Back to Journals » Clinical Ophthalmology » Volume 12
High myopic photorefractive keratectomy outcomes with the Alcon Wavelight® EX500 excimer laser
Authors Mifflin MD, Betts BS, Nguyen J, Pouly S
Received 30 January 2018
Accepted for publication 7 April 2018
Published 6 June 2018 Volume 2018:12 Pages 1041—1048
DOI https://doi.org/10.2147/OPTH.S164110
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Mark D Mifflin,1 Brent S Betts,2 Jason Nguyen,3 Severin Pouly4
1Department of Ophthalmology and Visual Sciences, John A. Moran Eye Center, University of Utah, Salt Lake City, UT, USA; 2Intermountain Eye and Laser Center, Boise, ID, USA; 3Department of Ophthalmology, West Virginia University School of Medicine, Morgantown, WV, USA; 4Little Rock Eye Clinic, Little Rock, AR, USA
Purpose: To present refractive outcomes from consecutive cases with the Alcon Wavelight® EX500 excimer laser using photorefractive keratectomy (PRK) in patients with high myopia.
Methods: A retrospective chart review of consecutive cases of high myopic eyes (≥6.0 Diopters [D]) undergoing PRK with the Alcon Wavelight EX500 excimer laser (Alcon Laboratories, Fort Worth, TX, USA) was done. Moderately high myopic eyes (6.0 to <8.0 D [6 D]) were compared with high myopic eyes (8.0 D or greater [8 D]). Outcomes measured included pre- and postoperative refractive error, uncorrected distance visual acuity (UDVA), corrected distance visual acuity, spherical equivalent correction (SEQ), haze incidence, and intraocular pressure (IOP).
Results: One hundred eighteen eyes of 63 patients were evaluated, with 59 eyes having 12 months of follow-up. Thirty-one eyes of 19 patients had 8.0 D or more of myopia. Twelve-month average LogMAR UDVA was –0.06 (20/17) for the 6 D group and –0.08 (20/16) for the 8 D group. Average 12-month SEQ was –0.18 D and preoperatively was –7.52 D for the 6 D group and –0.09 and –9.02 in the 8 D group. Sixty-five eyes (86%) and 24 eyes (96%) had an SEQ within 0.50 D of emmetropia at 3 months in the 6 and 8 D groups, respectively. One eye had visually significant haze developed at 8 months. Three eyes had IOP elevation that resolved with addition of short-term topical IOP-lowering medication.
Conclusion: High myopic PRK with the Alcon Wavelight EX500 excimer laser yields excellent refractive outcomes with a low incidence of complications.
Keywords: high myopia, PRK, wavefront-optimized, refractive surgery
Introduction
Photorefractive keratectomy (PRK) has increased in popularity over the last decade as it has been recognized that a wider range of corrections can be treated when compared to LASIK.1 Still, many USA surgeons shy away from PRK, particularly in high myopia, because of concerns about slow recovery, inconvenience, risk of haze, more postoperative visits, and longer postoperative medication regimen. Despite slower recovery, PRK for high myopia has structural and mechanical advantages as compared to LASIK. Advantages include greater residual stromal bed and, therefore, less risk of ectasia and more reserve tissue for enhancement surgery if needed. Avoidance of early or late flap complications, although rare with femtosecond laser, is another desirable advantage of PRK. The newest generation 500 Hz high-frequency excimer laser, the Alcon Wavelight® EX500 (Alcon Laboratories, Fort Worth, TX, USA), has been shown to safely and effectively treat high myopia with LASIK.2 Before obtaining this laser, we would preferentially recommended LASIK or phakic intraocular lenses (PIOLs) to highly myopic patients. Our excellent LASIK outcomes with the Wavelight EX500 caused us to reevaluate the success of high myopic PRK and offer this to appropriately screened patients. Herein, we compare outcomes with the Wavelight EX500 excimer laser for moderately high myopic eyes with 6.0 D of myopia, but <8.0 D of myopia (6 D) to a subset of eyes with myopia of 8.0 D or more (8 D).
Methods
The University of Utah Institutional Review Board approved the study and it was conducted in accordance with the tenets of the Declaration of Helsinki. All patients were provided with written informed consent. Eyes undergoing PRK with 6.0 D or greater of myopic spherical equivalent (SEQ) were included in the study for distance correction. A subset analysis of eyes with 8.0 D or more of myopia was performed. Patients underwent a standard refractive screening examination with 1 surgeon (MDM). Patients with a history of prior refractive surgery, keratoconus, form-fruste keratoconus, and an ablation that would leave <300 microns of residual stromal bed (RSB) were excluded.
Corneal epithelium was gently debrided after exposure to ethanol 20% in balanced salt solution for 35–40 seconds placed in an 8.0 mm well. Laser ablation was performed using the wavefront-optimized Alcon Allegretto Wavelight EX500 excimer laser according to the surgeon-optimized nomogram.3 Mitomycin C 0.02% (MMC) was used in all eyes because laser ablation depth was >60 microns, with application time being from 12 to 20 seconds based on the surgeon’s preference. Average MMC application time was 12.8±2.42 and 16.4±3.03 seconds in the 6 and the 8 D groups, respectively. Immediately after MMC, the ocular surface was rinsed with chilled saline for 30 seconds. One drop of prednisolone acetate 1%, a topical fluoroquinolone, and a topical nonsteroidal anti-inflammatory drug were instilled and then a soft bandage contact lens was placed over the eye.
Postoperatively patients used prednisolone acetate 1% suspension in the operative eye, starting at a frequency of 4 times per day for the first week, then 2 times per day for 3 weeks, and subsequently switched to fluorometholone 0.1% suspension. Fluorometholone was used 3 times per day for 1 month, then 2 times per day for 1 month, and then stopped.
The primary outcome measure was uncorrected distance visual acuity (UDVA). Secondary outcomes included intraocular pressure (IOP), corrected distance visual acuity (CDVA), manifest refraction SEQ, time of MMC application, and incidence and grade of postoperative corneal haze, based on the Fantes scale.4 Significant IOP elevation was defined as ≥10 mmHg above baseline, or any IOP over 21 mmHg.
Standard statistics were calculated and used to describe the treatment groups in terms of all study variables including UDVA, CDVA, manifest refraction, presence or absence and degree of postoperative corneal haze, and incidence of other postoperative complications. A P-value of 0.05 or less was considered statistically significant. Data analysis was performed using Microsoft Excel (Microsoft Corp, Redmond, WA, USA).
Results
Sixty-three patients were included in the study. Thirty-six patients (57%) were female and 27 (42%) were male. The average age was 32.7±7.76 (range 21–53). Six-month follow-up data were recorded on 83 eyes of 43 patients and 12-month follow-up was obtained on 59 eyes of 32 patients. Demographic and preoperative parameters of patients in both the 6 and 8 D groups are compared in Table 1.
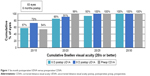
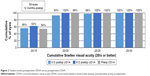
Average 6-month and 12-month UDVA values for both groups are shown in Figures 1 and 2, respectively. Average LogMAR UDVA at 6 months was −0.07 (20/17) in the 6 D group and −0.08 (20/16) in the 8 D group. UDVA was 20/20 or better in 52 eyes (85%) in the 6 D group and 20 eyes (91%) in the 8 D group at 6 months (P=0.14). At 12 months, 40 eyes (93%) and 16 eyes (100%) had UDVA of 20/20 or better in the 6 and 8 D groups, respectively (P=0.36). Twelve-month average LogMAR UDVA was −0.06 (20/17) for the 6 D group and −0.08 (20/16) for the 8 D group.
Thirty-seven eyes (61%) had no change in CDVA after PRK at 6 months, whereas 19 eyes (31%) gained 1 line of CDVA in the 6 D group. Seven eyes (32%) had no change in CDVA and 14 eyes (64%) gained 1 line of CDVA 6 months after PRK in the 8 D group. Five eyes (8%) and 1 eye (5%) lost 1 line of CDVA in the 6 and 8 D groups, respectively (Figure 3). Figure 4 shows safety outcomes at 12 months. In the 8 D group, 6 eyes (38%) had no change in CDVA, 8 eyes (50%) gained 1 line, and 2 eyes (12%) lost 1 line of CDVA. In the 6 D group, 24 eyes (56%), 14 eyes (33%), and 5 eyes (12%) had no change in CDVA, gained 1 line, or lost 1 line of CDVA, respectively.
  | Figure 3 Percentage of eyes at 6 months postoperatively that lost or gained lines of Snellen corrected distance visual acuity (CDVA). |
  | Figure 4 Percentage of eyes at 12 months postoperatively that lost or gained lines of Snellen CDVA. |
Postoperative spherical equivalent refraction (Figure 5) showed that 51 eyes (84%) and 20 eyes (91%) were within 0.50 D of emmetropia at 6 months in the 6 and 8 D groups. Fifty-nine eyes (97%) and 22 eyes (100%) were within 1 D of emmetropia in the 6 and 8 D groups, respectively. At 12 months, 37 eyes (86%) were within 0.5 D of emmetropia in the 6 D group, and 16 eyes (100%) in the 8 D group were within the same range (Figure 6).
  | Figure 5 Six-month postoperative spherical equivalent refractive accuracy. |
  | Figure 6 Twelve-month postoperative spherical equivalent refractive accuracy. |
Figure 7 shows the predictability of PRK corrections in both groups at 12 months. Three eyes (5%) were overcorrected by 0.50 D. Ten eyes (17%) were undercorrected by 0.50 D, with 2 eyes (3%) undercorrected by −1.00 D. All eyes over- or undercorrected by 0.50 D or more were in the 6 D group. Fifty-two eyes (88%) had 0.25 D or less of astigmatism at 12 months (Figure 8). One eye (2%) had 1.25 D of astigmatism at 12 months. Figure 9 shows refractive stability of corrections. Only 4% of eyes had a change in SEQ of 0.50 D or more from 3 to 12 months postoperatively.
  | Figure 7 Twelve-month postoperative predictability of SE with achieved SE (y-axis) and attempted SE (x-axis). |
  | Figure 9 Stability of spherical equivalent refraction in diopters (D) at 1, 3, 6, and 12 months. |
Four eyes with <8 D of myopia had visually insignificant trace to 1+ haze at the 1-month visit, with one of these eyes having persistent trace haze at 3 months with an UCVA of 20/15. One eye developed late haze at 8 months with loss of 1 line of CDVA.
Three eyes of 2 patients had a significant IOP elevation during the first 3 postoperative months. IOP returned to baseline in both cases when the topical corticosteroid (fluorometholone 0.1%) was stopped.
Discussion
Our analysis of 12-month outcomes of high myopic PRK with the Wavelight EX500 excimer laser shows the procedure to result in excellent UDVA and a low rate of complications. Kanellopoulos et al previously reported successful outcomes with the same excimer laser in cases of high myopic LASIK.2 Our visual acuity outcomes are comparable to this study. With low rates of postoperative haze, we prefer PRK in cases of high myopia. Higher regression rates with high myopic corrections have been documented previously and PRK leaves more residual stromal bed in case an enhancement surgery is indicated.5 PRK also negates concerns of LASIK flap complications and leaves more of the stronger anterior stroma intact. It has been suggested that the risk for ectasia may be lower with PRK.1 In cases of asymmetric inferior steepening with normal appearing posterior corneal elevation, we prefer PRK over LASIK.
The literature is mixed on whether LASIK or PRK results in more postoperative dry eye. Bower et al analyzed 143 patients before and after LASIK and PRK.6 They found that 5% of PRK and 0.8% of LASIK eyes developed chronic dry eye 12 months after treatment. Alternatively, other studies have concluded that LASIK is associated with neurotrophic epitheliopathy7 and higher myopic ablations have been shown to result in higher rates of dry eye after LASIK.8 In comparison to PRK, LASIK has been shown to decrease tear secretion to a greater degree.9 Due to our practice location in the arid western USA, we have observed a high incidence of dry eye in our screened refractive surgery candidates and more postoperative dry eye in our LASIK-treated eyes. Theoretically, this may be supported by the greater number of corneal nerves transected by a LASIK flap followed by ablation. If patients have preoperative dry eye signs and symptoms we tend to recommend PRK to help mitigate postoperative dry eye complications based on our personal experience. Others may be doing the same, and this may result in a bias confounding retrospective studies evaluating dry eye after LASIK and PRK.
Haze was uncommon in our study population with 5 eyes (4%) developing haze. Previous studies have reported the incidence of haze to be up to 20% after PRK, with visually significant haze in around 2% of eyes.10–12 Sia et al have shown that application of MMC following high myopic PRK leads to low rates of haze formation.11 Only 1 eye (0.8%) developed visually significant haze. The visually significant haze developed around 8 months after surgery. Late haze has been reported as a very rare complication, especially when using adjunctive MMC, which the affected eye received.13 There is also a correlation with higher depth of ablation and haze development.14 Our PRK patients undergo a 3-month steroid taper and this has been successful in preventing haze formation.15 We believe that a slow steroid taper and mitomycin application are especially important in high myopes based on their increased risk for haze formation.
The group of eyes with 8 D or more of myopia had particularly good outcomes with 100% of the eyes being within 0.5 D of emmetropia 12 months after surgery. Our criteria for treating these highly myopic eyes was very stringent and may have led to selecting eyes that were prone to better outcomes. Less ideal candidates were encouraged to consider PIOL as a vision correction option. The safety of conventional PRK in high myopia has been demonstrated previously.11 Wavefront-optimized treatments induce less higher-order aberrations and lead to better quality vision, but require more tissue per diopter of correction.16,17 The Wavelight EX500 is FDA-approved for higher myopic ablations than any other wavefront excimer laser in the USA, allowing a higher range of corrections to be safely treated with this excimer technology. Study of long-term follow-up in this group is warranted as these eyes are known to have more myopic regression.
PRK has limitations. We try to avoid overflattening corneas, even with wavefront-optimized ablation patterns. Ectasia still may be a risk and higher-risk patients may be selected for PRK. Extreme vigilance for risk factors is of paramount importance in high myopes as there is little margin for error. Compliance with postoperative steroid regimens is important in prevention of haze. Corticosteroid eye drops cause elevated IOP in some patients. There is some unknown risk with MMC long-term. Patient expectations must be managed appropriately as the recovery after PRK is much longer than that of LASIK. We did not measure contrast sensitivity or perform formal testing of night vision side effects in this study population. Further study of subjective symptoms after high myopic PRK is warranted.
Our study is limited by its retrospective nature, small proportion of patients with 12-month outcomes, and lack of systematic symptom analysis. Prospective, long-term studies of high myopic PRK patients along with a study of post-PRK symptoms would be beneficial.
PRK is effective in reducing spectacle dependence in high myopes with low complication rates. Eyes with normal structural parameters can be safely treated with PRK while mitigating the risks of LASIK because of higher residual stromal bed per correction and more accurately correct astigmatism than currently available PIOLs. Appropriate candidates with 6–11 D of myopia can be offered PRK as an excellent alternative to LASIK or PIOL for surgical vision correction.
Acknowledgments
This study was supported in part by an unrestricted grant from Research to Prevent Blindness, Inc., New York, New York, USA, to the Department of Ophthalmology and Visual Sciences, University of Utah, Salt Lake City, Utah, USA.
Disclosure
The authors report no conflicts of interest in this work.
References
Moisseiev E1, Sela T, Minkev L, Varssano D. Increased preference of surface ablation over laser in situ keratomileusis between 2008–2011 is correlated to risk of ecatasia. Clin Ophthalmol. 2013;7:93–98. | ||
Kanellopoulos AJ, Asimellis G. Refractive and keratometric stability in high myopic LASIK with high-frequency femtosecond and excimer lasers. J Refract Surg. 2013;29(12):832–837. | ||
Labiris G, Sideroudi H, Giarmoukakis A, Koukoulas S, Pagonis G, Kozobolis VP. Evaluation of the difference between intended and measured ablation and its impact on refractive outcomes of the wavefront optimize profile and the S001 Wellington nomogram in myopic spherocylindrical corrections. Clin Exp Ophthalmol. 2012;40(2):127–133. | ||
Fantes FE, Hanna KD, Waring GO 3rd, Pouliquen Y, Thompson KP, Savoldelli M. Wound healing after excimer laser keratomileusis (photorefractive keratectomy) in monkeys. Arch Ophthalmol. 1990;108(5):665–675. | ||
Oruçoglu F, Kingham JD, Kendüsim M, Ayoglu B, Toksu B, Göker S. Laser in situ keratomileusis application for myopia over minus 14 diopter with long-term follow-up. Int Ophthalmol. 2012;32(5):435–441. | ||
Bower KS, Sia RK, Ryan DS, Mines MJ, Dartt DA. Chronic dry eye in photorefractive keratectomy and laser in situ keratomileusis: manifestations, incidence, and predictive factors. J Cataract Refract Surg. 2015;41(12):2624–2634. | ||
Ambrósio R Jr, Tervo T, Wilson SE. LASIK-associated dry eye and neurotrophic epitheliopathy: pathophysiology and strategies for prevention and treatment. J Refract Surg. 2008;24(4):396–407. | ||
De Paiva CS, Chen Z, Koch DD, et al. The incidence and risk factors for developing dry eye after myopic LASIK. Am J Ophthalmol. 2006;141(3):438–445. | ||
Lee JB, Ryu CH, Kim J, Kim EK, Kim HB. Comparison of tear secretion and tear film instability after photorefractive keratectomy and laser in situ keratomileusis. J Cataract Refract Surg. 2000;26(9):1326–1331. | ||
Parekh P, Davis EA. Prevention and treatment of haze in refractive surgery. Int Ophthalmol Clin. 2008;48(1):29–40. | ||
Sia RK, Ryan DS, Edwards JD, Stutzman RD, Bower KS. The US. Army Surface Ablation Study: comparison of PRK, MMC-PRK, and LASEK in moderate to high myopia. J Refract Surg. 2014;30(4):256–264. | ||
Ang BC, Foo RC, Lim EW, et al. Risk factors for early-onset corneal haze after photorefractive keratectomy in an Asian population: outcomes from the Singapore Armed Forces Corneal Refractive Surgery Programme 2006 to 2013. J Cataract Refract Surg. 2016;42(5):710–716. | ||
Qazi, MA, Johnson, TW, Pepose, JS. Development of late-onset subepithelial corneal haze after laser-assisted subepithelial keratectomy with prophylactic intraoperative mitomycin-C; case report and literature review. J Cataract Refract Surg. 2006;32(9):1573–1578. | ||
Kuo IC, Lee SM, Hwang DG. Late-onset corneal haze and myopic regression after photorefractive keratectomy (PRK). Cornea. 2004;23(4):350–355. | ||
Mifflin MD, Leishman LL, Christiansen SM, Sikder S, Hsu M, Moshirfar M. Use of loteprednol for routine prophylaxis after photorefractive keratectomy. Clin Ophthalmol. 2012;6:653–659. | ||
El Awady HE, Ghanem AA, Saleh SM. Wavefront-optimized ablation versus topography-guided customized ablation in myopic LASIK: comparative study of higher order aberrations. Ophthalmic Surg Lasers Imaging. 2011;42(4):314–320. | ||
Moshirfar M, Churgin DS, Betts BS, et al. Prospective, randomized, fellow eye comparison of WaveLight Allegretto Wave Eye-Q versus VISX CustomVue™ STAR S4 IR™ in photorefractive keratectomy: analysis of visual outcomes and higher-order aberrations. Clin Ophthalmol. 2011;5:1185–1193. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.