Back to Journals » OncoTargets and Therapy » Volume 12
High feasibility of cytological specimens for detection of ROS1 fusion by reverse transcriptase PCR in Chinese patients with advanced non-small-cell lung cancer
Authors Zhang L, Wang Y, Zhao C, Shi J , Zhao S, Liu X, Jia Y, Zhu T, Jiang T, Li X, Zhou C
Received 19 December 2018
Accepted for publication 1 March 2019
Published 1 May 2019 Volume 2019:12 Pages 3305—3311
DOI https://doi.org/10.2147/OTT.S198827
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr William C. Cho
Limin Zhang,1,2,* Yan Wang,1,* Chao Zhao,3,* Jinpeng Shi,1 Sha Zhao,1 Xiaozhen Liu,1 Yijun Jia,1 Tao Zhu,4 Tao Jiang,1 Xuefei Li,3 Caicun Zhou1
1Department of Medical Oncology, Shanghai Pulmonary Hospital & Thoracic Cancer Institute, Tongji University School of Medicine, Shanghai 200433, People’s Republic of China; 2Department of Oncology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan 450052, People’s Republic of China; 3Department of Lung Cancer and Immunology, Shanghai Pulmonary Hospital, Tongji University School of Medicine, Shanghai 200433, People’s Republic of China; 4Department of Laboratory Medicine, Zhecheng People’s Hospital, Shangqiu, Henan 476200, People’s Republic of China
*These authors contributed equally to this work
Purpose: Our previous study demonstrated that cytological specimens can be used as alternative samples for detecting anaplastic lymphoma kinase (ALK) fusion with the method of reverse transcriptase PCR (RT-PCR) in patients with advanced non-small-cell lung cancer (NSCLC). The current study aimed to investigate the feasibility of cytological specimens for ROS proto-oncogene 1, receptor tyrosine kinase (ROS1) fusion detection by RT-PCR in advanced NSCLC patients.
Patients and methods: A total of 2,538 patients with advanced NSCLC, including 2,101 patients with cytological specimens and 437 patients with tumor tissues, were included in this study. All patients were screened for ROS1 fusion status by RT-PCR. The efficacy of crizotinib treatment was evaluated in ROS1 fusion-positive NSCLC patients.
Results: Among 2,101 patients with cytological specimens, the average concentration of RNA acquired from cytological specimens was 47.68 ng/µL (95% CI, 43.24–52.62), which was lower than the average of 66.54 ng/µL (95% CI, 57.18–76.60, P=0.001) obtained from 437 tumor tissues. Fifty-five patients harbored ROS1 fusion gene that was detected by RT-PCR, and 14 of them were treated with crizotinib. The incidence of ROS1 fusion was 1.95% (41/2,101) in 2,101 patients with cytological specimens, similar to the rate of 3.20% (14/437, P=0.102) for the 437 patients with tumor tissue. Regarding crizotinib treatment, no statistically significant differences were observed in the objective response rate (ORR) (81.8% vs 100%, P=0.604) between the cytological and tissue subgroups of ROS1-positive patients.
Conclusion: This study shows that cytological specimens can be utilized as alternative samples for ROS1 fusion detection by RT-PCR in advanced NSCLC patients.
Keywords: non-small-cell lung cancer, ROS proto-oncogene 1 receptor tyrosine kinase, ROS1, cytological specimens, reverse transcriptase polymerase chain reaction, RT-PCR, crizotinib
Introduction
The identification of oncogenic mutations, such as in epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK), and ROS proto-oncogene 1, receptor tyrosine kinase (ROS1), has raised great interest in small molecular tyrosine kinase inhibitors as therapeutics for non-small-cell lung cancer (NSCLC), with dramatic responses observed in patients harboring relevant driver mutations.1–6 In particular, crizotinib, the first small molecular inhibitor targeting ROS1/ALK/MET to be tested in the clinic, has dramatically changed the therapeutic landscape for ROS1 fusion-positive NSCLC.5–8 Therefore, the detection of ROS1 fusion status is a critical step in determining treatment strategy for this subgroup of patients.
ROS1 fusion gene represents a novel molecular subtype of NSCLC, accounting for ~1%–2.2% of NSCLC cases.7–12 Several methods were performed to detect this fusion gene, including fluorescence in situ hybridization (FISH), immunohistochemistry (IHC), and reverse transcriptase PCR (RT-PCR). FISH is considered to be the gold standard method for ROS1 fusion detection in clinical trials. IHC is a cost-effective screening tool to identify ROS1 fusion-positive NSCLC. In regards to ROS1 molecular testing guideline, the recommendation of the College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology guideline was that ROS1 testing should be performed on all patients with advanced lung adenocarcinoma, irrespective of their clinical characteristics, and the Expert Consensus Opinion was that IHC may be used as a screening test for ROS1 fusion status in patients with advanced lung adenocarcinoma. However, positive ROS1 IHC results should be reconfirmed by a molecular or cytogenetic method.13 Both FISH and IHC are not limited to histological tissue, but also work with cytological specimens.14–17 However, both methods require adequate quality and quantity of tumor cells; therefore, histological tissue is more suitable for screening than cytological specimens. However, advanced NSCLC patients are unsuitable for surgery or biopsy; in contrast, cytological specimens can be easily acquired. Several studies have shown that cytological specimens can be used for molecular testing in lung cancer.18,19 In addition, our previous study detected ALK fusion status from cytological specimens in as many as 79% of the NSCLC patients.20
RT-PCR is another alternative screening method that is easy to perform and highly sensitive to detect ROS1 fusion status. The Chinese Food and Drug Administration has approved the ADx ROS1 fusion gene diagnostic kit (Amoy Diagnostics, Xiamen, China) for assessing ROS1 fusion status in the clinic. Our previous studies reported a slightly higher incidence for ROS1 fusion when detected by RT-PCR than through the FISH or IHC analysis methods used by other studies.7–11,21 Furthermore, we have shown high feasibility for the detection of ALK fusion status from cytological samples by RT-PCR.20 However, the feasibility of detecting ROS1 fusion status from cytological specimens by RT-PCR remains unknown.
Hence, the purpose of this study was to investigate the feasibility of cytological samples as alternative specimens for ROS1 fusion testing by RT-PCR in advanced NSCLC patients. We compared RNA yields and the incidence of ROS1 fusion between cytological specimens and tumor tissue in 2,538 advanced NSCLC patients. Furthermore, we compared the efficacy of crizotinib treatment in ROS1-positive patients in light of different sample types.
Patients and methods
Patients and samples
This study included NSCLC patients who had histologically confirmed stage IV disease and were screened for ROS1 fusion status by RT-PCR between October 1, 2013 and June 30, 2016 at Shanghai Pulmonary Hospital, Tongji University School of Medicine. Clinical data for each patient were collected in detail as described in our previous study.8,22 Tumor responses were evaluated at 1 month after the first administration of crizotinib (250 mg, twice daily) and then after every two cycles thereafter on the basis of the Response Evaluation Criteria in Solid Tumors (version 1.1). An informed consent form was signed by each patient before the initiation of any study-related procedure. This study was approved by the Shanghai Pulmonary Hospital Ethics Committee. This study was conducted in accordance with the Declaration of Helsinki.
Specimen preparation and RNA extraction
All samples were confirmed by pathologists. Tumor tissues were stored in formalin-fixed, paraffin-embedded blocks until use. The details of all cytological specimens and tumor tissue preparation were listed in our previous studies.20,22 RNA was extracted from cytological specimens and tumor tissue using either an RNeasy Mini Kit (Qiagen, Hilden, Germany) or an AmoyDx RNA Kit (Amoy Diagnostics) according to the manufacturer’s protocol. The quantity and quality of RNA was subsequently determined on a NanoDrop 2000 Spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA).
ROS1 fusion detection
ROS1 fusion was detected by using an AmoyDx® ROS1 fusion gene detection kit (Amoy Diagnostics). Detailed methods are provided in our previous studies.8,9,22 Briefly, mRNA extracted from cytological specimens and tumor tissue was reverse transcribed to cDNA at 42°C, and then amplified by PCR. The RT-PCR conditions were as follows: 95°C for 5 minutes, 15 cycles of denaturation at 95°C for 25 seconds, annealing at 64°C for 20 seconds, and elongation at 72°C for 20 seconds to ensure specificity, and then up to 31 cycles at 93°C for 25 seconds, 60°C for 35 seconds (data collection), and 72°C for 20 seconds. Patterns of ROS1 fusion were screened as previously described.8,9
Statistical analysis
All statistical analyses were carried out using SPSS version 22.0 (IBM, Armonk, NY, USA). Student’s t-test was used for comparisons between two different groups, and a P-value of <0.05 was considered statistically significant in a two-way analysis.
Results
Summary of specimens acquired
From October 1, 2013 to June 30, 2016, 2,538 patients with advanced NSCLC who received ROS1 fusion screening by RT-PCR, including 437 (17.2%) with tumor tissue and 2,101 (82.8%) with cytological specimens, were enrolled into our study. A total of 55 (55/2,538, 2.2%) patients were ROS1 fusion-positive. Of these, 51 (51/55, 92.7%) were further verified by direct sequencing, and no false-positive cases were found. CD74-ROS1 fusion was found in 21 cases, EZR-ROS1 fusion in 13 cases, SLC34A2–ROS1 fusion in nine cases, SDC4-ROS1 in seven cases, and GOPC-ROS1 in one case.
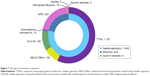
Among the 437 patients from whom tumor tissue was collected, 128 patients had paired cytological specimens and were only represented in the tumor tissue group for statistical analyses. Among the 2,101 patients with cytological specimens, 1,648 were collected by needle aspiration, 449 were by effusion samples, and four were by sputum samples. Of the samples collected by needle aspiration, 1,197 were by computed tomography-guided transthoracic needle aspiration (TTNA), 190 were by endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA), 251 were by needle aspiration of superficial lymph nodes (SLN-NA), and ten were by needle aspiration of subcutaneous nodules. Of the effusion samples, 429 were malignant pleural effusions (MPEs), 15 were pericardial effusions, and five were ascites. The types of specimen acquisition methods are also illustrated in Figure 1.
RNA concentrations and ROS1 fusion detection
In this study, we adopted spectrophotometry to evaluate the RNA concentrations obtained (Table 1). Among the 2,101 cytological specimens, the average, minimum, and maximum RNA concentrations per collection method were 36.13, 0.09, and 593.34 ng/μL for TTNA; 31.93, 0.09, and 485.54 ng/μL for EBUS-TBNA; 34.74, 0.31, and 446.63 ng/μL for SLN-NA; 26.14, 5.30, and 89.60 ng/μL for NA from subcutaneous nodules; 12.51, 5.12, and 20.60 ng/μL for sputum samples; 93.38, 0.60, and 3,768.04 ng/μL for MPE samples; 92.31, 0.64, and 296.06 ng/μL for pericardial effusions; and 76.80, 39.89, and 151.11 ng/μL for ascites. For the 437 tumor tissue samples, the average, minimum, and maximum RNA concentrations were 66.50, 0.14, and 786.62 ng/μL. Across all 2,101 cytological specimens, the average RNA concentration was 47.68 ng/μL (95% CI, 43.24–52.62), which was significantly lower than 66.54 ng/μL (95% CI, 57.18–76.60, P=0.001) obtained from the 437 tumor tissue samples (Figure 2). However, the incidence rates of ROS1 fusion were similar in both tissue types: 1.95% (41/2,101) in cytological specimens vs 3.20% (14/437, P=0.102) in tumor tissues.
  | Figure 2 Comparison of average concentrations of RNA between cytological group and tissue group. |
Among the 55 ROS1-positive patients, 14 had tumor tissue samples and 41 had cytological specimens, including 17 collected by TTNA, two collected by EBUS-TBNA, ten collected by SLN-NA, and 12 collected by MPE (Table 1). For all these ROS1-positive patients, the RNA concentrations for specimens ranged from 1.59 ng/μL to 355.85 ng/μL. For 14 ROS1-positive patients with tumor tissue, the range of RNA concentrations was from 6.37 ng/μL to 355.85 ng/μL. For 41 ROS1-positive patients, RNA concentrations ranged from 1.59 ng/μL to 191.28 ng/μL. The minimum RNA concentration obtained from an MPE specimen was determined, and its fusion status was reconfirmed by direct sequencing as SLC34A2–ROS1 (E4; E32).
In our study, 128 patients had paired cytological and tissue samples, including four ROS1 fusion-positive samples. In order to analyze the concordance rate of ROS1 fusion status between tissue specimens and paired cytological specimens, we chose the only 4 samples with ROS1-positive and 4 with ROS1-negative that were randomly selected, all of which were detected by tumor tissue. The consistency with regard to ROS1 fusion detection between tumor tissue samples and paired cytological samples was 100%.
The efficacy of crizotinib
Totally, 14 of the 55 patients identified as ROS1 RT-PCR-positive received the treatment of crizotinib, including eleven with cytological specimens and three with tissue. For eleven patients with cytological specimens, six were female and one had brain metastases. For three patients with tumor tissue, none were female and none had brain metastases. Among the eleven patients with cytological specimens, nine had a partial response and two got a stable disease. Among the three patients with tumor tissue, one showed a complete response and two showed a partial response. Three patients with cytological specimens were lost to follow-up in November 2016. Thus, the objective response rate (ORR) was 81.8% in patients with cytological specimens, similar to the ORR of 100% (P=0.604) obtained for the three patients with tumor tissue.
Discussion
To the best of our knowledge, this is the first large-scale retrospective study to comprehensively explore the feasibility of detecting ROS1 fusion status by RT-PCR from cytological specimens. We compared RNA yields and the incidence rates of ROS1 fusion between tumor tissue samples from 437 patients and cytological specimens from 2,101 patients. Among the cytological specimens, the average RNA concentration was 47.68 ng/μL (95% CI, 43.24–52.62), which was lower than the average of 66.54 ng/μL (95% CI, 57.18–76.60, P=0.001) obtained from tumor tissues. However, no statistically significant difference was observed in ROS1 fusion incidence between cytological specimens and tumor tissues. In addition, there were no statistically significant differences in ORR with respect to sample type for ROS1-positive patients treated with crizotinib. Taken together, these results support the fact that RT-PCR of cytological specimens can be used to detect ROS1 fusion status in advanced NSCLC patients.
A large retrospective survey of Asian populations indicated that EGFR mutation status was detectable using cytological samples in no fewer than 50% of NSCLC patients.23 Similarly, another study demonstrated that cytological samples can be used successfully for EGFR mutation analysis in lung cancer.24 In addition, we found in a previous study that EBUS-guided needle aspiration can be used to perform molecular analyses for ERCC1, RRM1, and BRCA1.25 Similarly, Zhao et al showed that pleural, ascitic, or pericardial effusions of advanced lung adenocarcinoma can be used for detecting ALK, ROS1, and RET fusion status.26 Finally, Wang et al indicated a high feasibility for the detection of ALK fusion status by RT-PCR from cytological specimens, which might also be considered as a feasible sample source for ALK detection in advanced NSCLC patients.20 Hence, cytological samples may be used for molecular analyses in clinical practice among NSCLC patients.
Till date, no studies have investigated whether ROS1 fusion testing can be performed in the cytological specimens of advanced NSCLC patients. We found that the RNA yields of cytological specimens were significantly lower than those from tumor tissue. Nevertheless, the incidence rates of ROS1 fusion were similar (1.95% vs 3.20%, P=0.102) between patients with cytological specimens and with tumor tissue. Thus, we can conclude that cytological specimens can be used for ROS1 fusion detection among patients with advanced NSCLC.
Numerous previous studies have found that FISH, IHC, and RT-PCR can all reliably detect ROS1 fusion status in advanced NSCLC patients, with concordance rates above 90%.27–31 Wang et al suggested that the high concordance (99.2%) between FISH and RT-PCR results supports considering RT-PCR as an alternative method for detecting ALK fusion status.32 They also reported that advanced NSCLC patients who are ALK fusion-positive, detected by RT-PCR, achieved similar clinical responses to crizotinib compared to those detected by FISH; furthermore, by using RT-PCR approach, two ALK-positive patients responded to crizotinib who would otherwise be missed by FISH testing.32 In a Phase II study of crizotinib, East Asian advanced NSCLC patients had their ROS1 fusion status assessed through RT-PCR, and the ROS1-positive patients who were treated with crizotinib achieved clinically marked benefits and durable responses.6 Building upon these findings, we adopted the RT-PCR approach for detecting ROS1 fusion status and found no statistically significant differences in ORR upon crizotinib treatment of patients from the cytological and tissue subgroups.
We must mention that there are several limitations to this study. Firstly, this was a retrospective study, and selection bias was inevitable. The ROS1 fusion incidence of 2.2% in our study was slightly higher than for several previous reports, which was partially attributable to some patients who were selected from the wild-type EGFR and ALK population. Secondly, due to the limited number of ROS1-positive patients who received crizotinib in our study, the conclusion on its efficacy should be viewed cautiously. Thirdly, more detection methods, such as FISH and IHC, should be adopted to validate our conclusions.
However, taken together, the results of the current study demonstrate that cytological specimens can be used as alternative samples for detecting ROS1 fusion status by RT-PCR in advanced NSCLC patients.
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (No 81672286), the Ministry of Science and Technology of the People’s Republic of China (No 2016YFC0902302), the Shanghai Committee of Science and Technology (Nos 16JC1405900 and 16411964400), and the Shanghai Hygiene and Health Committee’s Key Discipline Project of Respiratory Diseases (No 2017ZZ02012).
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–957. doi:10.1056/NEJMoa0810699 | ||
Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239–246. doi:10.1016/S1470-2045(11)70393-X | ||
Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735–742. doi:10.1016/S1470-2045(11)70184-X | ||
Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371(23):2167–2177. doi:10.1056/NEJMoa1408440 | ||
Shaw AT, Ou SH, Bang YJ, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med. 2014;371(21):1963–1971. doi:10.1056/NEJMoa1406766 | ||
Wu YL, Yang JC, Kim DW, et al. Phase II study of crizotinib in East Asian patients with ROS1-positive advanced non-small-cell lung cancer. J Clin Oncol. 2018;36(14):1405–1411. doi:10.1200/JCO.2017.75.5587 | ||
Bergethon K, Shaw AT, Ou SH, et al. ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol. 2012;30(8):863–870. doi:10.1200/JCO.2011.35.6345 | ||
Zhang L, Jiang T, Zhao C, et al. Efficacy of crizotinib and pemetrexed-based chemotherapy in Chinese NSCLC patients with ROS1 rearrangement. Oncotarget. 2016;7(46):75145–75154. | ||
Cai W, Li X, Su C, et al. ROS1 fusions in Chinese patients with non-small-cell lung cancer. Ann Oncol. 2013;24(7):1822–1827. doi:10.1093/annonc/mdt071 | ||
Takeuchi K, Soda M, Togashi Y, et al. RET, ROS1 and ALK fusions in lung cancer. Nat Med. 2012;18(3):378–381. doi:10.1038/nm.2658 | ||
Davies KD, Le AT, Theodoro MF, et al. Identifying and targeting ROS1 gene fusions in non-small cell lung cancer. Clin Cancer Res. 2012;18(17):4570–4579. doi:10.1158/1078-0432.CCR-12-0550 | ||
Scheffler M, Schultheis A, Teixido C, et al. ROS1 rearrangements in lung adenocarcinoma: prognostic impact, therapeutic options and genetic variability. Oncotarget. 2015;6(12):10577–10585. doi:10.18632/oncotarget.3387 | ||
Kalemkerian GP, Narula N, Kennedy EB, et al. Molecular testing guideline for the selection of patients with lung cancer for treatment with targeted tyrosine kinase inhibitors: American Society of Clinical Oncology Endorsement of the College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology Clinical Practice Guideline Update. J Clin Oncol. 2018;36(9):911–919. doi:10.1200/JCO.2017.76.7293 | ||
Bozzetti C, Nizzoli R, Tiseo M, et al. ALK and ROS1 rearrangements tested by fluorescence in situ hybridization in cytological smears from advanced non-small cell lung cancer patients. Diagn Cytopathol. 2015;43(11):941–946. doi:10.1002/dc.23318 | ||
Savic S, Bubendorf L. Role of fluorescence in situ hybridization in lung cancer cytology. Acta Cytol. 2012;56(6):611–621. doi:10.1159/000339792 | ||
Vlajnic T, Savic S, Barascud A, et al. Detection of ROS1-positive non-small cell lung cancer on cytological specimens using immunocytochemistry. Cancer Cytopathol. 2018;126(6):421–429. doi:10.1002/cncy.21983 | ||
Kuroda N, Tamiya H, Nakatani K, et al. Cytological findings of ROS1-rearranged lung adenocarcinoma. Diagn Cytopathol. 2018;46(4):336–339. doi:10.1002/dc.23845 | ||
Rooper LM, Nikolskaia O, Carter J, Ning Y, Lin MT, Maleki Z. A single EBUS-TBNA procedure can support a large panel of immunohistochemical stains, specific diagnostic subtyping, and multiple gene analyses in the majority of non-small cell lung cancer cases. Hum Pathol. 2016;51:139–145. doi:10.1016/j.humpath.2015.12.025 | ||
Carter J, Miller JA, Feller-Kopman D, Ettinger D, Sidransky D, Maleki Z. Molecular profiling of malignant pleural effusion in metastatic non-small-cell lung carcinoma. The effect of preanalytical factors. Ann Am Thorac Soc. 2017;14(7):1169–1176. | ||
Wang Y, Liu Y, Zhao C, et al. Feasibility of cytological specimens for ALK fusion detection in patients with advanced NSCLC using the method of RT-PCR. Lung Cancer. 2016;94:28–34. doi:10.1016/j.lungcan.2016.01.014 | ||
Rimkunas VM, Crosby KE, Li D, et al. Analysis of receptor tyrosine kinase ROS1-positive tumors in non-small cell lung cancer: identification of a FIG-ROS1 fusion. Clin Cancer Res. 2012;18(16):4449–4457. doi:10.1158/1078-0432.CCR-11-3351 | ||
Zhang L, Jiang T, Li X, et al. Clinical features of bim deletion polymorphism and its relation with crizotinib primary resistance in Chinese patients with ALK/ROS1 fusion-positive non-small cell lung cancer. Cancer. 2017;123(15):2927–2935. doi:10.1002/cncr.30677 | ||
Yatabe Y, Kerr KM, Utomo A, et al. EGFR mutation testing practices within the Asia Pacific region: results of a multicenter diagnostic survey. J Thorac Oncol. 2015;10(3):438–445. doi:10.1097/JTO.0000000000000422 | ||
Ozluk Y, Firat P, Yegen G, Hocaoglu J, Tas S, Yilmazbayhan D. EGFR mutation testing using archival-stained smears in non-small cell lung carcinoma. Cytopathology. 2017;28(1):35–45. doi:10.1111/cyt.12357 | ||
Schmid-Bindert G, Wang Y, Jiang H, et al. EBUS-TBNA provides highest RNA yield for multiple biomarker testing from routinely obtained small biopsies in non-small cell lung cancer patients – a comparative study of three different minimal invasive sampling methods. PLoS One. 2013;8(10):e77948. doi:10.1371/journal.pone.0077948 | ||
Zhao C, Li X, Li J, et al. Detecting ALK, ROS1 and RET fusion genes in cell block samples. Transl Oncol. 2014;7(3):363–367. doi:10.1016/j.tranon.2014.04.013 | ||
Bubendorf L, Buttner R, Al-Dayel F, et al. Testing for ROS1 in non-small cell lung cancer: a review with recommendations. Virchows Arch. 2016;469(5):489–503. doi:10.1007/s00428-016-2000-3 | ||
Wu J, Lin Y, He X, et al. Comparison of detection methods and follow-up study on the tyrosine kinase inhibitors therapy in non-small cell lung cancer patients with ROS1 fusion rearrangement. BMC Cancer. 2016;16:599. doi:10.1186/s12885-016-2582-9 | ||
Cao B, Wei P, Liu Z, et al. Detection of lung adenocarcinoma with ROS1 rearrangement by IHC, FISH, and RT-PCR and analysis of its clinicopathologic features. Onco Targets Ther. 2016;9:131–138. doi:10.2147/OTT.S94997 | ||
Kao HL, Yeh YC, Lin CH, et al. Diagnostic algorithm for detection of targetable driver mutations in lung adenocarcinomas: comprehensive analyses of 205 cases with immunohistochemistry, real-time PCR and fluorescence in situ hybridization methods. Lung Cancer. 2016;101:40–47. doi:10.1016/j.lungcan.2016.09.007 | ||
Shan L, Lian F, Guo L, et al. Detection of ROS1 gene rearrangement in lung adenocarcinoma: comparison of IHC, FISH and real-time RT-PCR. PLoS One. 2015;10(3):e0120422. doi:10.1371/journal.pone.0120422 | ||
Wang Y, Zhang J, Gao G, et al. EML4-ALK fusion detected by RT-PCR confers similar response to crizotinib as detected by FISH in patients with advanced non-small-cell lung cancer. J Thorac Oncol. 2015;10(11):1546–1552. doi:10.1097/JTO.0000000000000668 |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.