Back to Journals » Cancer Management and Research » Volume 12
High-Grade B-Cell Lymphomas, Not Otherwise Specified: A Study of 41 Cases
Authors Li J, Liu X, Yao Z, Zhang M
Received 26 December 2019
Accepted for publication 3 March 2020
Published 13 March 2020 Volume 2020:12 Pages 1903—1912
DOI https://doi.org/10.2147/CMAR.S243753
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Yong Teng
Jiayin Li, 1, 2 Xiaoyin Liu, 3 Zhihua Yao, 4 Mingzhi Zhang 1, 2
1Department of Oncology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan 450000, People’s Republic of China; 2Lymphoma Diagnosis and Treatment Center of Henan Province, Zhengzhou, Henan 450000, Republic of China; 3Department of Hematology, People’s Hospital of Zhengzhou University, Zhengzhou, Henan 450003, People’s Republic of China; 4Department of Internal Medicine, The Affiliated Cancer Hospital of Zhengzhou University (Henan Cancer Hospital), Zhengzhou, Henan 450008, People’s Republic of China
Correspondence: Mingzhi Zhang
Department of Oncology, The First Affiliated Hospital of Zhengzhou University, No. 1 Jianshe East Road, Zhengzhou, Henan 450052, People’s Republic of China
Tel +86 371 6629 5562
Fax +86 371 6629 5563
Email [email protected]
Purpose: To analyze the clinical and pathological characteristics, treatment, and prognosis of high-grade B-cell lymphomas, not otherwise specified (HGBL, NOS), and to increase awareness of this type of lymphoma.
Patients and Methods: We collected clinical and pathological data of 41 cases of newly diagnosed HGBL, NOS, and analyzed diagnosis, prognosis and treatment to examine progression-free survival (PFS) and overall survival (OS).
Results: Among the 41 cases studied, the median PFS was 6.0 months and the median OS was 18.0 months. Compared with patients treated with the R-CHOP regimen, patients treated with a high-intensity chemotherapy (DA-EPOCH-R, R-CODOX-M/IVAC, or R-Hyper-CVAD) had superior PFS and OS (PFS: χ 2=4.173, P=0.041; OS: χ 2=5.200, P=0.023). A subgroup analysis showed that the OS for the double-expressor lymphoma (DEL) was inferior to that for the non-DEL (χ 2=4.563, P=0.033), and this trend was also seen for the single-hit lymphoma with MYC rearrangement (SHL) and the non-SHL (χ 2=4.955, P=0.026). Patients with low International Prognostic Index (IPI) scores (≤ 2) had better survival rates than those with high scores (> 2) (PFS: χ 2=6.482, P=0.011; OS: χ 2=10.156, P=0.001).
Conclusion: HGBL, NOS is associated with a high degree of malignancy, short survival period, and substantial extranodal involvement. High-intensity chemotherapy may improve patient prognosis. While IPI scores statistically correlated with the prognosis, SHL and DEL correlated with an inferior survival rate. New and improved treatments will be needed for HGBL, NOS.
Keywords: HGBL, NOS, clinical and pathological, treatment, prognosis
Introduction
High-grade B-cell lymphoma (HGBL) is a newly introduced category in the updated 2016 revision of the World Health Organization (WHO) classification, which primarily replaces “B-cell lymphoma, unclassifiable, with features intermediate between a diffuse large B cell lymphoma (DLBCL) and the Burkitt lymphoma (BL) (BCLU, DLBCL/BL).” Currently, HGBL comprises 2 types of lymphomas: HGBL with MYC and BCL2 and/or BCL6 rearrangements (HGBL, R) and high-grade B-cell lymphomas, not otherwise specified (HGBL, NOS).1–5 HGBL, R is also called a double/triple-hit lymphoma (HGBL, DH/TH).6 HGBL, NOS do not contain MYC and BCL2 or BCL6 gene rearrangements, but present morphologies between DLBCL and BL.
HGBL, NOS belong to a type of gray zone lymphomas, as they are rarely encountered in the clinic, and their etiology and pathogenesis remain unclear.7 Therefore, these gray zone lymphomas have been listed separately, to facilitate better study of the pathological and clinical features of the tumors. While a standard treatment has not been established for HGBL, NOS,8 the following regimens have been used at the National Comprehensive Cancer Network member institutions: R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine), DA-EPOCH-R (rituximab, dose-adjusted doxorubicin, cyclophosphamide, vincristine, etoposide, prednisone), R-Hyper-CVAD (rituximab, cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with high-dose methotrexate and cytarabine), R-CODOX-M/IVAC (rituximab, cyclophosphamide, vincristine, doxorubicin and methotrexate alternating with ifosfamide, etoposide, and cytarabine), in consolidation with a high-dose therapy with an autologous hematopoietic stem cell transplantation (ASCT).
Although the pathological features and clinical characteristics of patients with HGBL, DH/TH have been well characterized in the literature,5,6 few studies have focused on patients with HGBL, NOS. We retrospectively analyzed a group of patients with HGBL, NOS and summarized the clinical and the pathological features, and explored the patient diagnosis, treatment, and prognosis. We hope to improve the medical community’s understanding of this type of tumor.
Methods
Clinical Characteristics
We collected data from 1802 patients with a newly diagnosed aggressive mature B-cell lymphomas, from January 01, 2013 to June 01, 2019, in The First Affiliated Hospital of Zhengzhou University, People’s hospital of Zhengzhou University, and The Affiliated cancer Hospital of Zhengzhou University (Henan Cancer Hospital). Of the included patients, 67 were diagnosed with HGBL. While 26 met the criteria of HGBL, DH/TH, 41 met the diagnostic criteria of HGBL, NOS. Pathological sections of the 41 HGBL, NOS patients were reread by 2 senior lymphoma pathologists, who referred to the WHO lymphohematopoietic system tumor classification (2016)3 to identify the morphological, immunophenotypic, and cytogenetic characteristics. If the opinions of the two experts differed, then the pathological sections were consulted in the superior hospital. The expression of MYC/BCL2/BCL6 was detected by immunohistochemistry (IHC), while MYC/BCL2/BCL6 rearrangement was detected by fluorescence in situ hybridization (FISH). For IHC, the positivity cutoff values were 40% for MYC and 50% for BCL-2.6,9 Lymphomas that do not harbor MYC and BCL2 and/or BCL6 rearrangements but exhibit the MYC and BCL2/BCL6 protein expression are known as double-expressor lymphoma (DEL).10,11 HGBL, NOS with MYC rearrangement have been defined as single-hit lymphomas (SHL) in this study. The Hans classification12 was used to analyze the cell of the origin subtype. Patient baseline clinical characteristics included sex of the individual, age, the eastern cooperative oncology group score (ECOG), the Ann Arbor stage, the international prognostic index (IPI) score, B symptoms, the serum lactate dehydrogenase (LDH) level, the β2 microglobulin, extranodal sites, the Ki-67 level, and the bone marrow involvement.
Regimens
All patients with HGBL, NOS received therapy after the results of FISH were available. For first-line therapy, 17 of the 41 (41.5%) patients were treated with R-CHOP and 24 of the 41 (58.5%) were treated with a high-intensity chemotherapy (DA-EPOCH-R, R-CODOX-M/IVAC, and R-Hyper-CVAD). Out of the 41 patients, 15 (36.6%) patients who were at a higher risk for CNS involvement13 received a CNS prophylaxis. Six (19.5%) patients received an ASCT after complete remission and 2 (4.9%) received the chimeric antigen receptor T-cell immunotherapy after relapse.
Observation of Efficacy and Follow-Up
According to the International Working Group’s efficacy evaluation criteria,14,15 efficacy is divided into complete remission (CR), partial remission (PR), disease stabilization (SD), and progressive disease (PD). The objective response rate (ORR) is calculated according to the percentage of CR+ PR patients among all patients. Progression-free survival (PFS) was defined as the period of time from HGBL, NOS diagnosis to PD or death of any cause. Overall survival (OS) was defined as the time from HGBL, NOS diagnosis to death from any cause or to the last follow-up. The follow-up deadline was October 01, 2019.
Statistical Analysis
All data were analyzed using the SPSS 24.0. The survival rates (PFS and OS) of each group were estimated using the Kaplan–Meier method, and the survival rates were compared between two groups by a Log rank test. All P values <0.05 were considered statistically significant.
Results
Morphological, Immunophenotypic, and Cytogenetic Features
The included 41 patient samples presented medium-sized cell characteristics, with thin, scattered nuclei or the BL-like nuclei, some with a “starry sky” phenomenon. These morphological changes were uncertain but overall, cell sizes and shapes of the HGBL, NOS samples were larger than those of the BL samples. Since most specimens were obtained from medium-sized tumor cells with a diffuse hyperplasia and a partial large cell infiltration, small lymphocytes were rare (Figure 1A and B). All cases showed similar morphological markers, which included the expression of mature B-cell markers (such as CD19, CD20, and CD22) that were detected by IHC. Thirty-one (75.6%) cases expressed CD10, twenty-six (63.4%) expressed MUM-1, and thirty-four (82.9%) expressed BCL6. Variable proliferation indices (Ki-67 rates) were observed: 32 (78.0%) cases had high Ki-67 (> 90%), and the median Ki-67 was 90%. According to the Hans classifier,12 the phenotype of 33 (80.5%) cases was germinal center B-cell (GCB); and that of 8 (19.5%) cases was non-GCB. MYC and BCL2 or BCL6 were positively detected by IHC in 24 (58.5%) of the 41 cases that were diagnosed with DEL. Of all cases, 70.7% strongly expressed the MYC protein. Sixteen (39.0%) of the cases were diagnosed with SHL by FISH. The full list of cytogenetic results detected by FISH has been tabulated in Table 1 (n=41). Overall, 13 (31.7%) patients had a MYC rearrangement only, 2 (4.9%) had a BCL2 rearrangement only, 3 (7.3%) had a BCL6 rearrangement only, and none of the patients had both BCL2 and BCL6 rearrangements. Three (7.3%) cases had both the copy number changes and gene rearrangements, which included 1 case with a MYC gene rearrangement and the copy number change of BCL2, 1 case with a BCL2 rearrangement and the MYC copy number change, and 1 case with both BCL2 copy number changes and rearrangements. Five (12.5%) cases had copy number changes without gene rearrangement, and the remaining cases had no cytogenetic abnormalities.
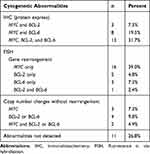 |
Table 1 Cytogenetic Findings of 41 Patients with HGBL, NOS (n=41) |
 |
Figure 1 The morphological features of one case of HGBL, NOS. (A) hematoxylin-eosin, original magnification ×200. (B) hematoxylin-eosin, original magnification ×400. |
Clinical Features
The characteristics of 41 cases of HGBL, NOS patients have been listed in Table 2. Twenty-six (63.4%) patients were male and 15 (36.6%) were female. The median patient age was 41 years (range 12–79 years). The first reported symptoms were: unexplained fever, lymph node swelling, night sweats, decreased body mass, abdominal pain, and abdominal distension. Twenty-three (56.1%) patients had the Ann Arbor stage grade III/IV, while 24 (58.5%) patients had an IPI score of >2. B symptoms were observed in 14 (34.1%) patients, an elevated LDH in 25 (61.0%), and an elevated β2 microglobulin in 14 (34.1%).
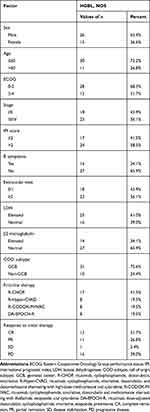 |
Table 2 Patients Characteristics (n=41) |
The median follow-up duration was 28.0 months and from the beginning of the study to the end of the follow-up period, there were a total of 20 deaths. The median PFS was 6.0 months (95% CI: 0.035–11.965 months) and the median OS was 18.0 months (95% CI: 14.772–21.228 months) (Figure 2A and B). Over a 2-year follow-up, the PFS rate was 41.5% and the OS rate was 36.7%.
 |
Figure 2 PFS (A) and OS (B) of patients with HGBL-NOS (n=41). Abbreviations: PFS, progression-free survival; OS, overall survival. |
All 41 patients received therapy which included R-CHOP regimen (n=17), DA-EPOCH-R regimen (n=8), R-CODOX-M/IVAC regimen (n=8), and R-Hyper-CVAD regimen (n=8). Fifteen (36.6%) patients, including 5 cases that received R-CHOP regimen, and 10 of other regimens, were treated with a CNS prophylaxis via an intrathecal injection. Six of the 41 (14.6%) patients received consolidation with an ASCT and 2 (4.9%) received a CAR-T therapy after disease relapse. The median number of chemotherapy cycles was 4 (range: 4 cycles–6 cycles). All 41 patients who were enrolled were evaluated for efficacy. Efficacy measures included CR (13 cases, 31.7%), PR (11 cases, 26.8%), SD (1 case, 2.4%), and PD (16 cases, 39.0%), and the ORR was 58.5%. The 41 patients were divided into 2 groups based on the therapeutic regimens (R-CHOP or high-intensity chemotherapy). Their baseline characteristics have been listed in Table 3. Compared to patients treated with the R-CHOP regimen, those treated with a high-intensity chemotherapy (DA-EPOCH-R, R-Hyper-CVAD, or R-CODOX-M/IVAC) had superior PFS and OS (PFS: χ2=4.173, P=0.041; OS: χ2=5.200, P=0.023) (Figure 3A and B).
 |
Table 3 Patients Characteristics (n=41) Based on Therapeutic Regimens |
We performed subgroup analyses on DEL and non-DEL of HGBL, NOS. The PFS and OS of the patients who were non-DEL were superior to those of the DEL group (χ2=7.785, P=0.005 for PFS; χ2=4.563, P=0.033 for OS) (Figure 4A and B). The patients were then divided into 2 groups, based on whether they were diagnosed with SHL. Analyses showed a significant tendency toward better PFS and OS for patients who were non-SHL than those who were diagnosed with SHL (χ2=4.247, P=0.039 for PFS, χ2=4.955, P=0.026 for OS) (Figure 5A and B). We also analyzed the association of PFS and OS with patient clinical features (such as sex of the individual, age, the ECOG, stage, the IPI score, the B symptoms, the LDH level, the β2 microglobulin level, and the extranodal sites), with results showing that patients with a high IPI scores (>2) had inferior PFS (χ2=6.482, P=0.011) and OS (χ2=10.156, P=0.001) (Figure 6A and B). The other factors were not independent prognostic factors (Table 4).
 |
Table 4 Univariate Analysis Results of the Clinical and Pathological Characteristics of Patients with HGBL, NOS |
Discussion
HGBL, NOS belong to a heterogeneous category of rare, invasively mature B-cell lymphomas that are often difficult to classify during pathological diagnosis.16,17 In 2001, the WHO classified HGBL, NOS as the “atypical Burkitt’s lymphoma” BL subtype.18 In 2008, the WHO revised the classification of non-Hodgkin’s lymphoma, which was classified separately as BCLU, DLBCL/BL. This new classification included lymphomas that were morphologically between BL and DLBCL but presented immunophenotypes that were close to those of the BL and the lymphomas that were morphologically similar to the BL, but were BCL2 positive and with or without MYC and BCL2 and/or BCL6 rearrangements.19 In 2016, the WHO reclassified lymphomas that did not contain MYC and BCL2 or BCL6 gene rearrangements, but presented morphologies between DLBCL and BL as HGBL, NOS.3 A few researchers investigated BCLU before the revised WHO lymphoma classification of 2016, and their studies mostly included HGBL, DH/TH and HGBL, NOS. No study has investigated the features and prognosis of HGBL, NOS after the 2016 WHO revision for tumor classification. To address the lack of evidence for this type lymphoma and to improve the understanding of this type of tumor, we hereby report a study summarizing the clinical and pathologic features, and exploring the diagnosis, treatment, and prognosis of HGBL, NOS.
The morphology of tumor cells is diverse. Some cases of HGBL, NOS present cells that are similar to the BL, but the immunophenotype and genetic characteristics are inconsistent with the BL. In other cases, the immunophenotype is consistent with the BL, but the size of the nuclei range between that in the BL and in the DLBCL.7,8 HGBL, NOS often shows a complex karyotype, with Ki-67 scores mostly >90% and no genetic rearrangement of MYC, BCL-2, or BCL-6. MYC plays an important role in the pathogenesis of cancer and its expression is elevated in up to 70% of all malignancies.20 MYC rearrangement usually results in a sustained expression of the MYC protein, which affects cell proliferation, migration, differentiation, and metabolism. Although MYC gene rearrangement is a characteristic of BL, it can also be found in the DLBCL and the BCLU.21 HGBL, DH/TH usually progresses more rapidly, is resistant to R-CHOP chemoimmunotherapy,6 and the mean OS fluctuates between 5 months and 2 years.22,23 In our study the median OS of HGBL, NOS was 18.0 months. Baryakh et al24 investigated 25 patients with BCLU and found that the 3-year OS was 62% and was lower in the DHL group than that in the non-DHL group (HGBL, NOS) (43% vs 75%, P<0.05). It seems, therefore, that HGBL, NOS has a better prognosis than HGBL, DH/TH.
HGBL, NOS appears as a blastoid or with a DLBCL/BL morphology (eventually more closely resembling BL than DLBCL), and in up to half of the DLBCL/BL cases, MYC rearrangements as one separate hit have been found.2 A retrospective study by Li et al25 found that over a median follow-up of 25 months, the OS of 61 patients with a single-hit lymphoma was poor and similar to that of patients with DHL (2-year OS: 41% vs 48%; P=0.35) and significantly worse than that of patients with DLBCL/BL without MYC rearrangements (P<0.05). Pedersen et al26 investigated an independent validation cohort of 28 patients with a MYC translocation-positive DLBCL in a retrospective analysis and found that MYC expression of >75% was associated with both reduced PFS (P=0.004) and OS (P=0.05). However, DH did not confer a worse outcome than MYC single-hit (SH). A prospective study by Landsburg et al27 found that patients with SHL appeared to have a poor prognosis (P<0.001 for PFS and OS) compared with to patients with DLBCL and BCLU with normal MYC. In our study, 16 cases of MYC rearrangements were detected by FISH, results which showed that patients with non-SHL of HGBL, NOS had a superior PFS (P=0.039) and OS (P=0.026) compared with that in the SHL. The expression of MYC and BCL2/BCL6 proteins by IHC is known as DEL. The retrospective study by Herrera et al5 reported that the 4-year PFS in patients with DEL, compared with those with non-DEL, was 48% vs 59% (P=0.049), and the 4-year OS was 56% vs 67% (P=0.10), respectively. DEL was independently associated with an inferior PFS in DLBCL. Teoh et al28 investigated 104 patients with DLBCL in a retrospective study and found that co-expression of MYC/BCL2 proteins was associated with a significantly inferior OS and an event-free survival (EFS) (P<0.05). In our study of HGBL, NOS, the results were similar to those of DLBCL. DEL has also been associated with an inferior survival, compared to that in the non-DEL.28 DEL and SHL of HGBL, NOS had poor clinical outcomes in the general population of our study.
Although the incidence of HGBL, NOS is low, accounting for 3% of the adult invasive B-cell lymphomas, and has a median age of onset of 55 (18–80) years.7 Incidences are higher in males than in females. Patients with HGBL, NOS often present with an elevated LDH, bone marrow and CNS involvement, and a high IPI. In our study, the median age of onset was 41 (12 to 79) years, and the ratio of males to females was 1.7:1. Extranodal involvement occurred in 29 (70.7%) patients, which included 4 cases of a gastrointestinal tract invasion, 13 (34.1%) of the involvement of bone marrow, and 5 (12.2%) of involvement of the central nervous system. These findings are consistent with those reported in the literature. Perry et al29 found that patients with BCLU with low IPI scores (0–2) and normal serum LDH levels had better survival rates. In agreement, our univariate analysis showed that for patients with HGBL, NOS, IPI score was an independent prognostic factor, while the other factors we investigated were not independent predictors of patient prognosis.
Due to the limited knowledge and research on HGBL, NOS, there is no international consensus on a standard therapeutic approach for this lymphoma.8 Lin et al17 investigated 52 cases of DLBCL/BL in a retrospective analysis, and showed that in cases with MYC rearrangement, the CODOX-M/IVAC or the Hyper-CVAD group had a better OS than that in the R-CHOP group. However, among patients without MYC gene rearrangements, there was no statistically significant difference between the different chemotherapy regimens. Corazzelli et al30 investigated 30 patients in a prospective analysis and found that the application of the CODOX-M/IVAC with the addition of rituximab and the liposome-encapsulated cytarabine had a better PFS outcome than that in the CODOX-M/IVAC (65% vs 37%; P=0.05). In another study, McPhail et al4 investigated 100 patients diagnosed with HGBL, R in a retrospective analysis, and showed that, compared to patients treated with other therapies, those treated with R-CODOX-M/IVAC had superior EFS12 (72% vs 39%, P=0.04), and although there was an improved OS (P=0.10), this was not significant. Taken together, these studies suggest that stronger chemotherapy regimens may improve the survival of patients with HGBL. In agreement, our study found that patients treated with intensive regimens (DA-EPOCH-R, R-CODOX-M/IVAC, or R-Hyper-CVAD) had superior PFS and OS.
Of all the patients examined in this study, 6 received transplantation after chemotherapy, resulting in a complete remission in 5 patients, and a relapse half-a-year after transplant in one patient. Of the 2 patients who received CAR-T after the disease relapsed, 1 patient received regular follow-up and 1 patient died. Herrera et al5 found that a relapsed or refractory DEL and DHL had inferior PFS after ASCT (P<0.05). For HGBL, NOS, intensive chemotherapy combined with ASCT seemed to improve the prognosis, but a larger sample size will be needed to verify this observation. CAR-T therapy is emerging as a novel treatment modality for lymphoma,31 and further investigations on applicability of CAR-T therapy in HGBL, NOS will be needed.
Conclusion
In summary, our retrospective study showed that HGBL, NOS were highly malignant tumors that were associated with short survival. We suggest that a high-intensity chemotherapy combined with ASCT may prolong the survival of patients and that DEL and SHL appear to be associated with poor prognoses. Moreover, we suggest that an IPI score >2 may be a poor prognostic factor. However, due to the limitations inherent in our small sample size and retrospective analysis, we propose further prospective and large-scale studies to clarify the clinical outcomes, effective treatments, and survival durations of patients with HGBL, NOS.
Ethics
This study was approved by the Ethics Committee of The First Affiliated Hospital of Zhengzhou University, People’s hospital of Zhengzhou University, and The Affiliated cancer Hospital of Zhengzhou University (Henan Cancer Hospital). Informed consent for the collection of medical information was obtained from all patients. All procedures performed in the study were in accordance with the ethical standards of the institutional research committee. The patient consent was written informed consent, and that this study was conducted in accordance with the Declaration of Helsinki.
Acknowledgments
The authors wish to thank Wencai Li, Guannan Wang, and Wugan Zhao for participating in pathological diagnosis.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Alsharif R, Dunleavy K. Burkitt lymphoma and other high-grade B-cell lymphomas with or without MYC, BCL2, and/or BCL6 rearrangements. Hematol Oncol Clin North Am. 2019;33(4):587–596. doi:10.1016/j.hoc.2019.04.001
2. Szumera-cieckiewicz A, Rymkiewicz G, Grygalewicz B, et al. Comprehensive histopathological diagnostics of aggressive B-cell lymphomas based on the updated criteria of the World Health Organisation’s 2017 classification. Polish J Pathol. 2018;69(1):1–19. doi:10.5114/pjp.2018.75332
3. Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–2390. doi:10.1182/blood-2016-01-643569
4. McPhail ED, Maurer MJ, Macon WR, et al. Inferior survival in high-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements is not associated with MYC/IG gene rearrangements. Haematologica. 2018;103(11):1899–1907. doi:10.3324/haematol.2018.190157
5. Herrera AF, Mei M, Low L, et al. Relapsed or refractory double-expressor and double-hit lymphomas have inferior progression-free survival after autologous stem-cell transplantation. J clin oncol. 2017;35(1):24–31. doi:10.1200/JCO.2016.68.2740
6. Hilton LK, Tang J, Ben-neriah S, et al. The double-hit signature identifies double-hit diffuse large B-cell lymphoma with genetic events cryptic to FISH. Blood. 2019;134(18):1528–1532. doi:10.1182/blood.2019002600
7. Li S, Lin P, Medeiros LJ. Advances in pathological understanding of high-grade B cell lymphomas. Expert Rev Hematol. 2018;11(8):637–648. doi:10.1080/17474086.2018.1494567
8. Novo M, Castellino A, Nicolosi M, et al. High-grade B-cell lymphoma: how to diagnose and treat. Expert Rev Hematol. 2019;12(7):497–506. doi:10.1080/17474086.2019.1624157
9. Swerdlow SH. Diagnosis of ‘double hit’ diffuse large B-cell lymphoma and B-cell lymphoma, unclassifiable, with features intermediate between DLBCL and Burkitt lymphoma: when and how, FISH versus IHC. Hematol Am Soc Hematol Educ Prog. 2014;2014(1):90–99. doi:10.1182/asheducation-2014.1.90
10. Rosenthal A, Younes A. High grade B-cell lymphoma with rearrangements of MYC and BCL2 and/or BCL6: double hit and triple hit lymphomas and double expressing lymphoma. Blood Rev. 2017;31(2):37–42. doi:10.1016/j.blre.2016.09.004
11. sarkozy C, Traverse-glehen A, Coiffier B. Double-hit and double-protein-expression lymphomas: aggressive and refractory lymphomas. Lancet Oncol. 2015;16(15):e555–e567. doi:10.1016/S1470-2045(15)00005-4
12. Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103(1):275–282. doi:10.1182/blood-2003-05-1545
13. Schmitz N, Zeynalova S, Nickelsen M, et al. CNS international prognostic index: a risk model for CNS relapse in patients with diffuse large B-cell lymphoma treated with R-CHOP. J clin oncol. 2016;34(26):3150–3156. doi:10.1200/JCO.2015.65.6520
14. Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J clin oncol. 2007;25(5):579–586. doi:10.1200/JCO.2006.09.2403
15. Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J clin oncol. 2014;32(27):3059–3068. doi:10.1200/JCO.2013.54.8800
16. Taylor J, Xiao W, Abdel-wahab O. Diagnosis and classification of hematologic malignancies on the basis of genetics. Blood. 2017;130(4):410–423. doi:10.1182/blood-2017-02-734541
17. Lin P, Dickason TJ, Fayad LE, et al. Prognostic value of MYC rearrangement in cases of B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and Burkitt lymphoma. Cancer. 2012;118(6):1566–1573. doi:10.1002/cncr.v118.6
18. McClure RF, Remstein ED, Macon WR, et al. Adult B-cell lymphomas with burkitt-like morphology are phenotypically and genotypically heterogeneous with aggressive clinical behavior. Am J Surg Pathol. 2005;29(12):1652–1660. doi:10.1097/01.pas.0000180442.87022.08
19. Hoeller S, Copie-bergman C. Grey zone lymphomas: lymphomas with intermediate features. Adv Hematol. 2012;2012:460801. doi:10.1155/2012/460801
20. Klapproth K, Wirth T. Advances in the understanding of MYC-induced lymphomagenesis. Br J Haematol. 2010;149(4):484–497. doi:10.1111/bjh.2010.149.issue-4
21. Johnson NA, Slack GW, Savage KJ, et al. Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J clin oncol. 2012;30(28):3452–3459. doi:10.1200/JCO.2011.41.0985
22. Cohen JB, Geyer SM, Lozanski G, et al. Complete response to induction therapy in patients with Myc-positive and double-hit non-Hodgkin lymphoma is associated with prolonged progression-free survival. Cancer. 2014;120(11):1677–1685. doi:10.1002/cncr.28642
23. Tomita N, Tokunaka M, Nakamura N, et al. Clinicopathological features of lymphoma/leukemia patients carrying both BCL2 and MYC translocations. Haematologica. 2009;94(7):935–943. doi:10.3324/haematol.2008.005355
24. Baryakh EA, Misyurina AE, Kovrigina AM, et al. Diagnosis and treatment in patients with B-cell lymphoma unclassified that is intermediate between diffuse large B-cell lymphoma and Burkitt’s lymphoma. Ter Arkh. 2015;87(8):77–85. doi:10.17116/terarkh201587877-85
25. Li S, Weiss VL, Wang XJ, et al. High-grade B-cell lymphoma with MYC rearrangement and without BCL2 and BCL6 rearrangements is associated with high P53 expression and a poor prognosis. Am J Surg Pathol. 2016;40(2):253–261. doi:10.1097/PAS.0000000000000542
26. Pedersen MO, Gang AO, Clasen-linde E, et al. Stratification by MYC expression has prognostic impact in MYC translocated B-cell lymphoma-Identifies a subgroup of patients with poor outcome. Eur J Haematol. 2019;102(5):395–406. doi:10.1111/ejh.13219
27. Landsburg DJ, Falkiewicz MK, Petrich AM, et al. Sole rearrangement but not amplification of MYC is associated with a poor prognosis in patients with diffuse large B cell lymphoma and B cell lymphoma unclassifiable. Br J Haematol. 2016;175(4):631–640. doi:10.1111/bjh.2016.175.issue-4
28. Teoh CS, Lee SY, Chiang SK, Chew TK, Goh AS. Impact of double expression of C-MYC/BCL2 protein and cell of origin subtypes on the outcome among patients with diffuse large B-cell lymphoma: a single Asian center experience. Asian Pac J Cancer Prev. 2018;19(5):1229–1236. doi:10.22034/APJCP.2018.19.5.1229
29. Perry AM, Crockett D, Dave BJ, et al. B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and burkitt lymphoma: study of 39 cases. Br J Haematol. 2013;162(1):40–49. doi:10.1111/bjh.2013.162.issue-1
30. Corazzelli G, Frigeri F, Russo F, et al. RD-CODOX-M/IVAC with rituximab and intrathecal liposomal cytarabine in adult Burkitt lymphoma and ‘unclassifiable’ highly aggressive B-cell lymphoma. Br J Haematol. 2012;156(2):234–244. doi:10.1111/bjh.2011.156.issue-2
31. Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531–2544. doi:10.1056/NEJMoa1707447
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




