Back to Journals » Neuropsychiatric Disease and Treatment » Volume 13
High levels of Nesfatin-1 in relation to the dysfunction of the hypothalamic–pituitary–adrenal and hypothalamus–pituitary–thyroid axes in depressed patients with subclinical hypothyroidism
Authors Xu YY, Liang J, Cao Y, Shan F, Liu Y, Xia QR
Received 5 April 2017
Accepted for publication 7 June 2017
Published 23 June 2017 Volume 2017:13 Pages 1647—1653
DOI https://doi.org/10.2147/NDT.S138954
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Wai Kwong Tang
Ya-Yun Xu,1,2 Jun Liang,1,2 Yin Cao,1,2 Feng Shan,1,2 Yang Liu,1,2 Qing-Rong Xia1,2
1Department of Pharmacy, Hefei Fourth People’s Hospital, Hefei, People’s Republic of China; 2Anhui Mental Health Center, Hefei, People’s Republic of China
Abstract: Despite the increasing amount of evidence suggesting a relationship between depression and subclinical hypothyroidism (SCH), the exact mechanism underlying this relationship remains unclear. The main purpose of this study was to investigate the roles of plasma Nesfatin-1 levels and dysfunction of the hypothalamic–pituitary–adrenal (HPA) and hypothalamus–pituitary–thyroid (HPT) axes in the comorbidity of depression and SCH. Dysfunctions of the HPA and HPT axes were detected by measuring plasma corticosterone and thyroid-stimulating hormone (TSH) concentrations, respectively. Subjects in the patient group were selected from patients hospitalized at the Anhui Mental Health Center, and subjects in the control group were recruited from healthy volunteers. Healthy control subjects were matched to the patients in terms of weight and body mass index. The Hamilton Depression Rating Scale was administered to both the groups. The enzyme-linked immunosorbent assay method was used to measure plasma Nesfatin-1, corticosterone, and TSH levels. A radioimmunoassay kit was used for the measurement of the plasma-free triiodothyronine and plasma-free thyroxine. The results showed that the Hamilton Depression Rating Scale scores and average Nesfatin-1, corticosterone, and TSH levels were significantly higher in depressed patients with SCH than in the control group. Moreover, positive relationships were observed between Nesfatin-1 levels and the concentrations of corticosterone (r=0.626, P<0.001) and TSH (r=0.229, P=0.036) in depressed patients with SCH. These findings indicate that Nesfatin-1 is involved in the comorbidity of depression and SCH, and the mechanism underlying this involvement might be related to the dysfunction of the HPA and HPT axes.
Keywords: corticosterone, depression, HPA axis, HPT axis, TSH, SCH
Introduction
Depression is the most common mood disorder and is estimated to affect 350 million people worldwide according to the World Health Organization. Although several behavioral and biologic factors may be involved in precipitating and perpetuating the symptoms of depression,1–3 the principal causes are not clear. A correlation between thyroid dysfunction and depression has been consistently reported in recent literature.4,5 Clinical hypothyroidism (also known as overt hypothyroidism) has traditionally been recognized as an important cause of depression,6 and thyroid hormones have been used as accelerators and augmenters of an antidepressant response for several decades.7,8 Moreover, dysfunctions of the hypothalamus–pituitary–thyroid (HPT) axis have been documented in patients with depression.9
Subclinical hypothyroidism (SCH) is characterized by elevation of the plasma thyroid-stimulating hormone (TSH) concentration above the statistically defined upper limit of the reference range when plasma-free triiodothyronine (fT3) and plasma-free thyroxine (fT4) concentrations are within their reference ranges, and it is a common thyroid dysfunction that occurs in 4%–20% of the adult population.10 Although the relationship between depression and SCH remains controversial, an increasing amount of evidence suggests that SCH is associated with neuropsychiatric disorders such as cognitive dysfunction11 and depression.12 Depression is reportedly observed more frequently among individuals with SCH than among those with overt hypothyroidism,13 and patients with SCH exhibit a twofold higher prevalence of depression-like symptoms relative to healthy individuals.14 Therefore, the guidelines of the American Association of Clinical Endocrinologists recommended that the diagnosis of SCH must be considered in every patient with depression.15
Nesfatin-1, a novel satiety factor, plays a role in integrating feeding, glucose homeostasis, and energy expenditure.16,17 Apart from the findings that Nesfatin-1 is distributed in stress-related brain regions, including the hypothalamus, and colocalized with stress-related substances,18,19 intracerebroventricular administration of Nesfatin-1 has been reported to be able to activate the hypothalamic–pituitary–adrenal (HPA) axis, the hyperactivity of which is proposed to be among the causal factors for triggering depressive episodes.20 Similarly, the results of our previous study confirmed that intraperitoneal administration of Nesfatin-1 in a single dose or chronically resulted in depression-like behavior and HPA axis hyperactivity in rats.21 Furthermore, an increased plasma concentration of Nesfatin-1 and an increased hypothalamic mRNA expression of Nesfatin-1 have been observed in animal models of depression.22 Consistent with the animal results, clinical studies have reported that the plasma Nesfatin-1 level is significantly higher in patients with major depressive disorder23 than in healthy controls and is associated with elevated scores of anxiety and depression.23,24 Given the roles of the HPA axis and Nesfatin-1 in the pathophysiology of depression, the plasma concentrations of Nesfatin-1 and corticosterone were further evaluated in depressed patients with SCH in the present study, and the relationship between Nesfatin-1 and corticosterone concentrations was also investigated.
Recent evidence has indicated a close relationship between Nesfatin-1 and thyroid function.25 The level of Nesfatin-1 has been found to be low in children with hyperthyroidism.26 In another study, the concentration of Nesfatin-1 was shown to be higher in hyperthyroid patients than in controls, and a negative correlation was observed between Nesfatin-1 and TSH concentrations.27 A similar negative correlation was reported in patients with type 2 diabetes in a recent study.25 However, to the best of our knowledge, little is known about the role of the plasma Nesfatin-1 levels in depressed patients with SCH. Considering the elevated plasma TSH concentration in depressed patients with SCH, another objective of this study was to investigate the association between Nesfatin-1 and TSH concentrations in depressed patients with SCH.
Given the dysfunction of the HPT and HPA axes, together with the important role of Nesfatin-1 in the pathophysiology of depression and SCH, we hypothesized that the plasma Nesfatin-1 levels should be increased in depressed patients with SCH and that this may be associated with the hyperactivity of the HPA axis and the overproduction of TSH. To test this hypothesis, subjects in the patient group were selected from patients hospitalized at the Anhui Mental Health Center, and subjects in the control group were recruited from healthy volunteers. The plasma Nesfatin-1, corticosterone, and TSH levels were measured using the enzyme-linked immunosorbent assay, and the plasma fT3 and fT4 levels were measured using a radioimmunoassay kit. Correlations were evaluated using a Pearson correlation test.
Materials and methods
Subjects
A total of 1,234 subjects with depression, according to the Diagnostic and Statistical Manual for Psychiatric Disorders – Fourth Version, who were hospitalized at the Anhui Mental Health Center from February 2016 to February 2017 were scanned in the study, and 83 depressed patients with a comorbidity of SCH were selected from the above patients on the basis of the following biochemical evidence: a TSH concentration greater than the reference range (TSH >4.50 mIU/L), with fT3 and fT4 within the reference range (fT3 between 1.7 and 3.7 pg/mL, fT4 between 0.8 and 2 ng/dL). The inclusion criteria were as follows: 1) all newly diagnosed patients with a Diagnostic and Statistical Manual for Psychiatric Disorders – Fourth Version diagnosis of major depressive disorder, minor depressive disorder, dysthymia, or adjustment disorder (with depressed mood); 2) depressed patients with comorbid SCH (defined by elevated plasma TSH levels and normal plasma fT3 and fT4 levels); and 3) patients who were not receiving an antidepressant, electroconvulsive therapy, or antithyroid drugs. The exclusion criteria included the following: 1) patients with a psychotic illness other than depressive disorders, 2) patients with thyroid diseases other than SCH, and 3) patients who were already on treatment for a depressive illness or thyroid dysfunction. In contrast, 60 healthy volunteers, selected on the basis of the biochemical evidence of normal thyroid function (TSH, fT3, and fT4 levels within the reference range), formed the control group. Figure 1 summarizes the recruitment process. In accordance with the principles of the Declaration of Helsinki, all subjects gave written informed consent prior to participation. This study was approved by the Ethics Committee of the Anhui Mental Health Center.
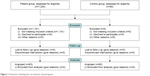  | Figure 1 Flowchart showing the recruitment of participants. |
Measurements
Weight and height measurements and the body mass index (BMI) calculation were carried out meticulously for all subjects. Study nurses administered questionnaires through face-to-face interviews. After an overnight fast, blood samples were drawn in the morning at approximately 8 a.m. from a forearm vein of the participants. Tubes that had a 5-mL capacity and contained EDTA were used for collecting blood. After centrifugation of the blood at 3,000× g for 5 min at 4°C, the plasma was obtained. The separated plasma was stored in a −80°C freezer until the time of assay.
The concentrations of Nesfatin-1, corticosterone, and TSH were measured using commercially available enzyme-linked immunosorbent assay kits (Nesfatin-1: Cusabio Biotech. Co., Ltd, Wuhan, Hubei, People’s Republic of China; corticosterone: Enzo Life Sciences, Inc., New York, NY, USA; TSH: Cusabio Biotech. Co., Ltd) according to the manufacturer’s instructions. The plasma fT3 and fT4 levels were measured using a radioimmunoassay kit (North Institute of Biological Technology, Beijing, People’s Republic of China) and apparatus from USTC Zonkia (USTC Zonkia Scientific Instruments Co., Ltd, Anhui, People’s Republic of China).
Statistical analysis
All of the statistical analyses were performed using Statistical Package for the Social Sciences version 12.0.1 (SPSS Inc., Chicago, IL, USA). Data are expressed as the mean ± standard error of the mean, and P<0.05 was considered statistically significant. A one-sample Kolmogorov–Smirnov test showed a normal distribution of the continuous variables (age, BMI, Hamilton Depression Rating Scale [HAM-D] scores, and the concentrations of Nesfatin-1, corticosterone, TSH, fT3, and fT4) in both the patient group and the control group. A Student’s t-test was used to evaluate differences between the groups (age, BMI, HAM-D scores, and the concentrations of Nesfatin-1, corticosterone, TSH, fT3, and fT4). To analyze the sex difference between groups, the χ2 test was used. The correlation analyses were performed using a Pearson correlation test.
Results
The demographic variables did not show any statistically significant difference between the groups. As shown in Table 1, differences between the groups were not statistically significant in terms of age, BMI, or sex. The mean HAM-D scores of the patient group were significantly higher than those of the control group (t(2,141) =−5.40, P<0.001, Cohen’s d=1.67, Table 1).
The Nesfatin-1 levels of the patient group were significantly higher than those of the control group (t(2,141) =−3.70, P<0.001, Cohen’s d=0.67, Table 2). Similarly, the depressed patients with SCH showed higher corticosterone levels than the healthy subjects (t(2,141) =−4.88, P<0.001, Cohen’s d=1.35, Table 2). In terms of thyroid hormones, the plasma concentrations of TSH were significantly higher in depressed patients with SCH than in the control group (t(2,141) =−17.33, P<0.001, Cohen’s d=4.13, Table 2), whereas no significant difference in the concentration of fT3 (t(2,141) =0.098, P=0.922) or fT4 (t(2,141) =1.391, P=0.168) was observed between the two groups.
Post hoc power analyses demonstrated that sufficient power was available to distinguish the above significant differences (HAM-D: power =1.00; Nesfatin-1: power =0.99; corticosterone: power =1.00; TSH: power =1.00).
The results of the Pearson correlation analysis showed that the plasma Nesfatin-1 concentrations were positively correlated with corticosterone concentrations among the depressed patients with SCH (r=0.626, P<0.001, Figure 2A). However, no such correlation was observed in the control group (r=0.012, P=0.926, Figure 2B).
Similarly, a positive correlation was found between the plasma Nesfatin-1 and TSH levels in the patient group (r=0.229, P=0.036, Figure 3A), but not in the control group (r=0.038, P=0.773, Figure 3B).
Discussion
In the present study, we demonstrated that the HAM-D scores and mean Nesfatin-1, corticosterone, and TSH concentrations were significantly higher in depressed patients with SCH than in healthy controls. Moreover, the plasma Nesfatin-1 concentrations were found to be positively correlated with corticosterone and TSH concentrations in depressed patients with SCH.
Accumulating evidence supports an association between depression and SCH.28 SCH has been reported to markedly increase the risk of depression,13 and the prevalence of depressive symptoms in the SCH population has been reported to be 63.5%.29 Consistent with this, in the present study, the prevalence of SCH in depressed patients was 6.7% (83/1,234), whereas the prevalence of SCH in healthy subjects was 0.0% (0/60) according to the results of the blood tests. These findings highlight the importance of a depressive evaluation in the management of patients with SCH and suggest that SCH might be considered as a possible risk factor for depression.
Nesfatin-1, a novel satiety molecule, is involved in various affective disorders, including anxiety disorder and depression.30,31 The results of clinical studies have demonstrated that the average Nesfatin-1 level is significantly higher in patients with major depressive disorder than in healthy controls.23 Similarly, the results of animal studies have indicated that water-avoidance stress, which is a model of acute depression, could increase the plasma Nesfatin-1 levels.22 Consistent with these findings, our results showed that the plasma Nesfatin-1 levels were significantly higher in depressed patients with SCH than in healthy volunteers. Moreover, considering the fact that stress and subsequent HPA axis hyperactivity have been characteristically associated with the pathogenesis of depression,32 plasma corticosterone concentrations were measured in our study. The results showed that the corticosterone concentrations were significantly higher in depressed patients with SCH, providing more data that link the HPA axis to the onset of depression. Additionally, recent studies have indicated that both intraperitoneal and intracerebroventricular administration of Nesfatin-1 can activate the HPA axis and result in depression-like behaviors in rats,20,21 suggesting that Nesfatin-1 is involved in the regulation of depression, and the underlying mechanism may relate to the relationship between Nesfatin-1 and hyperactivity of the HPA axis.33 Interestingly, the results of our study confirmed that plasma Nesfatin-1 levels were positively correlated with corticosterone levels in depressed patients with SCH. Given that the central administration of Nesfatin-1 infusion has been shown to increase corticotropin-releasing hormone levels in the hypothalamus,33 it is logical to hypothesize that the dysfunction of the HPA axis in depressed patients with SCH might be due to increased plasma Nesfatin-1 levels.
Recent studies corroborate the suggestion that Nesfatin-1 may have a close relationship with thyroid function. Correlations between Nesfatin-1 and TSH concentrations have been documented in several thyroid disorders.25,27 In our study, we found that there was a positive relation between Nesfatin-1 and TSH concentrations in depressed patients with SCH; however, the underlying mechanisms remain unclear. Recent studies have shown that Nesfatin-1 is colocalized with thyrotropin-releasing hormone (TRH) and affects the membrane potential of TRH neurons in the paraventricular nucleus,34,35 which is known to be involved in the regulation of thyroid function. Moreover, a central Nesfatin-1 infusion has been shown to increase the TRH levels in the paraventricular nucleus of the hypothalamus.33 In contrast, the central administration of anti-TRH has been shown to attenuate the Nesfatin-1-induced reduction in food intake, suggesting that TRH mediates the anorectic effect of Nesfatin-1 in the hypothalamus.33 Together with the observation that the plasma level of Nesfatin-1, which crosses the blood–brain barrier without saturation,36,37 is correlated with the level of Nesfatin-1 in the cerebrospinal fluid,38 we speculated that the increased plasma Nesfatin-1 levels might regulate thyroid function by adjusting TRH section in the hypothalamus, thereby resulting in SCH in depressed patients. Further research is required to explore this hypothesis.
Several limitations in this study should also be acknowledged. First, the study’s findings should be interpreted with caution until they have been replicated in larger patient groups as the sample size was small. Second, due to the cross-sectional study design, no causality can be determined. Third, although all of the patients were drug free for at least 2 weeks, we were unable to take into account the probable long-term effects of previous drugs on their plasma Nesfatin-1 levels.
Conclusion
In conclusion, this study is the first to reveal that Nesfatin-1 levels are significantly higher in depressed patients with SCH than in healthy controls, which might be related to dysfunction of the HPA and HPT axes. However, the roles of neuropeptides such as Nesfatin-1 in depression and SCH need to be investigated in further studies.
Acknowledgments
We thank Dr Chun-Song Wen, Dr Xiu-Yan Liu, and all nurses at Hefei Fourth People’s Hospital who kindly provided the data necessary for our analysis. This project was funded by the research projects of Hefei Fourth People’s Hospital (2017003).
Disclosure
The authors report no conflicts of interest in this work.
References
Almeida OP, Calver J, Jamrozik K, Hankey GJ, Flicker L. Obesity and metabolic syndrome increase the risk of incident depression in older men: the health in men study. Am J Geriatr Psychiatry. 2009;17(10):889–898. | ||
Draper B, Pfaff JJ, Pirkis J, et al. Long-term effects of childhood abuse on the quality of life and health of older people: results from the Depression and Early Prevention of Suicide in General Practice Project. J Am Geriatr Soc. 2008;56(2):262–271. | ||
Fond G, Brunel L, Boyer L. C-reactive protein as a differential biomarker of bipolar II depression versus major depressive disorder. World J Biol Psychiatry. 2017;18(1):71–72. | ||
Almeida OP, Alfonso H, Flicker L, Hankey G, Chubb SA, Yeap BB. Thyroid hormones and depression: the Health in Men study. Am J Geriatr Psychiatry. 2011;19(9):763–770. | ||
Ojha SP, Dhungana S, Chapagain M, Tulachan P. Association of thyroid dysfunction with depression in a teaching hospital. J Nepal Health Research Council. 2013;11(23):30–34. | ||
Guimaraes JM, de Souza Lopes C, Baima J, Sichieri R. Depression symptoms and hypothyroidism in a population-based study of middle-aged Brazilian women. J Affect Disord. 2009;117(1–2):120–123. | ||
Cooper R, Lerer B. [The use of thyroid hormones in the treatment of depression]. Harefuah. 2010;149(8):529–534, 549, 550. Hebrew. | ||
Pae CU, Mandelli L, Han C, et al. Thyroid hormones affect recovery from depression during antidepressant treatment. Psychiatry Clin Neurosci. 2009;63(3):305–313. | ||
Plaza A, Garcia-Esteve L, Ascaso C, et al. Childhood sexual abuse and hypothalamus-pituitary-thyroid axis in postpartum major depression. J Affect Disord. 2010;122(1–2):159–163. | ||
de Jongh RT, Lips P, van Schoor NM, et al. Endogenous subclinical thyroid disorders, physical and cognitive function, depression, and mortality in older individuals. Eur J Endocrinol. 2011;165(4):545–554. | ||
Bajaj S, Sachan S, Misra V, Varma A, Saxena P. Cognitive function in subclinical hypothyroidism in elderly. Indian J Endocrinol Metab. 2014;18(6):811–814. | ||
Demartini B, Ranieri R, Masu A, Selle V, Scarone S, Gambini O. Depressive symptoms and major depressive disorder in patients affected by subclinical hypothyroidism: a cross-sectional study. J Nerv Ment Dis. 2014;202(8):603–607. | ||
Chueire VB, Romaldini JH, Ward LS. Subclinical hypothyroidism increases the risk for depression in the elderly. Arch Gerontol Geriatr. 2007;44(1):21–28. | ||
Almeida C, Brasil MA, Costa AJ, et al. Subclinical hypothyroidism: psychiatric disorders and symptoms. Revista brasileira de psiquiatria. 2007;29(2):157–159. | ||
Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid. 2012;22(12):1200–1235. | ||
Aydin S. Role of NUCB2/nesfatin-1 as a possible biomarker. Curr Pharm Des. 2013;19(39):6986–6992. | ||
Celik F, Belviranli M, Okudan N. Circulating levels of leptin, nesfatin-1 and kisspeptin in postmenopausal obese women. Arch Physiol Biochem. 2016;122(4):195–199. | ||
Stengel A, Goebel M, Wang L, et al. Central nesfatin-1 reduces dark-phase food intake and gastric emptying in rats: differential role of corticotropin-releasing factor2 receptor. Endocrinology. 2009;150(11):4911–4919. | ||
Goebel-Stengel M, Wang L. Central and peripheral expression and distribution of NUCB2/nesfatin-1. Curr Pharm Des. 2013;19(39):6935–6940. | ||
Konczol K, Bodnar I, Zelena D, et al. Nesfatin-1/NUCB2 may participate in the activation of the hypothalamic-pituitary-adrenal axis in rats. Neurochem Int. 2010;57(3):189–197. | ||
Ge JF, Xu YY, Qin G, et al. Depression-like behavior induced by Nesfatin-1 in rats: involvement of increased immune activation and imbalance of synaptic vesicle proteins. Front Neurosci. 2015;9:429. | ||
Xu YY, Ge JF, Qin G, et al. Acute, but not chronic, stress increased the plasma concentration and hypothalamic mRNA expression of NUCB2/nesfatin-1 in rats. Neuropeptides. 2015;54:47–53. | ||
Ari M, Ozturk OH, Bez Y, Oktar S, Erduran D. High plasma nesfatin-1 level in patients with major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(2):497–500. | ||
Hofmann T, Stengel A, Ahnis A, Busse P, Elbelt U, Klapp BF. NUCB2/nesfatin-1 is associated with elevated scores of anxiety in female obese patients. Psychoneuroendocrinology. 2013;38(11):2502–2510. | ||
Liu F, Yang Q, Gao N, Liu F, Chen S. Decreased plasma nesfatin-1 level is related to the thyroid dysfunction in patients with type 2 diabetes mellitus. J Diabetes Res. 2014;2014:128014. | ||
Sawicka B, Bossowski A. Analiza stężenia nesfatyny w surowicy u dzieci i młodzieży z chorobami tarczycy autoimmunologicznej. [Analysis of serum levels of nesfatin-1 in children and adolescents with autoimmune thyroid diseases]. Pediatr Endocrinol Diabetes Metab. 2013;19(1):5–10. Polish. | ||
Tohma Y, Akturk M, Altinova A, et al. Circulating levels of Orexin-A, Nesfatin-1, agouti-related peptide, and neuropeptide Y in patients with hyperthyroidism. Thyroid. 2015;25(7):776–783. | ||
Najafi L, Malek M, Hadian A, Ebrahim Valojerdi A, Khamseh ME, Aghili R. Depressive symptoms in patients with subclinical hypothyroidism – the effect of treatment with levothyroxine: a double-blind randomized clinical trial. Endocrine Res. 2015;40(3):121–126. | ||
Demartini B, Masu A, Scarone S, Pontiroli AE, Gambini O. Prevalence of depression in patients affected by subclinical hypothyroidism. Panminerva Medica. 2010;52(4):277–282. | ||
Gunay H, Tutuncu R, Aydin S, Dag E, Abasli D. Decreased plasma nesfatin-1 levels in patients with generalized anxiety disorder. Psychoneuroendocrinology. 2012;37(12):1949–1953. | ||
Emmerzaal TL, Kozicz T. Nesfatin-1; implication in stress and stress-associated anxiety and depression. Curr Pharma Des. 2013;19(39):6941–6948. | ||
Swaab DF, Bao AM, Lucassen PJ. The stress system in the human brain in depression and neurodegeneration. Ageing Res Rev. 2005;4(2):141–194. | ||
Gotoh K, Masaki T, Chiba S, et al. Nesfatin-1, corticotropin-releasing hormone, thyrotropin-releasing hormone, and neuronal histamine interact in the hypothalamus to regulate feeding behavior. J Neurochemistry. 2013;124(1):90–99. | ||
Brailoiu GC, Dun SL, Brailoiu E, et al. Nesfatin-1: distribution and interaction with a G protein-coupled receptor in the rat brain. Endocrinology. 2007;148(10):5088–5094. | ||
Price CJ, Hoyda TD, Samson WK, Ferguson AV. Nesfatin-1 influences the excitability of paraventricular nucleus neurones. J Neuroendocrinol. 2008;20(2):245–250. | ||
Price TO, Samson WK, Niehoff ML, Banks WA. Permeability of the blood-brain barrier to a novel satiety molecule nesfatin-1. Peptides. 2007;28(12):2372–2381. | ||
Pan W, Hsuchou H, Kastin AJ. Nesfatin-1 crosses the blood-brain barrier without saturation. Peptides. 2007;28(11):2223–2228. | ||
Tan BK, Hallschmid M, Kern W, Lehnert H, Randeva HS. Decreased cerebrospinal fluid/plasma ratio of the novel satiety molecule, nesfatin-1/NUCB-2, in obese humans: evidence of nesfatin-1/NUCB-2 resistance and implications for obesity treatment. J Clin Endocrinol Metabolism. 2011;96(4):E669–E673. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




