Back to Journals » OncoTargets and Therapy » Volume 9
High levels of EGFR expression in tumor stroma are associated with aggressive clinical features in epithelial ovarian cancer
Received 13 September 2015
Accepted for publication 28 November 2015
Published 19 January 2016 Volume 2016:9 Pages 377—386
DOI https://doi.org/10.2147/OTT.S96309
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Professor Daniele Santini
Ke Wang, Dan Li, Lu Sun
Department of Gynecologic Cancer, National Clinical Research Center for Cancer, Tianjin Key Laboratory of Cancer Prevention and Therapy, Tianjin Medical University Cancer Institute and Hospital, Tianjin, People’s Republic of China
Purpose: The aim of this study was to investigate the clinical significance and biological function of epidermal growth factor receptor (EGFR) expressed in tumor stroma of epithelial ovarian cancer.
Methods: Immunohistological staining of EGFR was evaluated in 242 patients with epithelial ovarian cancer. The correlations of EGFR expression in tumor stroma with clinicopathological features and with the expression level of Ki-67 were analyzed by SPSS software. Kaplan–Meier analysis and the Cox proportional hazard model were used to analyze the effect of EGFR expression in tumor stroma on the prognosis of patients with epithelial ovarian cancer. Meanwhile, the activities of proliferation and migration of tumor cells were detected when EGFR overexpressed in stroma cells.
Results: EGFR expression in tumor stroma correlated significantly with clinical stage (χ2=7.002, P=0.008) and distant metastases (χ2=16.59, P<0.001). Furthermore, there was a significantly positive correlation between the level of EGFR expressed in tumor stroma and the level of Ki-67 expressed in tumor cells (χ2=6.120, P=0.013). Patients with high EGFR expression level in tumor stroma showed poor survival (P=0.002). Multivariate analysis showed that high expression of EGFR in tumor stroma was an independent predictor for epithelial ovarian cancer patients (hazard ratio =1.703; 95% confidence interval 1.125–2.578, P=0.012). Furthermore, stroma cells overexpressing EGFR could promote the proliferation and migration of adjacent tumor cells.
Conclusion: High expression of EGFR in tumor stroma correlates with aggressive clinical features in epithelial ovarian cancer, and is an independent prognostic factor.
Keywords: EGFR, epithelial ovarian cancer, tumor stroma, clinical features, overall survival, prognostic factor
Introduction
Since ovarian cancer, located deep within the pelvis, has no early typical symptoms, it is difficult to detect at an early stage. Because of the lack of effective therapies for advanced-stage disease, epithelial ovarian cancer is the deadliest gynecological malignancy and the second leading cause of cancer-related deaths among women worldwide.1 About 22,240 women were diagnosed with invasive epithelial ovarian cancer in the USA in 2013.2 In ovarian cancer, disease histotype, differentiation grade, age, and performance status are well-known clinicopathological prognostic factors.3 Although these parameters can reflect biological features of patients, they are not sensitive or sufficiently specific for the individual. Therefore, it is urgent to find new biomarkers, which should aid in a more accurate prediction of survival and therapeutic targets for patients with epithelial ovarian cancer.4,5
Ovarian surface epithelium (OSE) is a single layer of epithelial cells in the surface of the ovary.6 The stroma of ovarian tissue can produce growth factors and cytokines, which act on the OSE and maintain the normal function of the ovary.7 The altered cellular activity of the OSE contributes to the etiology of ovarian cancer, and the stroma play an important role in this process.8 Tumor invasion also requires an association with stromal tissue and most ovarian tumors have a stromal-like component.9 Therefore, stromal–epithelial cell interactions appear to have a critical role in the function and growth of ovarian cancer. The tumor stroma is increasingly perceived as a major contributor to the pathogenesis and disease progression in practically all cancer types.10
Epidermal growth factor receptor (EGFR) is one of the receptor tyrosine kinases, mediating responses of extracellular signals to control cell differentiation, proliferation, and migration, expressed in both tumor cells and tumor stroma.11 EGFR holds considerable promise as a therapeutic target.12 Not surprisingly, there are also many published papers attempting assess the relationship between EGFR overexpression and survival. However, the data regarding the prognostic role of EGFR expression are inconsistent.13 Many researchers are specifically concerned with EGFR expressed in tumor cells, but EGFR expressed in tumor stroma attracts little attention. We report here that high expression of EGFR in tumor stroma is associated with aggressive clinical features, and is a new prognosis marker for epithelial ovarian cancer patients.
Materials and methods
Patients and tissue samples
Two hundred forty-two epithelial ovarian cancer tissue sections were obtained from the Department of Pathology, Tianjin Cancer Hospital, Tianjin Medical University during 2005–2007, and all the patients received surgical therapy and similar chemotherapy (Taxol/cisplatin or paclitaxel/cisplatin). Written informed consent was obtained from all patients, and the study was approved by the Ethical Committee of Tianjin Cancer Hospital. All tissue sections were examined by specialists to make a final diagnosis. The classification of cancer stage and grade was according to the International Federation of Gynecology and Obstetrics (2009). Clinicopathological data were collected including age, histology type, pathological grade, ascites, metastasis status, and tumor clinical stage. All patients’ characteristics are summarized in Table 1.
Antibodies
The primary antibodies used in this study are listed as follows: anti-EGFR (sc-03; Santa Cruz Biotechnology Inc., Dallas, TX, USA), anti-Ki-67 (ab16667; Abcam, Cambridge, UK), anti-E-cadherin (ab1416; Abcam), antivimentin (ab92547; Abcam), anti-β-actin (sc-1616; Santa Cruz Biotechnology), and antigreen fluorescence protein (GFP; sc-8334; Santa Cruz Biotechnology). Antimouse antibodies (Santa Cruz Biotechnology) and antirabbit antibodies (Zhongshan Goldbridge Biotechnology, Beijing, China) were used for Western blot in this study.
Immunohistochemistry staining
Tissue sections were first deparaffinized and rehydrated with xylene and graded alcohol solutions, and then endogenous peroxidase activity was quenched by 3% hydrogen peroxide, followed by boiling in 10 mM citrate buffer (pH 6.0) for 3 minutes in an autoclave sterilizer. After rinsing with phosphate-buffered saline (PBS), sections were incubated with primary antibodies diluted 1:100 in antibody diluent (Zhongshan Goldbridge Biotechnology) overnight at 4°C. After rinsing with PBS, sections were incubated with secondary antibody PV6001 or PV6002 (Zhongshan Goldbridge Biotechnology) for 1 hour at 37°C and stained with 3,3′-diaminobenzidine. The slides were counterstained with hematoxylin, dehydrated with ethanol, cleared with xylene, and mounted in neutral gum. Negative control sections were incubated with PBS instead of a primary antibody.
EGFR and Ki-67 evaluation
We counted the cells with cytoplasmic/membrane staining at the area with the highest immunohistochemical expression of EGFR. Both the percentage and intensity were considered in a semiquantitative assessment, and epithelial cells and stromal cells were counted, respectively. The percentage of positive cells was scored as 0 (0% positive cells), 1 (1%–25% positive cells), 2 (26%–50% positive cells), 3 (51%–75% positive cells), or 4 (>75% positive cells). The intensity of EGFR immunostaining was scored as 0 (negative), 1 (weak), 2 (intermediate), and 3 (strong). The intensity score (0–3) was multiplied by the percentage score (0–4) and a final score was assigned 0 (negative), 1–4 (weak expression), 5–8 (moderate expression), and 9–12 (strong expression). Samples with scores of 0–4 were considered to be low expression, while those with scores of 5–12 were considered to be high expression for statistical analysis. For Ki-67, greater than 25% of cells positive was considered higher expression, and lower than 25% was considered lower expression. The cutoff between the two groups was defined by the mean value of Ki-67 expression in cancerous tissue. All immunohistochemical data of tissue sections were assessed by three assessors, and the average value was the final score.
Cell culture
Ovarian cancer cell line SKOV3, A2780, and human ovarian fibroblasts (HOF) were obtained from American Type Culture Collection (Rockville, MD, USA). The cell line SKOV3 was cultured in MycCo′5A and A2780 and HOF were cultured in minimum essential medium supplemented with 10% fetal bovine serum (Thermo Fisher Scientific, Waltham, MA, USA) inside an incubator containing 5% CO2 at 37°C. In this study, all experimental protocols were approved by the Ethical Committee of Tianjin cancer hospital.
Cloning EGFR expression vectors and cell transfection, transduction
Human EGFR cDNA open reading frame (ORF) was amplified by polymerase chain reaction and subcloned into plasmids pCDH-CMV-copGFP. The sequences pCDH-EGFR-copGFP were confirmed by DNA sequencing. Fibroblast cells were transfected with either pCDH-EGFR-copGFP or control-plasmid (pCDH-CMV-copGFP) for 48 hours. The stable cell clones were selected until individual colonies contained the transfected construct, which was confirmed by Western blot analysis. SKOV3 and A2780 cells were transfected with pCDH-CMV-m-cherry for 48 hours, and the stable cell clones were selected until individual colonies contained the transfected construct.
Western blot
All agents were purchased from Santa Cruz Biotechnology. Lysis buffer (1% sodium dodecyl sulfate, 10 mM Tris–HCl, pH 7.6, 1 mM aprotinin, 1 mM leupeptin, and 1 mM phenylmethyl sulfonyl fluoride) was used to obtain total protein and Bradford method was used to measure the protein concentration. Proteins were separated on a 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and blotted onto a polyvinylidene difluoride membrane. The membrane was blocked by 5% fat-free milk, and then incubated with antibody for 1 hour and labeled with horseradish peroxidase-conjugated secondary antibody for 1 hour at room temperature. β-Actin was used as an internal control. LI-COR Odyssey image reader (LI-COR Biosciences, Lincoln, NE, USA) was used to detect Western blot.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide assay for cell proliferation
Cells were seeded in 96-well plates, at a density of 3,000 cells per well and incubated for 0, 24, 48, 72, and 96 hours. At the end of incubation, 20 μL of 5 mg/mL 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide (MTT) assay solution (Sigma-Aldrich, St Louis, MO, USA) was added to each well. The plates were incubated at 37°C, under 5% CO2 for 4 hours, following which 150 μL dimethyl sulfoxide was added. The plates were gently agitated until the formazan was completely dissolved, and the absorbance was measured at 490 nm wavelength.
Cell migration assay
The cell migration analysis was carried out using transwell inserts (8.0 mm pore size; Costar, Cambridge, MA, USA). First, EGFR-fibroblast or empty vector-fibroblast cell suspensions were placed into the lower reservoir at the same density as normal media, and changed with serum-free media 24 hours later. The ovarian cancer cells were seeded at a density of 50,000 per well and then in 200 μL of serum-free medium for the stimulation. After incubation for 24 hours, noninvading cells on the top of each transwell were scraped off with a cotton swab. Cells that had migrated to the other side were fixed with 2.5% glutaraldehyde (Wako, Tokyo, Japan) and stained with crystal violet (Wako). The number of migrated cells was manually counted with a light microscope (KX4; Olympus, Tokyo, Japan). The sum of the numbers of cells in five areas was used as the migrated cell number, and expressed as a percentage of the control value. These experiments were repeated at least three times, and significant differences among treatments were assessed by analysis of variance followed by Tukey’s test.
Statistical methods
The data were analyzed using SPSS 16.0 software package (SPSS Inc., Chicago, IL, USA). The correlations of EGFR expression with different clinical characteristics were analyzed with chi-square test. Cox proportional hazard model was used to analyze the correlation of survival with various clinical characteristics and EGFR protein expression. The Kaplan–Meier method and Log-rank test were used to analyze the correlation of patient survival with EGFR expression. P<0.05 was considered significant.
Results
The correlation of EGFR expression in tumor cells and in tumor stroma
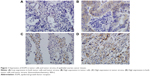
Immunohistochemistry was applied to investigate the expression of EGFR in 242 epithelial ovarian cancer tissues. The results showed that EGFR was expressed in both tumor cells and tumor stromal cells (myofibroblasts, endothelial cells, and leukocytes), and localized to cell cytoplasm and membrane (Figure 1). Sixty four cases had EGFR low expression in both tumor cells (named as EGFR-tc) and tumor stroma (named as EGFR-ts), 85 samples had EGFR higher expression only in tumor stroma, 39 samples showed EGFR higher expression only in tumor cells, and 54 samples showed EGFR higher expression in both tumor cells and tumor stroma (Table 2).
Then, we investigated the correlation between the expression level of EGFR-tc and the expression level of EGFR-ts. The results demonstrated that there was no significant correlation between EGFR-tc and EGFR-ts (Table 2).
We analyzed the associations of EGFR-tc expression levels with clinicopathological variables of cancer, including age, histology grade, clinical stage, ascites, and distant metastasis status. The results (summarized in Table 1) showed that there was no significant correlation between the expression level of EGFR-ts measured by means of immunohistochemistry and age, histological grade, and ascites. Significant correlations between the level of EGFR-tc and clinical stage (χ2=7.752, P=0.005), and distant metastasis (χ2=14.15, P<0.001) were found.
Then, we analyzed the associations of EGFR-ts expression level with clinicopathological variables. The results are summarized in Table 3. Significant correlations between the level of EGFR-tc and clinical stage (χ2=7.002, P=0.008), and distant metastasis (χ2=16.593, P<0.001) were found. There was no significant correlation between the expression level of EGFR-ts and age or histological grade. Furthermore, there was a positive correlation between high level of EGFR-ts and ascites, although it was not significant (χ2=3.244, P=0.072).
Ki-67 is a nuclear protein involved in cell proliferation regulation. Because of the correlation of EGFR and aggressive character of cancer, we detected the expression of Ki-67 in tumor cells of all samples and its correlation with the expression level of EGFR. The results showed that there were 148 cases with higher Ki-67 expression in tumor cells. Then, we measured the relationship between the expression levels of Ki-67 and the expression state of EGFR. The results showed a significantly positive correlation between Ki-67 level of tumor cells and EGFR-ts (χ2=6.12, P=0.013; Table 4), and the patients with higher levels of EGFR-ts often had higher expression of Ki-67 in tumor cells. But, there was no correlation between the expression of Ki-67 and EGFR-tc (Table 4).
Survival analysis
First, there was a statistically significant difference in ovarian cancer-specific survival between tumors with high versus low clinical stage (P<0.001), which was consistent with previous study.14 Then, Kaplan–Meier survival analysis showed that the survival rate was significantly lower in the EGFR-ts high-expression group than in the low-expression group (Figure 2). But, we did not find significant correlation between the expression level of EGFR-tc and the patients’ survival rate.
In the multivariate analysis, EGFR-ts was also a significant predictor of the survival (P=0.012) when entered into a model containing all clinicopathological variables (Table 5).
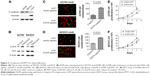
Our previous clinical data have shown that EGFR expressed in tumor stroma played a significant role in tumor aggressive features, and fibroblasts were one of the most abundant components of tumor stroma. To detect if fibroblasts overexpressing EGFR could promote the proliferation of adjacent cancer cells, we employed a coculture system consisting of ovarian cancer cells (A2780 or SKOV3) and fibroblasts (HOF). Figure 3A shows the high expression of epithelial protein marker E-cadherin in A2780 and SKOV3, and high expression of mesenchymal protein marker vimentin in HOF cells. To distinguish the fibroblasts from the cancer cells, we first built the fibroblast cells carrying either empty vector-GFP (EV-GFP) or EGFR-GFP (Figure 3B), and ovarian cancer cells carrying m-cherry tag. The same number of EGFR-GFP fibroblast cells and control cells were seeded respectively into cell dishes with the same number of ovarian cancer cells. After 48 hours, we determined the effects of EGFR-GFP-fibroblasts or EV-GFP-fibroblasts on proliferation of cocultured cells through counting ovarian cancer cells. The results showed that the number of A2780 was significantly more in HOF-EGFR-GFP than control HOF (Figure 3C). The same result was found in SKOV3 coculture system, and there were more SKOV3 cells in HOF-EGFR-GFP group (Figure 3D).
In addition, we collected the medium which had been used to culture HOF-EGFR-GFP and control HOF cells for 24 hours. Then, we utilized the collected medium to culture the ovarian cancer cell lines A2780 and SKOV3, which were determined by MTT assay. The MTT assay results demonstrated that the medium collected from HOF-EGFR-GFP cell dishes could promote the proliferation of both A2780 and SKOV3 (Figure 3E and F).
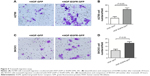
We also studied the functional role of EGFR-GFP-fibroblasts in ovarian cancer cell migration using a transwell migration assay. First, EGFR-GFP-fibroblast or EV-fibroblast cell suspensions were placed into the lower reservoir with normal media, and changed with serum-starved media 24 hours later, and then ovarian cancer cells were placed into the upper well of transwell filter apparatus in serum-free media. The cells that were found on the underside of the filter were counted 24 hours later. The results showed that EGFR-GFP-fibroblast could increase the migration of A2780 (Figure 4A and B) and SKOV3 (Figure 4C and D).
Discussion
Many researchers are just concerned about the high expression of EGFR in ovarian cancer cells for the present, but the data regarding the clinical and prognostic roles of EGFR expression are inconsistent.13 Especially, EGFR expressed in tumor stroma has attracted little attention. In this present study, we found that high levels of EGFR expression in tumor stroma were associated with aggressive clinical conditions in epithelial ovarian cancer, and higher expression of EGFR in tumor stroma was an independent prognosis factor for epithelial ovarian cancer patients, but the statistical significance of EGFR expressed in tumor cells was not found to relate to patients’ survival. In addition, there was a significantly positive correlation between the expression levels of EGFR in tumor stroma and the expression levels of Ki-67 in tumor cells. Also, overexpressing EGFR in fibroblasts could promote the cell growth and migration ability of adjacent ovarian cancer cells.
Epithelial ovarian cancer is the deadliest gynecological malignancy among women worldwide at the present time because of no early typical symptoms and no effective diagnostic methods.1 Although ovarian cancer is relatively uncommon in China, an increase in the incidence has been reported.15 So, discovering cancer at an early stage and handling it at the late stage in an efficient way are challenging in clinical care. Current diagnostic methods and evaluated prognosis for ovarian cancer are not sensitive or sufficiently specific to diagnose ovarian cancer at an early stage and to evaluate the prognosis of patients. Consequently, finding diagnostic markers and prognostic indicators with high sensitivity and specificity remains a major clinical challenge.
EGFR, a receptor tyrosine kinase, is often abnormally upregulated and activated in a variety of tumors, including ovarian cancer.12 Upregulation of EGFR often leads to the promotion of cell proliferation or migration, inhibition of cell death, or the induction of angiogenesis, through activating several downstream signaling pathways.13,14 EGFR overexpression is detected in up to 60% of epithelial ovarian cancers, and occurs in all histologic subtypes (serous, endometrioid, mucinous, clear cell, and undifferentiated).16,17 Many published reports attempted to assess the correlations between EGFR protein overexpression and clinicopathological features and survival of ovarian cancer.13,18–20 Increased EGFR expression has been associated with high tumor grade and high cell proliferation index of ovarian cancer.21 However, the prognostic impact of EGFR expression in ovarian cancers still remains controversial. A meta-analysis found that EGFR expression has a modest effect on prognosis compared with well-known clinicopathological prognostic factors such as tumor stage and residual tumor after primary surgery.13 Psyrri et al22 found that there was a correlation between EGFR overexpression and ovarian cancer patients’ survival. However, Demir et al23 found that EGFR expression was more frequent in advanced tumors, but was not related to poorer outcome. Elie et al24 also did not find any correlation between EGFR overexpression and patients’ survival. In our study, we did not find any correlation between EGFR expressed in tumor cells and survival of ovarian cancer patients. However, there was a significant relationship between EGFR overexpression in tumor stroma and patients’ survival in our study.
As we all know, tumor microenvironment plays an important role in promoting tumor growth and metastasis.25 The progression of breast and prostate cancer is highly dependent on cancer-associated fibroblasts, an important component of tumor stroma.26,27 Epithelial ovarian cancer was defined as arising either from the mesothelial lining of the ovaries (the epithelial surface lining or cortical ovarian cysts) or from the fallopian tube epithelium.28 The stroma of ovary could produce growth factors and cytokines that play an important role in the normal ovarian cycle and development of ovarian cancer.7 It can be seen that the interaction between ovarian epithelial cells and stroma appears to be important for the normal functioning of the ovary and ovarian cancer. Tumor cells could produce stroma-modulating growth factors into their stromal microenvironment, disrupting normal tissue homeostasis, and activate surrounding stromal cell types, such as fibroblasts, smooth muscle cells, and adipocytes. In particular, fibroblasts could affect the stromal microenvironment, leading to an increase in tumor aggressiveness.29
In this study, overexpression of EGFR in tumor stroma was associated with aggressive features of tumor, and the patients with higher EGFR expression in tumor stroma often had higher clinical stage and distant metastases. In addition, there was a significant relationship of EGFR expressed in tumor stroma and survival. In the multivariate survival analysis, apart from clinical stage, EGFR expressed in tumor stroma was also a significant predictor of the survival (P=0.019) when entered into a model containing all clinicopathological variables, including age, histological grade, histology type, clinical stage and distant metastasis, ascites, Ki-67, and EGFR expressed in tumor cells. In addition, there was a significantly positive correlation between the expression levels of EGFR in tumor stroma and the expression levels of Ki-67 in tumor cells. When EGFR was highly expressed in cells of tumor stroma, Ki-67 was often expressed highly in adjacent tumor cells.
As an important receptor tyrosine kinase, EGFR was expressed in both tumor cells and tumor stromal cells in ovarian cancer tissues. However, there were few papers discussing the role of EGFR expressed by tumor stroma in tumor progression. Fibroblasts were one of the most abundant components of tumor stroma, and the tumor stroma played an important role in limiting responsiveness to EGFR-tyrosine kinase inhibitors.30 In our study, we utilized a coculture system consisting of ovarian cancer cells, and fibroblasts carrying EGFR or control plasmid to evaluate if fibroblasts promoted the proliferation of adjacent cancer cells. The results showed that fibroblasts overexpressing EGFR could promote the proliferation of adjacent tumor cells. In addition, fibroblasts overexpressing EGFR could increase the ability of cell migration using transwell migration assay. So, we speculated that the tumor stroma played an extremely important role in tumor progression. In this paper, we only showed the function of EGFR expressed in tumor stroma; in order to identify the specific mechanisms of EGFR expressed in tumor stroma, further research is required.
Conclusion
We have demonstrated the biological and clinical significance of EGFR expressed in tumor stroma of ovarian cancer. There was a significantly positive correlation between the expression levels of EGFR in tumor stroma and the expression levels of Ki-67 in tumor cells. Overexpression of EGFR in tumor stroma was associated with aggressive clinical conditions in ovarian cancer. Furthermore, overexpression of EGFR in tumor stroma was an independent prognostic factor for ovarian cancer patients. Upregulation of EGFR in fibroblasts could promote the growth and migration ability of ovarian cancer cells.
Disclosure
The authors report no conflicts of interest in this work.
References
Coleman MP, Forman D, Bryant H, et al. Cancer survival in Australia, Canada, Denmark, Norway, Sweden, and the UK, 1995–2007 (the International Cancer Benchmarking Partnership): an analysis of population-based cancer registry data. Lancet. 2011;377(9760):127–138. | ||
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. | ||
Winter WE 3rd, Maxwell GL, Tian C, et al. Prognostic factors for stage III epithelial ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25(24):3621–3627. | ||
Xu L, Cai J, Yang Q, et al. Prognostic significance of several biomarkers in epithelial ovarian cancer: a meta-analysis of published studies. J Cancer Res Clin Oncol. 2013;139(8):1257–1277. | ||
Reade CJ, Riva JJ, Busse JW, Goldsmith CH, Elit L. Risks and benefits of screening asymptomatic women for ovarian cancer: a systematic review and meta-analysis. Gynecol Oncol. 2013;130(3):674–681. | ||
Caric A, Poljicanin A, Tomic S, Vilovic K, Saraga-Babic M, Vukojevic K. Apoptotic pathways in ovarian surface epithelium of human embryos during embryogenesis and carcinogenesis: close relationship of developmental plasticity and neoplasm. Acta Histochem. 2014;116(2):304–311. | ||
Doraiswamy V, Parrott JA, Skinner MK. Expression and action of transforming growth factor alpha in normal ovarian surface epithelium and ovarian cancer. Biol Reprod. 2000;63(3):789–796. | ||
Murdoch WJ. Ovarian surface epithelium, ovulation and carcinogenesis. Biol Rev Camb Philos Soc. 1996;71(4):529–543. | ||
Scully RE. Pathology of ovarian cancer precursors. J Cell Biochem Suppl. 1995;23:208–218. | ||
Guldner IH, Zhang S. A journey to uncharted territory: new technical frontiers in studying tumor-stromal cell interactions. Integr Biol (Camb). 2015;7(2):153–161. | ||
Yarden Y, Pines G. The ERBB network: at last, cancer therapy meets systems biology. Nat Rev Cancer. 2012;12(8):553–563. | ||
Dinh P, Harnett P, Piccart-Gebhart MJ, Awada A. New therapies for ovarian cancer: cytotoxics and molecularly targeted agents. Crit Rev Oncol Hematol. 2008;67(2):103–112. | ||
de Graeff P, Crijns AP, de Jong S, et al. Modest effect of p53, EGFR and HER-2/neu on prognosis in epithelial ovarian cancer: a meta-analysis. Br J Cancer. 2009;101(1):149–159. | ||
Cui Y, Yang S, Fu X, Feng J, Xu S, Ying G. High levels of KAP1 expression are associated with aggressive clinical features in ovarian cancer. Int J Mol Sci. 2014;16(1):363–377. | ||
Wang B, Liu SZ, Zheng RS, Zhang F, Chen WQ, Sun XB. Time trends of ovarian cancer incidence in China. Asian Pac J Cancer Prev. 2014;15(1):191–193. | ||
Brustmann H. Epidermal growth factor receptor expression in serous ovarian carcinoma: an immunohistochemical study with galectin-3 and cyclin D1 and outcome. Int J Gynecol Pathol. 2008;27(3):380–389. | ||
Glaysher S, Bolton LM, Johnson P, et al. Targeting EGFR and PI3K pathways in ovarian cancer. Br J Cancer. 2013;109(7):1786–1794. | ||
Jeong KJ, Cho KH, Panupinthu N, et al. EGFR mediates LPA-induced proteolytic enzyme expression and ovarian cancer invasion: inhibition by resveratrol. Mol Oncol. 2013;7(1):121–129. | ||
Sheng Q, Liu J. The therapeutic potential of targeting the EGFR family in epithelial ovarian cancer. Br J Cancer. 2011;104(8):1241–1245. | ||
Annunziata CM, Walker AJ, Minasian L, et al. Vandetanib, designed to inhibit VEGFR2 and EGFR signaling, had no clinical activity as monotherapy for recurrent ovarian cancer and no detectable modulation of VEGFR2. Clin Cancer Res. 2010;16(2):664–672. | ||
Lassus H, Sihto H, Leminen A, et al. Gene amplification, mutation, and protein expression of EGFR and mutations of ERBB2 in serous ovarian carcinoma. J Mol Med (Berl). 2006;84(8):671–681. | ||
Psyrri A, Kassar M, Yu Z, et al. Effect of epidermal growth factor receptor expression level on survival in patients with epithelial ovarian cancer. Clin Cancer Res. 2005;11(24 Pt 1):8637–8643. | ||
Demir L, Yigit S, Sadullahoglu C, et al. Hormone receptor, HER2/NEU and EGFR expression in ovarian carcinoma: is here a prognostic phenotype? Asian Pac J Cancer Prev. 2014;15(22):9739–9745. | ||
Elie C, Geay JF, Morcos M, et al. Lack of relationship between EGFR-1 immunohistochemical expression and prognosis in a multicentre clinical trial of 93 patients with advanced primary ovarian epithelial cancer (GINECO group). Br J Cancer. 2004;91(3):470–475. | ||
Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6(5):392–401. | ||
Karnoub AE, Dash AB, Vo AP, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449(7162):557–563. | ||
Orimo A, Gupta PB, Sgroi DC, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121(3):335–348. | ||
Siwak DR, Carey M, Hennessy BT, et al. Targeting the epidermal growth factor receptor in epithelial ovarian cancer: current knowledge and future challenges. J Oncol. 2010;2010:568938. | ||
Dvorak HF, Weaver VM, Tlsty TD, Bergers G. Tumor microenvironment and progression. J Surg Oncol. 2011;103(6):468–474. | ||
Mink SR, Vashistha S, Zhang W, Hodge A, Agus DB, Jain A. Cancer-associated fibroblasts derived from EGFR-TKI-resistant tumors reverse EGFR pathway inhibition by EGFR-TKIs. Mol Cancer Res. 2010;8(6):809–820. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.