al. Strong programmed death ligand 1 expression predicts poor response and de novo resistance to EGFR tyrosine kinase inhibitors among NSCLC patients with EGFR mutation. J Thorac Oncol. 2018;13(11):1668–1675. doi:10.1016/j.jtho.2018.07.016
36. Hsu KH, Huang YH, Tseng JS, et al. High PD-L1 expression correlates with primary resistance to EGFR-TKIs in treatment naive advanced EGFR-mutant lung adenocarcinoma patients. Lung Cancer. 2019;127:37–43. doi:10.1016/j.lungcan.2018.11.021
37. Inoue A, Yoshida K, Morita S, et al. Characteristics and overall survival of EGFR mutation-positive non-small cell lung cancer treated with EGFR tyrosine kinase inhibitors: a retrospective analysis for 1660 Japanese patients. Jpn J Clin Oncol. 2016;46(5):462–467. doi:10.1093/jjco/hyw014
38. Kang HS, Shin AY, Yeo CD, et al. Clinical significance of anemia as a prognostic factor in non-small cell lung cancer carcinoma with activating epidermal growth factor receptor mutations. J Thorac Dis. 2020;12(5):1895–1902. doi:10.21037/jtd-19-3932
39. Tang Y, Fang W, Zhang Y, et al. The association between PD-L1 and EGFR status and the prognostic value of PD-L1 in advanced non-small cell lung cancer patients treated with EGFR-TKIs. Oncotarget. 2015;6(16):14209–14219. doi:10.18632/oncotarget.3694
40. Zhu JQ, Zhong WZ, Zhang GC, et al. Better survival with EGFR exon 19 than exon 21 mutations in gefitinib-treated non-small cell lung cancer patients is due to differential inhibition of downstream signals. Cancer Lett. 2008;265(2):307–317. doi:10.1016/j.canlet.2008.02.064
41. Chen N, Fang W, Zhan J, et al. Upregulation of PD-L1 by EGFR activation mediates the immune escape in EGFR-driven NSCLC: implication for optional immune targeted therapy for NSCLC patients with EGFR mutation. J Thorac Oncol. 2015;10(6):910–923. doi:10.1097/JTO.0000000000000500
42. Ota K, Azuma K, Kawahara A, et al. Induction of PD-L1 expression by the EML4-ALK oncoprotein and downstream signaling pathways in non-small cell lung cancer. Clin Cancer Res. 2015;21(17):4014–4021. doi:10.1158/1078-0432.CCR-15-0016
43. Sumimoto H, Takano A, Teramoto K, Daigo Y. RAS-mitogen-activated protein kinase signal is required for enhanced PD-L1 expression in human lung cancers. PLoS One. 2016;11(11):e0166626. doi:10.1371/journal.pone.0166626
44. Kim Y, Lee B, Shim JH, et al. Concurrent genetic alterations predict the progression to target therapy in EGFR-mutated advanced NSCLC. J Thorac Oncol. 2019;14(2):193–202. doi:10.1016/j.jtho.2018.10.150
45. Matsumoto Y, Sawa K, Fukui M, et al. Impact of tumor microenvironment on the efficacy of epidermal growth factor receptor-tyrosine kinase inhibitors in patients with EGFR-mutant non-small cell lung cancer. Cancer Sci. 2019;110(10):3244–3254. doi:10.1111/cas.14156
46. Isomoto K, Haratani K, Hayashi H, et al. Impact of EGFR-TKI treatment on the tumor immune microenvironment in EGFR mutation-positive non-small cell lung cancer. Clin Cancer Res. 2020;26(8):2037–2046. doi:10.1158/1078-0432.CCR-19-2027
47. Li J, Gu J. PD-L1 expression and EGFR status in advanced non-small-cell lung cancer patients receiving PD-1/PD-L1 inhibitors: a meta-analysis. Future Oncol. 2019;15(14):1667–1678. doi:10.2217/fon-2018-0639
48. Cho JH, Jung HA, Lee SH, et al. Impact of EGFR mutation on the clinical efficacy of PD-1 inhibitors in patients with pulmonary adenocarcinoma. J Cancer Res Clin Oncol. 2019;145(5):1341–1349. doi:10.1007/s00432-019-02889-0
49. Nosaki K, Satouchi M, Kurata T, et al. Re-biopsy status among non-small cell lung cancer patients in Japan: a retrospective study. Lung Cancer. 2016;101:1–8. doi:10.1016/j.lungcan.2016.07.007
50. Matsuo N, Azuma K, Sakai K, et al. Association of EGFR exon 19 deletion and EGFR-TKI treatment duration with frequency of T790M mutation in EGFR-mutant lung cancer patients. Sci Rep. 2016;6:36458. doi:10.1038/srep36458
51. Goag EK, Lee JM, Chung KS, et al. Usefulness of bronchoscopic rebiopsy of non-small cell lung cancer with acquired resistance to epidermal growth factor receptor-tyrosine kinase inhibitor. J Cancer. 2018;9(6):1113–1120. doi:10.7150/jca.21650
52. Chmielecki J, Foo J, Oxnard GR, et al. Optimization of dosing for EGFR-mutant non-small cell lung cancer with evolutionary cancer modeling. Sci Transl Med. 2011;3(90):90ra59. doi:10.1126/scitranslmed.3002356
53. Iwama E, Takayama K, Harada T, et al. Highly sensitive and quantitative evaluation of the EGFR T790M mutation by nanofluidic digital PCR. Oncotarget. 2015;6(24):20466–20473. doi:10.18632/oncotarget.4058
54. Zheng D, Ye X, Zhang MZ, et al. Plasma EGFR T790M ctDNA status is associated with clinical outcome in advanced NSCLC patients with acquired EGFR-TKI resistance. Sci Rep. 2016;6:20913. doi:10.1038/srep20913
55. Hata A, Katakami N, Yoshioka H, et al. Rebiopsy of non-small cell lung cancer patients with acquired resistance to epidermal growth factor receptor-tyrosine kinase inhibitor: comparison between T790M mutation-positive and mutation-negative populations. Cancer. 2013;119(24):4325–4332. doi:10.1002/cncr.28364
56. Hata A, Katakami N, Nanjo S, et al. Programmed death-ligand 1 expression and T790M status in EGFR-mutant non-small cell lung cancer. Lung Cancer. 2017;111:182–189. doi:10.1016/j.lungcan.2017.07.022
57. Haratani K, Hayashi H, Tanaka T, et al. Tumor immune microenvironment and nivolumab efficacy in EGFR mutation-positive non-small-cell lung cancer based on T790M status after disease progression during EGFR-TKI treatment. Ann Oncol. 2017;28(7):1532–1539. doi:10.1093/annonc/mdx183
58. Hosomi Y, Morita S, Sugawara S, et al. Gefitinib alone versus gefitinib plus chemotherapy for non-small-cell lung cancer with mutated epidermal growth factor receptor: NEJ009 study. J Clin Oncol. 2020;38(2):115–123. doi:10.1200/JCO.19.01488
59. Hirsch FR, McElhinny A, Stanforth D, et al. PD-L1 immunohistochemistry assays for lung cancer: results from phase 1 of the blueprint PD-L1 IHC assay comparison project. J Thorac Oncol. 2017;12(2):208–222. doi:10.1016/j.jtho.2016.11.2228
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.