Back to Journals » Cancer Management and Research » Volume 14
High-Intensity Focused Ultrasound Enhanced Anti-Tumor Activities of Paclitaxel in Breast Cancer in vitro and in vivo
Authors Chen S, Bian H, Duan J
Received 14 November 2021
Accepted for publication 31 January 2022
Published 30 March 2022 Volume 2022:14 Pages 1303—1312
DOI https://doi.org/10.2147/CMAR.S349409
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Yong Teng
Sha Chen,1 Hao Bian,2 Jingyu Duan1
1Department 2 of Ultrasound, Cangzhou Central Hospital, Cangzhou, 061000, Hebei, People’s Republic of China; 2Magnetic Resonance Imaging Department, Cangzhou Central Hospital, Cangzhou, 061000, Hebei, People’s Republic of China
Correspondence: Sha Chen, Department 2 of Ultrasound, Cangzhou Central Hospital, No. 16, Xinhua West Road, Yunhe District, Cangzhou, 061000, Hebei, People’s Republic of China, Tel +86-18232858958, Email [email protected]
Background: Paclitaxel (PTX) is an important oncologic chemotherapeutic agent against breast cancer, but breast cancer patients develop significant resistance to PTX during chemotherapy. Alterations in tubulin and associated proteins have been implicated in resistance to PTX. High-intensity focused ultrasound (HIFU) induces deep tumor penetration of anti-tumor agents in solid tumors.
Methods: We investigated the influence of HIFU on the anti-tumor activities of PTX in breast cancer. Both in vivo and in vitro experiments were performed in this research: mice were treated with 2 mg/Kg PTX through tail vein injection, while breast cancer cells were treated with 400 nM PTX. Cell viability was analyzed through Cell Counting Kit-8. Cell apoptosis was evaluated through Annexin-V/PI Apoptosis Analysis Kit. The activities of catalase (CAT) and superoxide dismutase (SOD) and the concentration of malondialdehyde (MDA) were evaluated by relative commercial kits.
Results: HIFU enhanced PTX-inhibited breast cancer cell viability and PTX-induced cell apoptosis. Simultaneous treatment of HIFU and PTX decreased the activities of CAT and SOD and increased the concentration of MDA. In mice bearing MDA-MB-231 tumors, the treatment of HIFU and PTX significantly decreased tumor size, increased body weight and elevated animal survival. HIFU enhanced the distribution of PTX in tumor tissues.
Conclusion: The performance of HIFU promoted the distribution of PTX and enhanced its anti-tumor activities in breast cancer.
Keywords: breast cancer, paclitaxel, high-intensity focused ultrasound, cancer therapy
Introduction
Breast cancer accounts for about 7–10% of tumors in female population, and is the number one malignant tumor in Chinese women aged 30–59 years.1 Chemotherapy is an effective therapy for breast cancer, but many patients experience chemotherapeutic reactions such as nausea, hair loss, abnormal sensation, and bone marrow suppression when undergoing chemotherapy. These side effects hinder clinical treatment and increase patients’ fear of the chemotherapy.2 Till now, diverse treatments are performed to enhance the anti-tumor activities of chemotherapeutic agents and to decrease the extent of side effects, including crocin administration, silymarin administration, and melatonin coadministration.3–6
Paclitaxel (PTX) is an important oncologic chemotherapeutic agent and has become the first-line drug in chemotherapy for breast cancer patients.7 PTX can bind to β-microtubulin and affect the dynamic balance between microtubulin dimers and microtubules. PTX promotes the assembly of microtubulin into microtubules, as well as aggregates and blocks the depolymerization of assembled ones. Abnormal microtubule formation inhibits cell mitosis and leads to cell death.8–10 PTX resistance in breast cancer patients has received increasing attention. PTX is 30–60% effective as a first-line agent, while it is 20–40% effective as a second-line agent or single-agent in chemotherapy.11 The development of resistance to PTX in breast cancer involves multiple molecular mechanisms. Altered expression of β-tubulin isotypes and changes in microtubule-associated proteins, such as microtubule-associated protein-4 and tau, affect microtubule dynamics and modulate the resistance to PTX.12 PTX and the microtubule-binding protein Tau bind to microtubules through the same site. Tau competes with PTX to inhibit its role in promoting microtubule aggregation, thereby causing resistance to PTX.13 Altered expression levels of specific isotype microtubule proteins can also lead to PTX resistance.14
Because of its non-ionizing and noninvasive properties, high-intensity focused ultrasound (HIFU) is a relatively safe treatment.15,16 Since the repeated treatment of HIFU is possible and the complication occurrence rate is low, HIFU has become an attractive option for breast cancer therapy.17 The combination of HIFU with nanoparticles has a substantial potential to enhance the efficacy of drug delivery and reduce side effects of drugs in the therapy of tumors and other diseases.18 Moreover, HIFU technology provides the targeted release of drugs encapsulated in a low-temperature thermo-sensitive liposome PTX coated in animal model.19 Thus, HIFU may also promote PTX delivery and its anti-tumor activity.
In this research, we aimed to investigate whether HIFU could enhance the anti-tumor effect of PTX in breast cancer.
Methods
Cell Culture
MDA-MB-231 and MCF-7 cells (ATCC, Manassas, VA) were used in this research. DMEM (Invitrogen, MA, USA) containing 10% fetal bovine serum (Gibco, MA, USA) and 100 U/mL penicillin and streptomycin (Thermo Fisher, MA, USA) was used for cell culture.
PTX was purchased from Aladdin (Shanghai, China). Cells were separated into the control group, control + PTX group, HIFU group, and HIFU + PTX group. After PTX treatment, cell viability was analyzed.
HIFU
1 × 106 cells were placed in the test tube and radiated at 1 MHz of frequency for 30s. For mouse model, HIFU was applied at 1.5 MHz of frequency for 5 min at the tumor site (interval 2 mm, time per spot 30s). Mice were anesthetized with inhalation isoflurane throughout the HIFU exposure process. Each mouse was positioned in a holder.
Measurement of Cell Viability
1000 cells per well were plated in 96-well plates. Cell Counting Kit-8 assay (Bimake, Shanghai, China) was employed for evaluating cell viability according to the manufacturers’ instruction.
Measurement of Cell Apoptosis
Cell apoptosis was analyzed by Annexin-V/PI Apoptosis Analysis Kit (Yeasen, Shanghai, China) according to the manufacturer’s protocol.
Detection of Antioxidant Activities
Superoxide dismutase (SOD) was measured by SOD analysis kit (KeyGen Biotech, Nanjing, China) using the Xanthine oxidase method, measured at 550 nm, and expressed as U/mL. Catalase (CAT) was evaluated by CAT analysis kit (KeyGen Biotech) using H2O2 and ammonium molybdate, measured at 405 nm, and expressed as U/g protein. The concentration of malondialdehyde (MDA) was measured by MDA analysis kit (KeyGen Biotech) by thiobarbituric acid (TBA) method, measured at 532 nm, and expressed as nmol/L.
Animal Model
Female BALB/c mice (5-week old) were xenografted with 5×106 MDA-MB-231 cells into the right flank. Mice were kept until tumor size reached 5 to 8 mm. Mice bearing tumors were treated with 2 mg/Kg PTX through tail vein injection. Mice were separated into the saline group, PTX group, HIFU group, and HIFU + PTX group. The tumor size, body weight, and survival rate were analyzed. Animal studies were performed in strict accordance with the NIH guidelines for the care and use of laboratory animals (8th edition, NIH), and were approved by the ethics committee of Cangzhou Central Hospital (2021.06.17.c4).
Pharmacokinetics of PTX
Blood samples (0.3 mL) and tumor tissue samples were collected at 0.2, 0.5, 1, 2, 4, 8, 12, and 24 h after PTX treatment. Tissues were homogenized by Omni Bead Ruptor 24 Homogenizer (Kennesaw, GA, USA). To quantify the PTX concentration in samples, solid phase extraction was applied before the measurement. PTX concentration was detected by liquid chromatography/mass spectrometry (LC/MS).
Statistical Analysis
In this research, statistical analysis was performed by SPSS Statistics Version 11.0. Data were shown as mean ± standard deviation (SD). Statistical significance was analyzed by one-way ANOVA or two-way ANOVA followed by appropriate post hoc tests, and defined by P < 0.05.
Results
Effect of HIFU on PTX-Inhibited Breast Cancer Cell Viability
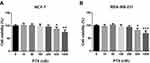
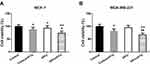
In Figure 1A and B, 400 nM or 1000 nM PTX significantly inhibited the viability of MDA-MB-231 (400 nM: 81.0%±7.5%, p=0.030; 1000 nM: 56.1%±8.2%, p<0.001) and MCF-7 (400 nM: 85.8%±6.8%, p=0.048; 1000 nM: 72.1%±7.8%, p=0.009) cells. Thus, the PTX concentration used in subsequent experiments was chosen as 400 nM. HIFU inhibited the viability of MCF-7 cells (Figure 2A) (86.5%±6.6%, p=0.049). In both cell lines, cells treated with the combination of HIFU and PTX exhibited significantly lower cell viability than those treated with PTX alone (MCF-7: 65.7%±7.4%, p=0.024; MDA-MB-231: 60.1%±6.4%, p=0.008) (Figure 2A and B).
Effect of HIFU on PTX-Induced Oxidative Stress in Breast Cancer Cells
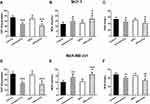
In both cell lines, both HIFU and PTX reduced the activity of CAT (Figure 3A, −13.1 U/g protein, p=0.028; −27.8 U/g protein, p<0.001 and Figure 3D, −12.3 U/g protein, p=0.047; −36.1 U/g protein, p<0.001). Furthermore, cells in the HIFU + PTX group exhibited significantly lower CAT activity than those in the control + PTX group (Figure 3A, −12.2 U/g protein, p=0.039 and Figure 3D, −9.3 U/g protein, p=0.049). The MDA concentrations of both cell lines in the control + PTX group were significantly elevated compared with the control group (Figure 3B, 3.5 nmol/L, p=0.028; Figure 3E, 4.8 nmol/L, p=0.008). Importantly, the MDA concentration in the HIFU + PTX group was significantly higher than in the control + PTX group (Figure 3B, 3.8 nmol/L, p=0.049; Figure 3E, 3.0 nmol/L, p=0.047). Furthermore, the activity of SOD in the HIFU + PTX group was significantly lower than in the control + PTX group (Figure 3C, −8.5 U/mL, p=0.048 and Figure 3F −8.1 U/mL, p=0.040). Thus, HIFU and PTX exhibited synergistic effect on the induction of oxidative stress in breast cancer cells.
Effect of HIFU on PTX-Induced Breast Cancer Cell Apoptosis
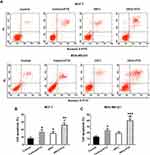
In MCF-7 cells, both HIFU (14.8%±2.4% vs 7.8%±2.4%, p=0.047) and PTX (17.1%±3.2% vs 7.8%±2.4%, p=0.011) enhanced cell apoptosis (Figure 4A and B). Compared with the control + PTX group, MCF-7 cells in the HIFU + PTX group (26.6%±2.8%, p=0.009) showed significantly elevated extent of apoptosis (Figure 4A and B). MDA-MB-231 cells in the HIFU + PTX group (38.4%±3.6% vs 24.2%±2.7%, p=0.001) also exhibited significantly higher apoptosis than in the control + PTX group (Figure 4A and C). Thus, HIFU and PTX showed synergistic effect on breast cancer cell apoptosis.
Effect of HIFU on the Anti-Tumor Effect of PTX in Mice Bearing MDA-MB-231 Tumors
In mice bearing MDA-MB-231 tumors, the effects of HIFU and PTX on tumor tissues were also evaluated. Tumor size in the PTX group (218.63 ± 32.98 mm3 vs 282.14 ± 36.71 mm3, p=0.001) was significantly smaller than that of the saline group (Figure 5A). In the HIFU + PTX group (166.74 ± 30.11 mm3 vs 218.63 ± 32.98 mm3, p=0.012), the tumor size was significantly smaller than that in the PTX group (Figure 5A). Compared with mice in the saline group, those in the HIFU + PTX group displayed significantly higher body weight (20.9 ± 2.2 g vs 17.2 ± 1.9 g, p=0.017) (Figure 5B). Furthermore, the HIFU + PTX group exhibited the highest survival rate among all four groups (Figure 5C). Thus, HIFU and PTX exhibited synergistic anti-tumor effect against breast cancer.
Effect of HIFU on the Distribution of PTX in Tumor Tissues
Pharmacokinetic studies of PTX with and without HIFU were conducted. In both the PTX group and the HIFU + PTX group, PTX concentrations in the plasma dropped rapidly and showed no significant difference (Figure 6A). In mice bearing MDA-MB-231 tumors, PTX concentrations in the tumor tissues were increased in the first 4 hours after injection and subsequently decreased (Figure 6B). 24 hours post PTX injection, the HIFU + PTX group showed significantly higher PTX concentrations in tumor tissues then the PTX group (108.66 ± 20.17 ng/g vs 72.15± 12.04 ng/g, p=0.012) (Figure 6B).
Discussion
In this research, we investigated the therapeutic and drug delivery efficacy of HIFU-triggered PTX against breast cancer through both in vivo and in vitro experiments. Intravenously administered PTX could be well accumulated in the tumor tissues of mice bearing MDA-MB-231 xenograft tumors through HIFU treatment. Compared to PTX without HIFU treatment, in vitro HIFU-triggered PTX in breast cancer cells displayed enhanced effects in inhibiting cell viability, inducing oxidative stress, and promoting apoptosis. According to these results, treatment of HIFU could promote the accumulation efficiency of PTX in breast cancer tissues and its anti-tumor effect.
Rapid growth of tumor tissues leads to leakage from the vascular system. In the complex tumor microenvironment, limited deep tumor penetration greatly hinders the delivery of anti-tumor drugs to tumor sites.20 It is well known that heterogeneous tumors have different vascular structures and perfusion rates.21 In tumor tissues, the thick extracellular matrix (ECM) can generate a physical barrier to inhibit drug accumulation.22
Several studies have attempted to promote anti-tumor drug delivery by remodeling ECM in the tumor microenvironment.23 Matrix metalloprotease (MMP) can break down the structure of ECM thereby improve anti-tumor drug delivery and therapeutic efficacy.24 HIFU can physically break down dense tumor ECM structures with no toxicity25 and improve the delivery of high molecular weight antibodies and anti-tumor drugs to tumor tissues.26,27 The exact mechanism of HIFU-mediated nanoparticle delivery in tumors has been reported.25,28 The dense ECM structure of tumor tissues can be successfully disrupted by exposure to noninvasive pulsed HIFU. In addition, normalizing the tumor vasculature can reduce the interstitial flow pressure in ECM-rich tumors.29 Intravenously administered drugs can successfully accumulate in tumor tissues exposed to HIFU.30
In addition to radiotherapy, surgery and hormonal therapy, chemotherapy has become an effective treatment for breast cancer.31 Most patients respond to the initial chemotherapy, but the sensitivity and response rate against chemotherapeutic agents gradually decline during the process of chemotherapy as a result of the development of multidrug resistance, which can eventually lead treatment failure.32 The mechanisms by which drug resistance arises are complex and can be mediated by a variety of factors. Evidence suggests that drug resistance is closely associated with increased drug efflux transporter protein activity, reduced levels of cellular drug uptake, upregulation of the detoxification system, or adaptation to metabolic reprogramming.33–35
There is growing interest in the use of neoadjuvant treatments for earlier inhibition of micro-metastasis to improve the treatment outcome.36 Neoadjuvant chemotherapy is the gold standard treatment for breast cancer to reduce the extent of subsequent surgical treatment.37 HIFU is an option for neoadjuvant therapy against pancreatic cancer and prostate cancer.38,39 In preclinical data, higher concentration of chemotherapy in tumors is correlated with increased tumor response.40 Research has reported that mild local hyperthermia induced by magnetic resonance guided-HIFU and lyso-thermosensitive liposomal doxorubicin could be used in the neoadjuvant chemotherapy of patients with breast cancer.41
As a taxane compound, PTX has been widely used for the chemotherapy of breast cancer. Through interfering with microtubule polymerization, PTX inhibits cell division and induces the apoptosis of tumor cells.42 Evidence has shown that PTX has potent therapeutic effect against breast cancer. However, approximately 50% of breast cancer patients develop significant resistance to PTX within 6–10 months of chemotherapy.43 We therefore explored whether HIFU could promote the anti-tumor activity of PTX in breast cancer.
PTX binds to microtubules, stabilizes microtubule polymerization and promotes the prolongation of tubulin polymers.44,45 PTX polymerizes free microtubule unattached or preexisting ones in the microtubule tissue center.46 The ability of cell division is inhibited by insufficient requirement for mitotic checkpoints.47 PTX interferes with the kinetics of microtubule polymerization and delays mitotic progression, which ultimately leads to mitotic arrest and apoptosis.48,49 In this research, we investigated the effect of PTX or HIFU alone or the combination of both on breast cancer viability and apoptosis. Results demonstrated that treatment of HIFU had no effect on the viability or apoptosis of MDA-MB-231 cells, whereas inhibited the viability and enhanced the apoptosis of MCF-7 cells. The combination of HIFU and PTX conferred more potent inhibition of cell viability and induction of cell apoptosis than PTX alone in breast cancer cells.
It has been illustrated that treatment with PTX can induce the production of hydroperoxides and cause oxidative stress.50 Oxidative stress is an imbalance of oxidative and anti-oxidant factors, which peroxidizes membrane lipids, damages organelles, and leads to cellular damage.51 It is caused by overproduced oxidative stress species or reduced anti-oxidant capacity.52 The key parameters are anti-oxidant enzymes such as SOD and CAT, as well as end products of lipid peroxidation such as MDA.53 In this research, we also evaluated the oxidative stress in breast cancer cells. In MCF-7 and MDA-MB-231 cells, the administration of PTX inhibited the activity of SOD and CAT and increased the level of MDA. Meanwhile, the combination of HIFU and PTX exerted more potent inhibition of SOD and CAT and elevation of MDA level than PTX alone in breast cancer cells. These results indicated that HIFU could increase the anti-tumor activity of PTX in breast cancer cells.
Furthermore, we also evaluated the effect of HIFU on PTX treatment in mice bearing MDA-MB-231 tumors. These in vivo experiments showed that the combination of HIFU and PTX significantly enhanced the effect of PTX in decreasing tumor size and increasing body weight and survival rate. Intravenously administered drugs can successfully accumulate in ECM-rich tumors exposed to noninvasive HIFU treatment. In this research, we also analyzed PTX pharmacokinetics in mice bearing MDA-MB-231 tumors. Based on these results, the accumulation of PTX in the tumor tissues was significantly enhanced by the treatment of HIFU.
However, there were some limitations in this study. The number of mice used in the in vivo experiments was relatively small. We have already observed the effects of HIFU on the anti-tumor activities of PTX in both cell and mouse models, but difference still exists between laboratory and clinical trials, and results in this research were not directly applicable in humans therefore should be further validated.
In conclusion, treatment of HIFU promoted the distribution of PTX and enhanced the anti-tumor activities of PTX in breast cancer cell lines and mouse model. These findings will provide an experimental basis for HIFU as an adjuvant therapy in patients with breast cancer.
Funding
There is no funding to report.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Fan L, Strasser-Weippl K, Li JJ, et al. Breast cancer in China. Lancet Oncol. 2014;15(7):e279–89. doi:10.1016/S1470-2045(13)70567-9
2. Assi S, Torrington E, Cheema E, Hamid AA. Adverse drug reactions associated with chemotherapeutic agents used in breast cancer: analysis of patients’ online forums. J Oncol Pharm Pract. 2021;27(1):108–118. doi:10.1177/1078155220915767
3. Salek R, Dehghani M, Mohajeri SA, Talaei A, Fanipakdel A, Javadinia SA. Amelioration of anxiety, depression, and chemotherapy related toxicity after crocin administration during chemotherapy of breast cancer: a double blind, randomized clinical trial. Phytother Res. 2021;35(9):5143–5153. doi:10.1002/ptr.7180
4. Moezian GSA, Javadinia SA, Sales SS, Fanipakdel A, Elyasi S, Karimi G. Oral silymarin formulation efficacy in management of AC-T protocol induced hepatotoxicity in breast cancer patients: a randomized, triple blind, placebo-controlled clinical trial. J Oncol Pharm Pract. 2021;10781552211006182. doi:10.1177/10781552211006182
5. Wang Y, Chen Z. Mutation detection and molecular targeted tumor therapies. STEMedicine. 2020;1(1):e11. doi:10.37175/stemedicine.v1i1.11
6. Sedighi Pashaki A, Mohammadian K, Afshar S, et al. A randomized, controlled, parallel-group, trial on the effects of melatonin on fatigue associated with breast cancer and its adjuvant treatments. Integr Cancer Ther. 2021;20:1534735420988343. doi:10.1177/1534735420988343
7. Le XF, Bast RC
8. Wall ME, Wani MC, Taylor H. Plant antitumor agents, 27. Isolation, structure, and structure activity relationships of alkaloids from Fagara macrophylla. J Nat Prod. 1987;50(6):1095–1099. doi:10.1021/np50054a014
9. McGrogan BT, Gilmartin B, Carney DN, McCann A. Taxanes, microtubules and chemoresistant breast cancer. Biochim Biophys Acta. 2008;1785(2):96–132. doi:10.1016/j.bbcan.2007.10.004
10. Mortal S. Microtubule dynamics in cytoskeleton, neurodegenerative and psychiatric disease. STEMedicine. 2021;2(6):e81. doi:10.37175/stemedicine.v2i6.81
11. Perez EA. Paclitaxel in breast cancer. Oncologist. 1998;3(6):373–389. doi:10.1634/theoncologist.3-6-373
12. Rivera E, Gomez H. Chemotherapy resistance in metastatic breast cancer: the evolving role of ixabepilone. Breast Cancer Res. 2010;12(Suppl 2):S2. doi:10.1186/bcr2573
13. Smoter M, Bodnar L, Duchnowska R, Stec R, Grala B, Szczylik C. The role of Tau protein in resistance to paclitaxel. Cancer Chemother Pharmacol. 2011;68(3):553–557. doi:10.1007/s00280-011-1696-7
14. Ganguly A, Yang H, Cabral F. Class III beta-tubulin counteracts the ability of paclitaxel to inhibit cell migration. Oncotarget. 2011;2(5):368–377. doi:10.18632/oncotarget.250
15. Maloney E, Hwang JH. Emerging HIFU applications in cancer therapy. Int J Hyperthermia. 2015;31(3):302–309. doi:10.3109/02656736.2014.969789
16. Zhang H, Han K. High intensity focused ultrasound enhances anti-tumor immunity through promoting CD4 Th1 effector T cell response. STEMedicine. 2020;1(4):e65. doi:10.37175/stemedicine.v1i4.65
17. Feril LB, Fernan RL, Tachibana K. High-intensity focused ultrasound in the treatment of breast cancer. Curr Med Chem. 2021;28(25):5179–5188. doi:10.2174/0929867327666201111143206
18. Tharkar P, Varanasi R, Wong WSF, Jin CT, Chrzanowski W. Nano-enhanced drug delivery and therapeutic ultrasound for cancer treatment and beyond. Front Bioeng Biotechnol. 2019;7:324. doi:10.3389/fbioe.2019.00324
19. Farr N, Wang YN, D’Andrea S, et al. Hyperthermia-enhanced targeted drug delivery using magnetic resonance-guided focussed ultrasound: a pre-clinical study in a genetic model of pancreatic cancer. Int J Hyperthermia. 2018;34(3):284–291. doi:10.1080/02656736.2017.1336675
20. Jain RK. Delivery of molecular and cellular medicine to solid tumors. Adv Drug Deliv Rev. 2001;46(1–3):149–168. doi:10.1016/s0169-409x(00)00131-9
21. Junttila MR, de Sauvage FJ. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature. 2013;501(7467):346–354. doi:10.1038/nature12626
22. Netti PA, Berk DA, Swartz MA, Grodzinsky AJ, Jain RK. Role of extracellular matrix assembly in interstitial transport in solid tumors. Cancer Res. 2000;60(9):2497–2503.
23. Wong C, Stylianopoulos T, Cui J, et al. Multistage nanoparticle delivery system for deep penetration into tumor tissue. Proc Natl Acad Sci U S A. 2011;108(6):2426–2431. doi:10.1073/pnas.1018382108
24. Parodi A, Haddix SG, Taghipour N, et al. Bromelain surface modification increases the diffusion of silica nanoparticles in the tumor extracellular matrix. ACS Nano. 2014;8(10):9874–9883. doi:10.1021/nn502807n
25. Lee S, Han H, Koo H, et al. Extracellular matrix remodeling in vivo for enhancing tumor-targeting efficiency of nanoparticle drug carriers using the pulsed high intensity focused ultrasound. J Control Release. 2017;263:68–78. doi:10.1016/j.jconrel.2017.02.035
26. Oh KS, Han H, Yoon BD, et al. Effect of HIFU treatment on tumor targeting efficacy of docetaxel-loaded Pluronic nanoparticles. Colloids Surf B Biointerfaces. 2014;119:137–144. doi:10.1016/j.colsurfb.2014.05.007
27. Wang S, Shin IS, Hancock H, et al. Pulsed high intensity focused ultrasound increases penetration and therapeutic efficacy of monoclonal antibodies in murine xenograft tumors. J Control Release. 2012;162(1):218–224. doi:10.1016/j.jconrel.2012.06.025
28. You DG, Yoon HY, Jeon S, et al. Deep tissue penetration of nanoparticles using pulsed-high intensity focused ultrasound. Nano Converg. 2017;4(1):30. doi:10.1186/s40580-017-0124-z
29. Choi Y, Han H, Jeon S, et al. Deep tumor penetration of doxorubicin-loaded glycol chitosan nanoparticles using high-intensity focused ultrasound. Pharmaceutics. 2020;12(10):Oct. doi:10.3390/pharmaceutics12100974
30. Phenix CP, Togtema M, Pichardo S, Zehbe I, Curiel L. High intensity focused ultrasound technology, its scope and applications in therapy and drug delivery. J Pharm Pharm Sci. 2014;17(1):136–153. doi:10.18433/j3zp5f
31. Cortazar P, Geyer CE
32. Costea T, Vlad OC, Miclea LC, Ganea C, Szollosi J, Mocanu MM. Alleviation of multidrug resistance by flavonoid and non-flavonoid compounds in breast, lung, colorectal and prostate cancer. Int J Mol Sci. 2020;21(2):401. doi:10.3390/ijms21020401
33. Ambudkar SV, Dey S, Hrycyna CA, Ramachandra M, Pastan I, Gottesman MM. Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu Rev Pharmacol Toxicol. 1999;39(1):361–398. doi:10.1146/annurev.pharmtox.39.1.361
34. Shen DW, Goldenberg S, Pastan I, Gottesman MM. Decreased accumulation of [14C]carboplatin in human cisplatin-resistant cells results from reduced energy-dependent uptake. J Cell Physiol. 2000;183(1):108–116. doi:10.1002/(SICI)1097-4652(200004)183:1<108::AID-JCP13>3.0.CO;2-4
35. Liu YY, Han TY, Giuliano AE, Cabot MC. Ceramide glycosylation potentiates cellular multidrug resistance. FASEB J. 2001;15(3):719–730. doi:10.1096/fj.00-0223com
36. Fazilat-Panah D, Vakili Ahrari Roudi S, Keramati A, et al. Changes in cytokeratin 18 during neoadjuvant chemotherapy of breast cancer: a prospective study. Iran J Pathol. 2020;15(2):117–126. doi:10.30699/ijp.2020.116238.2261
37. Buonomo OC, Grasso A, Pistolese CA, et al. Evaluation of concordance between histopathological, radiological and biomolecular variables in breast cancer neoadjuvant treatment. Anticancer Res. 2020;40(1):281–286. doi:10.21873/anticanres.13950
38. Stanislavova N, Karamanliev M, Ivanov T, Yotsov T, Zhou K, Dimitrov D. Is high-intensity focused ultrasound (HIFU) an option for neoadjuvant therapy for borderline resectable pancreatic cancer patients? - a systematic review. Int J Hyperthermia. 2021;38(2):75–80. doi:10.1080/02656736.2021.1909150
39. Chaussy CG, Thuroff S. High-intensity focused ultrasound for the treatment of prostate cancer: a review. J Endourol. 2017;31(S1):S30–S37. doi:10.1089/end.2016.0548
40. Besse HC, Barten-van Rijbroek AD, van der Wurff-jacobs KMG, Bos C, Moonen CTW, Deckers R. Tumor drug distribution after local drug delivery by hyperthermia, in vivo. Cancers. 2019;11(10):Oct. doi:10.3390/cancers11101512
41. de Maar JS, Suelmann BBM, Braat M, et al. Phase I feasibility study of magnetic resonance guided high intensity focused ultrasound-induced hyperthermia, lyso-thermosensitive liposomal doxorubicin and cyclophosphamide in de novo stage IV breast cancer patients: study protocol of the i-GO study. BMJ Open. 2020;10(11):e040162. doi:10.1136/bmjopen-2020-040162
42. Zhu L, Chen L. Progress in research on paclitaxel and tumor immunotherapy. Cell Mol Biol Lett. 2019;24(1):40. doi:10.1186/s11658-019-0164-y
43. Jones SE, Erban J, Overmoyer B, et al. Randomized Phase III study of docetaxel compared with paclitaxel in metastatic breast cancer. J Clin Oncol. 2005;23(24):5542–5551. doi:10.1200/JCO.2005.02.027
44. Weaver BA. How Taxol/paclitaxel kills cancer cells. Mol Biol Cell. 2014;25(18):2677–2681. doi:10.1091/mbc.E14-04-0916
45. Sackett D, Fojo T. Taxanes. Cancer Chemother Biol Response Modif. 1997;17:59–79.
46. De Brabander M, Geuens G, Nuydens R, Willebrords R, De Mey J. Taxol induces the assembly of free microtubules in living cells and blocks the organizing capacity of the centrosomes and kinetochores. Proc Natl Acad Sci USA. 1981;78(9):5608–5612. doi:10.1073/pnas.78.9.5608
47. Abu Samaan TM, Samec M, Liskova A, Kubatka P, Busselberg D. Paclitaxel’s mechanistic and clinical effects on breast cancer. Biomolecules. 2019;9(12):789. doi:10.3390/biom9120789
48. Jordan MA, Toso RJ, Thrower D, Wilson L. Mechanism of mitotic block and inhibition of cell proliferation by taxol at low concentrations. Proc Natl Acad Sci USA. 1993;90(20):9552–9556. doi:10.1073/pnas.90.20.9552
49. Jordan MA, Wilson L. Microtubules and actin filaments: dynamic targets for cancer chemotherapy. Curr Opin Cell Biol. 1998;10(1):123–130. doi:10.1016/s0955-0674(98)80095-1
50. Alexandre J, Batteux F, Nicco C, et al. Accumulation of hydrogen peroxide is an early and crucial step for paclitaxel-induced cancer cell death both in vitro and in vivo. Int J Cancer. 2006;119(1):41–48. doi:10.1002/ijc.21685
51. Islam MT. Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders. Neurol Res. 2017;39(1):73–82. doi:10.1080/01616412.2016.1251711
52. Lenaz G, D’Aurelio M, Merlo Pich M, et al. Mitochondrial bioenergetics in aging. Biochim Biophys Acta. 2000;1459(2–3):397–404. doi:10.1016/s0005-2728(00)00177-8
53. Lv Q, Hu Q, Zhang W, et al. Disturbance of oxidative stress parameters in treatment-resistant bipolar disorder and their association with electroconvulsive therapy response. Int J Neuropsychopharmacol. 2020;23(4):207–216. doi:10.1093/ijnp/pyaa003
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.