Back to Journals » Journal of Inflammation Research » Volume 15
High Level of Serum Uric Acid induced Monocyte Inflammation is Related to Coronary Calcium Deposition in the Middle-Aged and Elder Population of China: A five-year Prospective Cohort Study
Authors Wang X, Liu X, Qi Y, Zhang S, Shi K, Lin H, Grossfeld P, Wang W, Wu T, Qu X, Xiao J, Ye M
Received 5 January 2022
Accepted for publication 3 March 2022
Published 12 March 2022 Volume 2022:15 Pages 1859—1872
DOI https://doi.org/10.2147/JIR.S353883
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Xiaojun Wang,1,2,* Xuanqi Liu,3,* Yiding Qi,4,* Shuyi Zhang,4 Kailei Shi,4 Huagang Lin,5 Paul Grossfeld,6 Wenhao Wang,1,2 Tao Wu,1,2 Xinkai Qu,4 Jing Xiao,5 Maoqing Ye2,4
1Department of Traditional Chinese Medicine, Huadong Hospital Affiliated to Fudan University, Shanghai, 200040, People’s Republic of China; 2Shanghai Key Laboratory of Clinical Geriatric Medicine, Huadong Hospital Affiliated to Fudan University, Shanghai, 200040, People’s Republic of China; 3Department of Respiratory and Critical Care Medicine, Huadong Hospital Affiliated to Fudan University, Shanghai, 200040, People’s Republic of China; 4Department of Cardiology, Huadong Hospital Affiliated to Fudan University, Shanghai, 200040, People’s Republic of China; 5Department of Nephrology, Huadong Hospital Affiliated to Fudan University, Shanghai, 200040, People’s Republic of China; 6Division of Cardiology UCSD School of Medicine, Rady Children’s Hospital of San Diego, La Jolla, CA, 92093, USA
*These authors contributed equally to this work
Correspondence: Maoqing Ye, Department of Cardiology, Shanghai Key Laboratory of Clinical Geriatric Medicine, Huadong Hospital Affiliated to Fudan University, Shanghai, 200040, People’s Republic of China, Tel +86-18930721396, Email [email protected] Jing Xiao, Department of Nephrology, Huadong Hospital Affiliated to Fudan University, Shanghai, 200040, People’s Republic of China, Tel +86-13817100256, Email [email protected]
Background: Serum uric acid (SUA) is suspected to be associated with atherosclerosis and calcium deposition in atherosclerosis is known to related poor prognosis, yet there is no cohort study on the aged in China. We aimed to investigate the relationships between SUA levels and coronary calcium deposition in the middle-aged and elderly populations in China.
Methods: A total of 326 participants between the ages of 50 and 85 who had undergone a coronary CT scan in 2015 at the Huadong Hospital Affiliated to Fudan University (Shanghai, China) were included in this study. Univariate and multivariate binary logistic regression was performed to analyze the correlation between SUA levels and coronary artery calcium score (CACS). The changes in CACS during a five-year follow-up were analyzed through Kaplan–Meier survival and binary cox regression analysis. An observational study was done on another 104 asymptomatic middle-aged and elderly patients to compare relative mRNA expressions of proinflammatory factors in peripheral blood mononuclear cells (PBMCs) from 104 subjects.
Results: Based on the first year of follow-up data analysis, the elevation of SUA levels (P< 0.001) is an independent risk factor for the increase of CACS after coordinating the confounding factors. According to five-year follow-up data, cox regression analysis proved that SUA was a risk factor for CACS (HR =5.86, P< 0.001). The mRNA expression of IL-6 and CXCL8 in the HUA and HUA patients with CAC (HUA-CAC) groups was significantly higher than that in the normal control (NC) and coronary calcium deposition (CAC) groups.
Conclusion: Taken together, the findings in this study indicate that high SUA levels (P< 0.001) are an independent risk factor for CACS and elevated SUA levels increase the risk of developing coronary calcium deposition among middle-aged and old people in the Chinese population, which may be related to an increase of pro-inflammatory cytokines in the PBMCs.
Keywords: serum uric acid, calcium deposition, coronary artery calcium score, monocyte inflammation
Introduction
In the past decade, the rapid urbanization and accompanying lifestyle changes in China, such as a high-fat, high-calorie diet and decreased physical activity, have contributed to a significant increase in metabolic syndromes, like obesity, diabetes, hypertension, hypertriglyceridemia, low and high- density lipoprotein cholesterol levels, and hyperuricemia (HUA). Uric acid (UA) is the final product of purine metabolism in humans, and disorders of purine metabolism can lead to increased serum uric acid (SUA) levels and HUA. Previous experimental and clinical studies indicate that elevated levels of SUA are associated with higher mortality and increased cardiovascular events. Therefore, a strong and potentially critical connection may exist between HUA and cardiovascular diseases.1–3 Atherosclerosis is no longer a simple accumulation of cholesterol in blood vessels but a continuous, dynamic, inflammatory process affecting the vasculature and often, leading to cardiovascular events.4 Several Clinical studies have confirmed the association of HUA with the progression of atherosclerosis, thereby leading to its addressal as a possible risk factor for atherosclerosis.5,6 However, most HUA patients have complex metabolic or cardiovascular comorbidities such as obesity, diabetes, hypertension, or cardiac insufficiency; these confounding factors hinder our understanding of the association of HUA and atherosclerosis.
Because of contrasting clinical study results, the importance of SUA as a risk factor and prognostic indicator of atherosclerosis is still controversial.5,7 The underlying molecular mechanisms of atherosclerosis caused by SUA are unclear, but several speculated mechanisms of HUA involvement in atherosclerosis, including inflammation, oxidative stress, endothelial dysfunction, smooth muscle cells proliferation, and angiotensin II production, have been discussed.2,8–11Growing evidence has suggested that the central role of inflammation in the association between HUA and atherosclerosis. It is speculated that SUA works as a danger signal by inducing inflammasome-dependent inflammation via the suppression of AMP-activated protein kinase (AMPK) activity.10 In addition, prolonged high SUA levels lead to the crystallization of UA to form monosodium urate (MSU) crystals that induce an acute inflammation mediated by NLR family pyrin domain containing 3 (NLRP3) inflammasomes and interleukin (IL)-1.12 Meanwhile, inflammation is recognized as the major in underlying cause of atherosclerosis and an essential component during various stages of disease progression13. Mononuclear cells and cytokines also playing a critical role from the initial stages of atherosclerosis.14,15 However, questions like whether the increasing SUA related inflammation could independently induce atherosclerosis, what are the underlying mechanisms, and whether HUA should be treated to prevent the development of atherosclerosis need to be further investigated.
It is well-established that calcium deposition in atherosclerosis is related to disease severity and poor prognosis, and coronary artery calcium score (CACS) is an independent risk predictor of coronary events.16,17 The scores can be determined through a non-contrast cardiac computed tomography (CT) and they provide incremental utility for stratifying the risk among participants with no coronary heart disease (CHD).18,19 Based on the 2018 A American College of Cardiology (ACC) and American Heart Association (AHA) guidelines, CACS testing has been incorporated into the risk stratification of cardiovascular disease and treatment guidance. The 2020 Chinese guidelines for primary prevention of cardiovascular diseases have also adopted CACS as a risk factor for the condition.20 Hence, CACS can be used as an indicator for early detection of subclinical atherosclerotic calcium deposition. Coronary artery calcification (CAC) is usually considered as the natural process of atherosclerosis and a kind of vascular degenerative disease. It is mainly due to the abnormal deposition of calcium salt on the coronary artery wall, involving the vascular middle membrane and intima, resulting in the hardening of the vascular wall, reducing the vasoconstrictive response and affecting the coronary blood flow. At the same time, intimal injury causes various inflammatory reactions and promotes the formation of plaque.21 The results of some cohorts also proved that higher inflammation levels can aggravate the disease process of coronary calcification.22,23 Meanwhile, many studies have shown that the pathogenesis of HUA is related to inflammation, oxidative stress, insulin resistance and so on.8,24
Therefore, we aimed to investigate the relationships between SUA levels and coronary calcium deposition, taking inflammation as a mediator, in the middle-aged and elderly populations. In this five-year prospective cohort study, the association between SUA and CACS was investigated in 326 asymptomatic middle-aged and older patients. In addition, another 104 asymptomatic middle-aged and elderly patients were included for an observational study to further explore the effects of SUA on patients with calcium deposition in coronary atherosclerosis (CAS).
Materials and Methods
Participants of the Study
A total of 326 asymptomatic middle-aged and elderly patients (including 210 males and 116 postmenopausal females) from January 1, 2015 to December 31, 2015 who underwent physical examination in the Huadong Hospital Affiliated to Fudan University (Shanghai, China) were included in this prospective cohort study. In addition, 104 asymptomatic middle-aged and elderly patients from June 1, 2020 to March 31, 2021 were included in an observational study. All participants underwent routine physical examination, including screening for cancer, cardiovascular disease and other age-related diseases. The asymptomatic HUA patients exclusion criteria were as follows: (1) serum creatinine >1.5 mg/dL; (2) two-fold elevation of ALT compared with the normal upper limit; (3) serious stiffness or deformity due to gouty arthropathy; (4) clinically significant arrhythmia; (5) those having serious concurrent diseases in the hematopoietic system, liver, cerebrovascular system or kidney, mental diseases, malignant cancers and immune system; (6) those taking salicylate or aspirin (>325mg/d)-containing medications, hypouricemic medications, 6-mercaptopurine or azathioprine; (7) those being involved in additional clinical trials in the last three months were also excluded. At the same time, patients who had taken medications (such as digitalis, propranolol, phenytoin and hydroxybenzylamine) that may affect cardiovascular disease in the past 12 months or more were excluded from this analysis. In addition, patients with coronary heart disease undergoing percutaneous coronary intervention (PCI) were excluded. Patients with systolic blood pressure (SBP) greater than 130 mmHg, or patients with hypertension (SBP of greater than 130 mmHg) receiving medical therapy were also excluded (Supplement 1). All included patients with SUA levels higher than 420 µmol/L were diagnosed with HUA. Coronary calcium deposition is defined by elevated CACS or coronary CT angiography. The elevated CACS was defined as score >0. The study was conducted in accordance with the Helsinki Declaration and the protocol was reviewed and approved by the Institutional Ethics Committee of Shanghai Huadong Hospital (20150126). All written informed consents were available and this trial is registered with ChiCTR2100050192.
Biochemical and CACS Determination
Blood samples were collected from each participant in the morning after 12 h of fasting, and assayed at the Huadong Hospital Laboratory. The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation was used to determine glomerular filtration rate (eGFR; mL/min/1.73 m2) to assess renal function. In addition, triglycerides (TG), total cholesterol (TC), alkaline phosphatase (ALP), serum creatinine (Scr), UA, fasting blood glucose (FBG), hemoglobin A1c (HbA1c), and high and low-density lipoprotein (HDL and LDL) levels in serum were measured. Body height and weight were determined and body weight (kg) was divided by body height (m2) to calculate the body mass index (BMI). The Agatston calcium score, which was obtained from the 64-row 128-slice spiral CT coronary angiography examination (Siemens, Erlangen, Germany), was used to assess CACS.
Assessment of the Framingham Risk Scores (FRS)
The risk for cardiovascular disease was assessed using FRS calculated based on the six coronary risk factors including age, gender, TC, HDL-cholesterol, systolic blood pressure, and smoking habits. The cutoffs for calculating FRS were as follows: TC < 160, 160–199, 200–239, 240–279, and ≥ 280 mg/dL; for systolic blood pressure: < 120, 120–129, 130–139, 140–159, and ≥ 160 mmHg; and for HDL-C: < 40, 40–49, 50–59, and ≥ 60 mg/dL; for age:20–34, 35–39, 40–44, 45–49, 50–54, 55-59, 60–64, 65-69,7 0–74, 75-79; for smoking status:20–39 non-smoker, 20–39 smoker, 40–49 non-smoker, 40–49 smoker, 50–59 non-smoker, 50–59 smoker, 60–69 non-smoker, 60–69 smoker, 70–79 non-smoker, 70–79 smoker.
Bioinformatics Analysis
Disease-associated targets were identified from DisGeNET (http://www.disgenet.org) and National Center for Biotechnology Information (NCBI). The KEGG signal pathway enrichment of the important targets of HUA with CAS was analyzed by using the DAVID 6.8 database. Hub genes were determined using the Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) and Cytoscape software. A protein-to-protein interaction (PPI) network was established based on the STRING database, including 5 nodes and 18 edges. A P<0.05 was considered to be a statistically significant difference.
Collection of Human Peripheral Blood Mononuclear Cells (PBMCs)
All experiments with human blood were approved by the local Ethics Committee of Huadong Hospital affiliated with Fudan University (No. 20190037). Ficoll-Paque density gradient centrifugation was used to isolate the PBMCs. Whole blood was centrifuged at room temperature at 500 x g for 5 min to separate the plasma. The pellet was diluted with an equal volume of 1XPBS (at room temperature), underlaid with Ficoll-Paque (at room temperature), and centrifuged (2000 rpm, 30 min, 21 °C) in a Heraeus Multifuge X3R (Thermo Fisher Scientific) with acceleration set to five and deceleration set to zero. After collecting the PBMCs from the Ficoll-Paque-plasma interface, they were transferred to a 15mL tube and washed (1500 rpm, 10 min, 4 °C) twice with 1XPBS.
Quantitative Reverse Transcription PCR (RT-qPCR)
Total RNA was extracted from PBMCs using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.). cDNA was synthesized by a reverse-transcription reaction with the PrimeScript RT Reagent kit (Takara Biotechnology). The specific cDNA fragments were amplified using a Real-time PCR Detection System and Power SYBR-Green (Takara Biotechnology) with relevant primers. The results were expressed as a relative expression to the β-actin gene. The relative expression level of each target gene was analyzed using the 2-ΔΔCq method and normalized to β-actin. The PCR primer sequences are presented in Supplement 2.
Statistical Analysis
Data were analyzed using STATA15.1 and GraphPad Prism software. Continuous variables were presented as mean ± standard deviation (SD) while categorical variables were expressed as percentages. An independent sample’s t-test was adopted to compare measurement variables between the two groups. Univariate and multivariate binary logistic regression was performed to analyze the correlation between elevated SUA levels and CACS after adjusting for the covariates. A receiver operating characteristic (ROC) curve was constructed to measure the prognostic value of SUA. The changes in CACS levels during the five-year follow-up were analyzed through Kaplan-Meier survival estimate and binary cox regression analysis. Cluster analysis was performed for variables and participants through system cluster and hierarchical clustering and the R software (Version 3.6.3) was used for data analyses. The difference of P<0.05 was deemed to be statistically significant.
Results
Demographic Characteristics and Risk Factors Related to the Changes in CACS Levels Among Participants
Summary-level data related to the primary outcomes of the participants is provided in Table 1. A total of 326 asymptomatic patients were included in this analysis (mean age was 62.17 years; 62.42% males; 86.2% non-smokers; and 7.98% alcohol consumers) (Table 2). A small group of patients developed comorbidities, such as hypertension (10.12%) and diabetes (5.52%). To understand the underlying risk factors of coronary calcification, binary logistic regression model constructed, and the results of the model are described in Table 2.
 |
Table 1 Matrix of Outcome Measures and Assessments |
 |
Table 2 Univariate and Multivariate Logistic Regression Between CACS and Hemogram Indices |
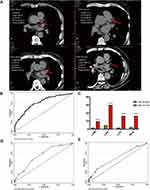
As shown in Figure 1A, CAC score is composed of four indicators including LAM, LAD, LCX and RCA. In univariate logistic regression, the main demographic parameters of age, BMI, gender, and alcoholic abuse, as well as hemogram indices such as UA, Scr, HBAC1, TG, HDL, LDL, FBG and ALP, were related to the changes in CACS. Furthermore, the results revealed that SUA (P<0.001) and ALP (P=0.006) were independent risk factors of coronary calcium deposition even after coordinating the confounding factors. When compared with normal patients, those with coronary calcium deposition were older (P=0.043) (Table 2). In an additional analysis, the four items of CACS were analyzed respectively, and the results demonstrated that the level of SUA is significantly associated with each item significantly (Figure 1C).
Predictive Role of SUA in Coronary Calcification Deposition
It has been reported that ALP is an independent risk factor for CACS [23]. To evaluate the prognostic value of SUA and ALP in coronary calcification deposition, ROC curve was constructed to predict the abnormal status of CACS in the first year of follow-up. As shown in Figure 1B, the area under the curve (AUC) values of SUA and ALP were 0.7596 and 0.4087, respectively (Supplement 3). Therefore, the predictive ability of SUA for the changes in CACS far exceeds that of ALP. This indicates that SUA is a useful predictive factor for the dynamic changes in CACS in the first year of follow-up. Moreover, FRS and erythrocyte sedimentation rate (ERS), which are critical markers of cardiovascular risk events and systematic inflammation, of the participants were documented. Based on the AUC value of FRS (Figure 1D) and ESR (Figure 1E), both FRS and ESR exhibited relatively poor predictive ability for CACS as compared with SUA.
Five-Year Survival Analysis Among the Asymptomatic Population
To further investigate the clinical predictive value of SUA for coronary calcification, the participants were followed up for five years. In the fifth year, information regarding increased CACS in the participants and the time of its occurrence was collected for 288 patients. To compare CACS between the participants with and without high SUA, the Kaplan-Meier survival analysis was performed. Results indicated that population with a higher value of SUA is usually the one to show the appearance of coronary calcification deposition earlier as compared with those with a lower value of SUA (Figure 2A). Statistical difference was not observed between the male and female groups (Supplement 4). To control the confounding factors, it was postulated that high SUA might be a predictive factor (Figure 2B) in the forest plot. For this purpose, a Cox regression analysis was performed, which indicated that SUA was indeed a risk factor related to high CACS (P<0.001) (Supplement 5).
Potential Targets of Comorbidity in HUA and CAS
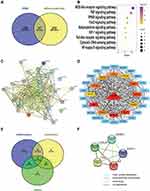
Until now, the mechanisms underlying the relationship between HUA and CAS have not been fully elucidated. To find the potential target genes related to HUA (CUI number:C0740394) and atherosclerosis (CUI number:C0004153), a total of HUA 196 genes and 239 atherosclerosis genes (confidence score is ≥0.3 in CAS) were screened from DisGeNET. In the VENN map, 37 genes were overlapping between CAS and HUA (Figure 3A). The KEGG pathway annotation indicated that these overlapping genes were enriched in 35 enriched pathways. Among these, nine signaling pathways with the highest p-adjust values (nominal p values) are presented in Figure 3B. These include the NOD-like receptor signaling pathway, inflammatory pathway and the Cytokine-cytokine receptor interaction pathway. A PPI network was also constructed, and the key modules and hub genes were determined using the STRING and Cytoscape software (Figure 3C and D). The above 37 genes (Figure 3A) overlapped between HUA and CAS were re-enriched in another 31 KEGG pathways which mainly enriched in inflammation related pathways, metabolism related pathways and cancer related pathways on the basis of KEGG pathway annotation. 18 targets related to the inflammation pathways, 11 targets related to metabolic pathways, and 10 targets related to cancer pathways were also obtained from the KEGG pathway annotation. Five of these overlapping targets which we consider may be the most import targets belonged to three pathways described in the VENN map (Figure 3E). A PPI network was also constructed using the STRING platform (Figure 3F), indicating that the tumor necrosis factor-α (TNF-α), cysteine X chemokine ligand (CXCL) 8, interleukin (IL)-6, caspase-3 (CASP3) and signal transducer and activator of transcription-3 (STAT3) were important pathogenic targets.
Gene Expression Analysis of Proinflammatory Cytokines in PBMCs of 104 Patients in the Observational Study
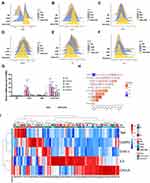
To verify our bioinformatics results stated above, we enrolled another set of 104 participants and divided them into four groups, namely, normal control (NC), HUA patients, patients with coronary calcium deposition (CAC), and HUA patients with CAC (HUA-CAC), for gene analysis in their PBMCs by RT-qPCR. The corresponding criteria of HUA and CAC was listed in Methods. As shown in Supplement 6, the clinical characteristics, including baseline features and hemogram indices, were collected for all these four groups. Furthermore, we followed up and documented the results of our laboratory examinations. A discriminant multivariate analysis showed that the patients did not differ much in all the clinical parameters between groups. Figure 4A–F gives a visual representation of the differences in clinical parameters across the four different groups. For patients with CAC, the value of SUA was more elevated as compared with normal controls.
Moreover, HUA-CAC patients harbored significantly higher levels of blood urea nitrogen (BUN) as compared with that the patients in the CAC group. All variables (IL-6, CXCL8, TNF, STAT3 and CASP3) were analyzed by ordinary one-way ANOVA statistical analysis (Tukey’s multiple comparisons test). The mRNA expression levels of inflammatory cytokines IL-6 (P < 0.0001) and CXCL8 (P < 0.0001) increased significantly from the HUA group to the NC group. Likewise, a significant increment was also observed in the mRNA expression levels of IL-6 (P < 0.0001) and CXCL8 (P < 0.0001) from the HUA with CAC group to the NC group (Figure 4G). To identify the differences in the five gene mRNA expressions of these genes among four groups, mRNA expression profiling was performed on the results obtained from system cluster and hierarchical cluster analyses. Results revealed that IL-6 and CXCL8 mRNAs were differentially abundant in the four groups (Figure 4G). Subsequently, these four groups were divided into further two groups on the basis of RT-PCR cluster analysis (the HUA and HUA-CAC groups were combined into one group, whereas the NC and CAC group were combined into the other group). The analysis further confirmed that IL-6 and CXCL8 are the chief pro-inflammatory cytokines mediating the inflammatory response (Figure 4I). A crucial result to be noted is that the relative mRNA expression of IL-6 was more positively correlated to the SUA level (r=0.76) according to the correlation analysis (Figure 4H).
Discussion
The present research is a five-year prospective cohort and observational study to explore whether a high SUA level is an independent risk factor for coronary calcification. First, a cross-sectional study was conducted on 326 asymptomatic participants, and the results suggested that SUA level is indeed strongly related to CACS. The one-year follow-up study confirmed that SUA has a better predictive value than FRS and ESR for coronary calcium deposition based on the ROC prediction model. To further verify this finding, a five-year follow-up consecutive study was performed using the Kaplan Meier survival analysis and Cox regression analysis. Next, bioinformatics analysis was performed on data from the DisGeNET database, suggesting that TNF-α, CXCL8, IL-6, CASP3 and STAT3 could be the common pathogenic gene targets of HUA and CAS. Also, an observational study was conducted and the relative mRNA expression of possible pathogenic factors was analyzed in PBMCs from the four groups of patients (NC, HUA, CAC and HUA-CAC). Results indicated that IL-6 and CXCL8 is highly expressed in the HUA and HUA-CAC groups; however, significant differences were not observed between their expressions in the NC and CAC groups.
Currently, SUA is suspected to25 affect the pathogenesis in subclinical arteriosclerosis, but this remains unclear, particularly for coronary calcification. Although several cohort studies have assessed the role of HUA, less research has been conducted in a continuous long-term prospective manner on the relationship between HUA and CACS, mainly because asymptomatic patients are reluctant to undergo CT examinations. As reported in a cross-sectional study, silent deposition of MSU crystals in patients with asymptomatic HUA was associated with the degree of CAC,26 but whether HUA is independently associated with CAC has not been established. In this study, a risk prediction model was established and then the area under the ROC curve was calculated to detect the forecast value of SUA for CACS. The results demonstrated that SUA has more predictive value than traditional inflammatory markers such as the ESR and FRS which can only predict the incidence of cardiovascular adverse events. After five years of follow-up, Kaplan Meier survival analysis was performed and Cox proportional hazards model was constructed to test and verify the prognostic value of SUA for CACS. Moreover, excluding the confounding factors, our results suggest that SUA is indeed an independent risk factor for CACS.
Inflammation is a major driver of atherosclerosis and the underlying pathology of cardiovascular diseases. Therefore, therapeutic targeting of inflammatory pathways is suggested to improve cardiovascular outcomes in patients with cardiovascular diseases.27 The dominant cellular players in chronic inflammation are the tissue macrophages and PBMCs. As inflammation progresses, pro-inflammatory cytokines such as IL-6, CXCL8, and TNF are secreted from the inflammatory cells.28 A previous study has indicated that STAT3 is a representative transcription factor known to that play a critical role in inflammation-associated diseases through multi-level participation.29 Moreover, inflammation is a crucial factor mediating high UA levels as well as CAS.30 Chronic inflammation can accelerate the process of aging. Some studies have found that the increase relative gene expression of proinflammatory cytokines from peripheral blood derived monocytes is related to the increase of serum uric acid levels,31 but no study has reported the role of inflammatory monocytes in the development of asymptomatic hyperuricemia and coronary calcification so far. In this study, the expressions of IL-6, CXCL8, TNF, CASP3, and STAT3 in PBMCs from the NC, HUA, CAC, and HUA-CAC patients were analyzed along with the gene expression changes. For cluster analysis of these five genes the HUA and HUA-CAC samples were grouped. Results indicated that the mRNA expression of IL-6 and CXCL8 in these two groups was significantly higher than that of the other two groups (NC group and CAC group). These results strongly suggested that IL-6 and CXCL8 seem to act as pro-inflammatory factors in PBMCs of HUA and HUA with CAC patients. Atherosclerosis is considered to be an inflammatory response, and pro-inflammatory cytokines undoubtedly aggravate the inflammation.32 Results of this study suggests that high SUA levels seem to aggravate the inflammatory state of PBMCs in patients with coronary calcification.
Although HUA is considered to be an adverse factor in cardiovascular events, the general treatment of asymptomatic HUA to reduce cardiovascular risk is not recommended.33 This study is the first to assess the mediating effects of chronic inflammation in PBMCs on the association between high SUA levels and coronary calcium deposition. Chronic inflammation is recognized as the major underlying cause of many pathologies including cardiovascular and cerebrovascular events, with mononuclear cells and cytokines playing a critical role in the initial stages of these diseases.34 Asymptomatic HUA is generally associated with inflammatory disorders, including cardiovascular disease.35 Most studies investigating the morbidity associated with SUA have only determined the effects associated with the appearance of MSU crystals instead of UA itself.36,37 Moreover, it is challenging to decipher the independent role of SUA at the clinical level due to the concurrence of other risk factors. The participants included in this study were patients with HUA without any other concomitant comorbidity (gouty arthritis, renal calculus and gout), clinical condition or antecedent of gout. Furthermore, the measurement of gene expression levels of cytokines was performed in PBMCs directly drawn from such individuals, without any kind of cell manipulation. Results of this study demonstrated that high SUA levels could promote the inflammation response, mediated by pro-inflammatory cytokines, in subclinical CAS patients in real clinical practice. Thus, the results elucidate a role for chronic inflammation in the association between high SUA levels and coronary calcification. This indicates that for patients with asymptomatic HU and coronary calcification, early UA-lowering therapy may reduce the proinflammatory cytokines in PBMCs, which may, in turn, reduce the incidence of adverse cardiovascular events.
This work is crucial for multiple reasons. First, rigorous exclusion criteria were used, based on routine laboratory results and medical histories, and the potential confounding factors were carefully adjusted for the sake of appropriate investigation of the physiological effects of SUA alone on coronary calcium deposition. In the study, we focused on the middle-aged and elderly people in China, while simultaneously confirming the predictive value of SUA levels for the changes of CACS in a five-year prospective cohort study. Second, although some studies have shown that HUA is associated with coronary calcification, most of them are cross sectional studies and do not focus on the middle-aged and elderly population.38–40 In this study, we focused on asymptomatic middle-aged and elderly participants because aging is considered as a chronic inflammatory state which plays a vital role in the pathogenesis and development of asymptomatic hyperuricemia and coronary calcification.22,24 The results of our five-year follow-up and another observational study have shown that high serum uric acid levels are an independent risk factor for coronary calcification in asymptomatic middle-aged and elderly population. The relative mRNA expression levels in PBMCs of the four groups of patients (NC, HUA, CAC and HUA-CAC patients) were compared to verify the results of previous bioinformatics analysis on the common target genes of HUA and CAS. The results confirmed that patients with HUA-CAC had the highest level of inflammation, which may be caused by high SUA according to the results of cluster analysis. Third, based on the data analysis of participants who were followed up for five years and the results of the relative mRNA expression levels by RT-qPCR in PBMCs of other subjects who participated in the observational study, it was demonstrated that the possibility of early UA-lowering therapy might be significant for coronary calcium deposition in patients with HUA.
Nonetheless, this study has certain limitations. First, this is a single-center prospective study with a relatively small sample cohort. Therefore, the results of this study cannot be extrapolated to larger populations. Second, we focused on the effects of SUA on CACS without correlating clinical symptoms with CACS. Third, although we conducted a five-year follow-up study and additional observational research to confirm the effects of high UA levels on coronary calcium deposition, we did not do a follow-up interventional study to make coronary calcium deposition patients with HUA undergo UA-lowering therapy.
In conclusion, the findings demonstrate that elevated SUA levels increase the risk of developing coronary calcium deposition, which may be related to the elevated pro-inflammatory cytokines in PBMCs of the patients. Our study focused on middle-aged and older females and males in China. Nonetheless, studies with a larger sample size and additional external validations are needed to confirm this conclusion.
Data Sharing Statement
The raw data supporting the conclusion in the article will be made available by the authors Xiaojun Wang ([email protected]) without reservation.
Acknowledgment
This work was supported by the National Natural Science Foundation of China (91949126, 81970378, 81770420), and the Science and Technology Commission of Shanghai Municipality (20140900600).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Culleton BF, Larson MG, Kannel WB, Levy D. Serum uric acid and risk for cardiovascular disease and death: the Framingham Heart Study. Ann Intern Med. 1999;131(1):7–13.
2. Fang J, Alderman MH. Serum uric acid and cardiovascular mortality the NHANES I epidemiologic follow-up study, 1971-1992. National Health and Nutrition Examination Survey. JAMA. 2000;283(18):2404–2410.
3. Wu AH, Gladden JD, Ahmed M, Ahmed A, Filippatos G. Relation of serum uric acid to cardiovascular disease. Int J Cardiol. 2016;213:4–7.
4. Ruparelia N, Chai JT, Fisher EA, Choudhury RP. Inflammatory processes in cardiovascular disease: a route to targeted therapies. Nat Rev Cardiol. 2017;14(5):314.
5. Kawamoto R, Tomita H, Oka Y, Ohtsuka N. Relationship between serum uric acid concentration, metabolic syndrome and carotid atherosclerosis. Intern Med. 2006;45(9):605–614.
6. Rodrigues TC, Maahs DM, Johnson RJ, et al. Serum uric acid predicts progression of subclinical coronary atherosclerosis in individuals without renal disease. Diabetes Care. 2010;33(11):2471–2473.
7. Takayama S, Kawamoto R, Kusunoki T, Abe M, Onji M. Uric acid is an independent risk factor for carotid atherosclerosis in a Japanese elderly population without metabolic syndrome. Cardiovasc Diabetol. 2012;11:2.
8. Jayachandran M, Qu S. Harnessing hyperuricemia to atherosclerosis and understanding its mechanistic dependence. Med Res Rev. 2021;41(1):616–629.
9. Kimura Y, Yanagida T, Onda A, Tsukui D, Hosoyamada M, Kono H. Soluble Uric Acid Promotes Atherosclerosis via AMPK (AMP-Activated Protein Kinase)-Mediated Inflammation. Arterioscler Thromb Vasc Biol. 2020;40(3):570–582.
10. Puddu P, Puddu GM, Cravero E, Vizioli L, Muscari A. Relationships among hyperuricemia, endothelial dysfunction and cardiovascular disease: molecular mechanisms and clinical implications. J Cardiol. 2012;59(3):235–242.
11. Rao GN, Corson MA, Berk BC. Uric acid stimulates vascular smooth muscle cell proliferation by increasing platelet-derived growth factor A-chain expression. J Biol Chem. 1991;266(13):8604–8608.
12. Rock KL, Kataoka H, Lai JJ. Uric acid as a danger signal in gout and its comorbidities. Nat Rev Rheumatol. 2013;9(1):13–23.
13. Zimmer S, Grebe A, Latz E. Danger signaling in atherosclerosis. Circ Res. 2015;116(2):323–340.
14. Flores-Gomez D, Bekkering S, Netea MG, Riksen NP. Trained Immunity in Atherosclerotic Cardiovascular Disease. Arterioscler Thromb Vasc Biol. 2021;41(1):62–69.
15. Lee S, Bartlett B, Dwivedi G. Adaptive Immune Responses in Human Atherosclerosis. Int J Mol Sci. 2020;21:23.
16. Hou ZH, Lu B, Gao Y, et al. Prognostic value of coronary CT angiography and calcium score for major adverse cardiac events in outpatients. JACC Cardiovasc Imaging. 2012;5(10):990–999.
17. Chaikriangkrai K, Velankar P, Schutt R, et al. Additive prognostic value of coronary artery calcium score over coronary computed tomographic angiography stenosis assessment in symptomatic patients without known coronary artery disease. Am J Cardiol. 2015;115(6):738–744.
18. Dzaye O, Dudum R, Mirbolouk M, et al. Validation of the Coronary Artery Calcium Data and Reporting System (CAC-DRS): dual importance of CAC score and CAC distribution from the Coronary Artery Calcium (CAC) consortium. J Cardiovasc Comput Tomogr. 2020;14(1):12–17.
19. Polonsky TS, McClelland RL, Jorgensen NW, et al. Coronary artery calcium score and risk classification for coronary heart disease prediction. JAMA. 2010;303(16):1610–1616.
20. Qazi AH, Zallaghi F, Torres-Acosta N, Thompson RC, O’Keefe JH. Computed Tomography for Coronary Artery Calcification Scoring: mammogram for the Heart. Prog Cardiovasc Dis. 2016;58(5):529–536.
21. Wexler L, Brundage B, Crouse J, et al. Coronary artery calcification: pathophysiology, epidemiology, imaging methods, and clinical implications. A statement for health professionals from the American Heart Association. Writing Group. Circulation. 1996;94(5):1175–1192.
22. Kamimura D, Cain-Shields LR, Clark D, et al. Physical Activity, Inflammation, Coronary Artery Calcification, and Incident Coronary Heart Disease in African Americans: insights From the Jackson Heart Study. Mayo Clin Proc. 2021;96(4):901–911.
23. Ong KL, McClelland RL, Allison MA, et al. Lipoprotein (a) and coronary artery calcification: prospective study assessing interactions with other risk factors. Metabolism. 2021;116:154706.
24. Lee TS, Lu TM, Chen CH, Guo BC, Hsu CP. Hyperuricemia induces endothelial dysfunction and accelerates atherosclerosis by disturbing the asymmetric dimethylarginine/dimethylarginine dimethylaminotransferase 2 pathway. Redox Biol. 2021;46:102108.
25. Mannarino MR, Pirro M, Gigante B, et al. Association Between Uric Acid, Carotid Intima-Media Thickness, and Cardiovascular Events: prospective Results From the IMPROVE Study. J Am Heart Assoc. 2021;10(11):e020419.
26. Drivelegka P, Forsblad-d’Elia H, Angeras O, et al. Association between serum level of urate and subclinical atherosclerosis: results from the SCAPIS Pilot. Arthritis Res Ther. 2020;22(1):37.
27. Geng S, Chen K, Yuan R, et al. The persistence of low-grade inflammatory monocytes contributes to aggravated atherosclerosis. Nat Commun. 2016;7:13436.
28. Wesselink E, van Baar H, van Zutphen M, et al. Inflammation Is a Mediating Factor in the Association between Lifestyle and Fatigue in Colorectal Cancer Patients. Cancers. 2020;12:12.
29. Michiels C, Puigdevall L, Cochez P, et al. A Targetable, Noncanonical Signal Transducer and Activator of Transcription 3 Activation Induced by the Y-Less Region of IL-22 Receptor Orchestrates Imiquimod-Induced Psoriasis-Like Dermatitis in Mice. J Invest Dermatol. 2021;2:987.
30. Tercan H, Riksen NP, Joosten LAB, Netea MG, Bekkering S. Trained Immunity: long-Term Adaptation in Innate Immune Responses. Arterioscler Thromb Vasc Biol. 2021;41(1):55–61.
31. Luis-Rodriguez D, Donate-Correa J, Martin-Nunez E, et al. Serum urate is related to subclinical inflammation in asymptomatic hyperuricaemia. Rheumatology. 2021;60(1):371–379.
32. Fragoulis GE, Soulaidopoulos S, Sfikakis PP, Dimitroulas T, DK G. Effect of Biologics on Cardiovascular Inflammation: mechanistic Insights and Risk Reduction. J Inflamm Res. 2021;14:1915–1931.
33. Braga TT, Davanso MR, Mendes D, et al. Sensing soluble uric acid by Naip1-Nlrp3 platform. Cell Death Dis. 2021;12(2):158.
34. Roman MJ, Shanker BA, Davis A, et al. Prevalence and correlates of accelerated atherosclerosis in systemic lupus erythematosus. N Engl J Med. 2003;349(25):2399–2406.
35. Baker JF, Krishnan E, Chen L, Schumacher HR. Serum uric acid and cardiovascular disease: recent developments, and where do they leave us? Am J Med. 2005;118(8):816–826.
36. Duewell P, Kono H, Rayner KJ, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464(7293):1357–1361.
37. Li-Yu J, Clayburne G, Sieck M, et al. Treatment of chronic gout. Can we determine when urate stores are depleted enough to prevent attacks of gout? J Rheumatol. 2001;28(3):577–580.
38. Han M, Kim H, Kim HJ, et al. Serum uric acid is associated with coronary artery calcification in early chronic kidney disease: a cross-sectional study. BMC Nephrol. 2021;22(1):247.
39. Liang L, Hou X, Bainey KR, et al. The association between hyperuricemia and coronary artery calcification development: a systematic review and meta-analysis. Clin Cardiol. 2019;42(11):1079–1086.
40. Kiss LZ, Bagyura Z, Csobay-Novak C, et al. Serum Uric Acid Is Independently Associated with Coronary Calcification in an Asymptomatic Population. J Cardiovasc Transl Res. 2019;12(3):204–210.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.