Back to Journals » Cancer Management and Research » Volume 12
High Expression of Nuclear Transcription Factor-κB is Associated with Cisplatin Resistance and Prognosis for Ovarian Cancer
Received 8 June 2020
Accepted for publication 5 August 2020
Published 9 September 2020 Volume 2020:12 Pages 8241—8252
DOI https://doi.org/10.2147/CMAR.S265531
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Chien-Feng Li
Yanyan Kan,1– 4 Juntian Liu,1– 4 Fangxuan Li1– 4
1Department of Cancer Prevention, Tianjin Medical University Cancer Institute and Hospital, Tianjin, People’s Republic of China; 2National Clinical Research Center for Cancer, Tianjin, People’s Republic of China; 3Key Laboratory of Cancer Prevention and Therapy, Tianjin, People’s Republic of China; 4Tianjin’s Clinical Research Center for Cancer, Tianjin, People’s Republic of China
Correspondence: Fangxuan Li
Department of Cancer Prevention, Tianjin Medical University Cancer Institute and Hospital, Huanhuxi Road, Hexi District, Tianjin 300060, People’s Republic of China
Tel/Fax +86-22-23340123
Email [email protected]
Background: Abnormal activation of the nuclear transcription factor-κB (NF-κB) signaling pathway plays a crucial role in the chemoresistance of tumor cells. This study aimed to explore the significance of NF-κB in the chemoresistance of ovarian cancer.
Materials: We performed immunohistochemical staining for evaluating the expression of NF-κB in cancer tissues. The MTT assay was performed for analyzing cell proliferation, Western blotting was performed to quantify NF-κB p65, and flow cytometry was used to determine the apoptosis rate.
Results: Nuclear NF-κB p65 over-expression was closely associated with ovarian cancer with advanced FIGO stage, residual disease ≥ 1 cm, low histologic grade, platinum resistance and refractory, chemotherapy resistance (P< 0.05). FIGO stage I–II and residual disease < 1 cm were associated with complete response (CR) to chemotherapy, while FIGO stage I–II, residual disease < 1cm and absence of lymph node (LN) metastasis were associated with platinum sensitivity. In multivariate logistic regression, residual disease ≥ 1 cm was a risk factor for response to chemotherapy, while the over-expression of nuclear NF-κB p65 was a risk factor for sensitivity to chemotherapy. In the ROC curves, nuclear NF-κB p65 expression had the discriminative ability for sensitivity to chemotherapy (AUC = 0.637, P = 0.021). Furthermore, nuclear NF-κB p65 expression was an independent prognostic factor. Western blotting showed that NF-κB p65 level in cisplatin-resistant cells (C13* and A2780cp) was significantly higher than that in cisplatin-sensitive cells (OV2008 and A2780s) (P < 0.05), and this increased expression could be suppressed by NF-κB inhibitor-PDTC treatment. The proliferation inhibitory rates of cisplatin in C13* and A2780cp cells increased after PDTC treatment in a concentration-dependent manner. PDTC treatment could also enhance cisplatin-induced apoptosis.
Conclusion: NF-κB was associated with the clinicopathological features, chemoresistance, and prognosis of ovarian cancer. The NF-κB inhibitor PDTC can enhance cisplatin sensitivity of platinum-resistant C13* and A2780cp ovarian cancer cells.
Keywords: ovarian cancer, NF-κB, cisplatin resistance, chemoresistance, PDTC
Background
Ovarian cancer is the most lethal gynecologic malignant tumor all over world, as well as the fourth cause of cancer-related deaths in female.1 Due to insidious symptoms and lack of an effective screening regimen, most women are detected in advanced stages (involving stage III and IV).2 Ovarian cancer is mainly treated by complete surgical debulking combined with platinum-based chemotherapy.3 Although platinum-based combination chemotherapies are effective in the most of ovarian carcinoma, resistance against these chemotherapies develops usually, which drags down the therapy effects and results in poor clinical outcomes. It has been reported that 5-year survival rate for unstaged ovarian carcinoma is approximately 19%.4 Therefore, chemotherapy resistance has been an issue that needs to be addressed immediately for effective ovarian cancer treatment.5
Tumorigenesis and development of chemotherapy resistance occur due to a series of cellular changes and physiological processes involving abnormal gene regulation and signal pathway activation. Nuclear transcription factor-κB (NF-κB) has been a pivotal multifunctional transcription factor, plays a series of biological functions.6 NF-κB could activate or inhibit gene expression via binding to distinct promoters or enhancers of DNA sequences. Activation of downstream NF-κB transcription factors is able to significantly increase the cancer proliferation, inhibit apoptosis and autophagy, facilitate neovascularization that results invasion and metastasis.7,8 Recent research has demonstrated that alteration in NF-κB pathway also play a crucial role in the chemoresistance of cancer.
Recently, an increasing number of studies are focusing on the importance of NF-κB family in ovarian cancer. For example, there is a study about the role of NF-κB cluster in the progression of ovarian carcinoma.9 Some studies confirmed the expression of NF-κB subunits in ovarian carcinoma tissues.10,11 Furthermore, several reports had studied the association between NF-κB and chemoresistance in ovarian cancer. Wang et al found that over-expression of NF-κB is risk factors for platinum resistance in ovarian carcinoma.12 Koti et al used DNA microarray analysis to find that the IGF-1/PI3K/NF-κB/ERK signaling pathways were involved in chemoresistance.13 Shuang et al explored that over-expression of nuclear NF-κB is a significant risk factor related with chemotherapy resistance and the poor prognosis of serous epithelial ovarian cancer in cancer tissues.14 Wang et al found that the inhibitor of PARP-1, PJ34, could significantly depress the proliferation and invasion of C13* cells, which may result in PARP-1-regulated NF-κB activity.15 Peng et al explored in ovarian cancer that chemotherapy induces immune toleration via NF-κB regulated PD-L1 over-expression.5
However, there is a rare experimental study directly validating the increasing expression of NF-κB in the chemoresistance of ovarian carcinoma. Thus, our study aimed to explore the significance of NF-κB in ovarian cancer chemoresistance in patients and by in vitro experiments. First, we analyzed the association between NF-κB p65 expression and chemotherapy response, chemotherapy sensitivity, and prognosis of ovarian cancer. Next, we verified the up-regulated NF-κB p65 expression in cisplatin resistant cells (including C13* and A2780cp). Furthermore, in vitro, we also investigated the impact of NF-κB inhibitors on proliferation, apoptosis, and cisplatin resistance in C13* and A2780cp cells.
Methods
Patients
The study patients consisted of 114 patients with serous epithelial ovarian cancer diagnosed in Tianjin Medical University Cancer Institute and Hospital from January 2011 to December 2014. All human subjects were identified (or diagnosed) primary cancer with no preoperative chemotherapy. Additional malignant, inflammatory, or ischemic disease wounds were ruled out. The patients received debulking surgery and 6–8 cycles of chemotherapy (paclitaxel, combined carboplatin) and no patient was treated with anti-angiogenic therapy. Clinical data included age, pathological stage, grade, pathological subtype, and lymph node (LN) metastasis, following up data until death or March 31, 2018. Patients only with complete clinical, pathological, and follow-up data were included.
Tumor Tissues
Tumor tissues were collected from women during primary debulking surgery. Tumor samples were stored at −80°C. Patients were sensitive to chemotherapy and radiotherapy prior to debulking and standard carboplatin/paclitaxel chemotherapy.
Histopathological examination for the tumor sections was performed by a pathologist and the presence of more than 70% tumor tissue was confirmed in all samples. Histological classification of the tumors was carried according to the WHO criteria, and the disease staging was performed according to the International Federation of Gynecology and Obstetrics (FIGO) guidelines. Based on the FIGO international system, Stage I ovarian cancer is defined as those are restricted to the ovaries. Stage II is defined as which involves one or both ovaries with pelvic extension (below the pelvic brim) or primary peritoneal cancer. Stage III is defined as tumor which occurs in one or both ovaries with cytologically or histologically confirmed spread of the peritoneum outside the pelvis and/or metastasis to the retroperitoneal LN. Stage IV is defined as distant metastasis excluding peritoneal metastasis.
Assessment of Chemotherapy Response
Patients’ sensitivity to chemotherapy: According to the NCCN guideline, based on disease-free survival (DFS) after chemotherapy finished, patients are divided as platinum-sensitive (DFS > 12 months) or platinum-resistant (DFS < 6 months). Those patients who progress between 6 and 12 months after chemotherapy are defined as the tumor’s partial sensitivity to platinum.12,16,17
Tumors’ response to chemotherapy: Based on the Response Evaluation Criteria in Solid Tumors (RECIST 1.1), a clinically complete response (CR) was the disappearance of all known lesions; a ≥30% reduction in the sum of the longest diameter of the primary lesion was classified as clinically partial response (PR), ≥20% increasing of the primary lesion was classified as progressive disease (PD), stable disease (SD) was neither sufficient reduction to classified as PR nor sufficient increasing to classified as PD.18,19
Immunohistochemical Staining
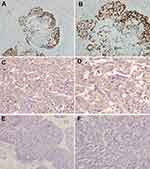
Tumor tissues were sectioned at 4 μm. Immunohistochemical staining was progressed based on the instruction manual. Firstly, heating for 30min at 56°C, then it was deparaffinized by xylene and rehydrated via graded alcohol. After heating in citrate buffer for 20 minutes and endogenous peroxidase activity was quenched in methanol and hydrogen peroxide for half an hour. The slides were incubated with NF-κB p65 rabbit anti-human polyclonal antibody (Abcam, Cambridge, UK) at a dilution of 1:50 at 4°C overnight. The primary antibody was detected by a goat anti-mouse IgG-HRP, sc-2302 (Santa Cruz, CA, USA). A DAB staining kit was used for the visualization of immunoreactive cells. The PBS was the negative control.
Nuclear expression of NF-κB p65 was ranking as following: negative is 0, weak or focal is 1, strong focal or widespread, moderate staining is 2, strong or widespread staining is 3. Tumors that got two or three were defined as nuclear positive. The NF-κB p65 cytoplasmic expression was scored as following: firstly, the color intensity was four levels, including no staining=0, light yellow=1, brown yellow=2, and dark brown=3. Then, positive cells were counted and ranked as following: the positive rate less than 5%=0, positive rate among 5%~25%=1, positive rate among 26%~50%=2, 51–75% was 3, positive rate more than 75%=4. The immunoreactive intensity and positive cell rate were then multiplied and ranked ultimately: 0~2 were classified as negative (-), 3~4 were classified as weakly positive (+), 5~8 were classified as mildly positive (++), and 9~12 were classified as strongly positive (+++).
The slides were evaluated via 2 independent pathologists to get the scoring decision.12 The representative figure of NF-κB p65 immunohistochemical staining is shown in Figure 1.
Cell Lines and Cell Culture
The C13* and A2780cp cell lines were cisplatin-resistant ovarian cancer cell lines, OV2008 and A2780s cell lines were their sensitive variant, respectively. C13* and OV2008 were gifted from Obstetrics and Gynecology Research Institute, Fudan University, China, who obtained from Chinese Type Culture Collection, Chinese Academy of Sciences.20 A2780 was purchased from the Chinese Academy of Sciences (Shanghai, China); The cisplatin-resistant ovarian cancer cell line A2780cis was purchased from ECACC (European Collection of Cell Cultures, Salisbury, UK). The four ovarian cancer cell lines were cultured in RPMI-1640 (Gibco) medium supplemented with FBS (10%), Penicillin (100 U/mL) and streptomycin (100 µg/mL) maintained at 5% CO2 at 37 °C.
Western Blotting
C13*OV2008, A2780cp, A2780s cells were treated with 30 µM PDTC and seeded in 6-well plates with a density of 25,000 cells per well. After incubation for 48h, cells were washed with PBS firstly and lysed in cell lysis buffer on ice (lysis buffer: 50 mM Tris-HCl, pH 7.4, 1% NP-40, 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM EDTA, 1 mM Na3VO4, 1 mM PMSF, 1 mM NaF, 1 μg/mL aprotinin, leupeptin and pepstatin). Following centrifugation, the dissolved cellular protein concentration was examined by a Micro BCA Protein Assay Kit (Pierce Biotechnology, Inc., USA), then solubilized in SDS buffer.
In Western blotting, proteins were separated by SDS-PAGE, then transferred to PVDF membranes. The PVDF membranes were incubated by mouse anti-human NF-κB p65 monoclonal antibody and mouse anti-human polyclonal β-actin antibody (Santa Cruz Biotechnology, Inc., USA) overnight, at 4 °C. The PVDF membrane was then incubated with the secondary antibody (Zhongshanjinqiao, Beijing, China) for 60 min. Bound HRP was examined on Super Signal West Pico chemiluminescent substrate (Pierce Biotechnology, Inc., USA).
Cell Proliferation Assay
Three thousand cells were seeded per well in 96-well plates. Each well was treated by 0 µM PDTC+ cisplatin (0, 1.25, 2.5, 5, 10 µg/mL), 10 µM PDTC + cisplatin (0, 1.25, 2.5, 5, 10 µg/mL), 30 µM PDTC + cisplatin (0, 1.25, 2.5, 5, 10 µg/mL), 50 µM PDTC + cisplatin (0, 1.25, 2.5, 5, 10 µg/mL), and same volume of cell culture medium as control. Each experiment was performed in triplicate. After 24 hours, 20 µL MTT was applied at 0, 12, 24, 48, and 72 hours. Incubated until the purple precipitate can been seen, the media was removed, then 150 µL DMSO was added. Cell proliferation at different time points was examined by the microplate reader at 490 nm.
Cell Apoptosis Analysis
Cells were cultrated in dishes and treated by RPMI 1640, 2.5 µg/mL cisplatin + RPMI 1640, 30 μM PDTC + RPMI 1640, 30 μM PDTC + 2.5 µg/mL cisplatin+ RPMI 1640. Five-microliter Annexin V-FITC and 5 µL propidium iodide (PI) solution were added to the cells resuspended with 100ul Binding Buffering (BD Pharmingen), followed by a 20-minute incubation at room temperature in dark. The stained cells were counted by flow cytometry within 30 minutes. Apoptosis rate was defined as early apoptosis (Q4) plus late apoptosis (Q2).
Statistical Analysis
The Chi-square test and Fisher’s exact test were applied for comparison of categorical variables. Continuous variables were described as mean ± SD. Statistic difference between groups was compared by independent t-test. Logistic regression was employed for the correlation between clinical features and chemotherapy response and sensitivity. The receiver operating characteristic (ROC) curve calculation was used for NF-κB expression nomogram to predict the chemotherapy response and sensitivity. The survival curves were drawn by Kaplan-Meier and Log-rank analysis. Cox proportional-hazard analysis was used for multivariate analysis to reveal the independent risk factor for survival. A P value < 0.05 was defined as statistically significance. The statistical analysis was processed via SPSS 20.0 software package.
Results
The Association Between Clinicopathological Features and NF-κB Expression in Ovarian Cancer Tissues
As shown in Table 1, over-expression of nuclear NF-κB p65 was significantly related to ovarian cancer tissues with advanced FIGO stage, residual disease ≥1 cm, low histologic grade, chemoresistance, and platinum resistance and refractory (all P < 0.05), which suggest that over-expression of nuclear NF-κB p65 is related to ovarian cancer phenotype with higher malignancy. These associations were not detected with cytoplasmic NF-κB expression.
 |
Table 1 The Association Between the Clinicopathological Feature and NF-κB P65 Expression in Ovarian Cancer Tissues |
Clinicopathological Features with Respect to Chemotherapy Response and Sensitivity in Ovarian Cancer Patients
Table 2 shows that the FIGO stage I–II and residual disease <1 cm were positively associated with CR of chemotherapy for ovarian cancer. FIGO stage I–II, no LN metastasis, as well as residual disease <1 cm were associated with platinum sensitivity positively. In addition to nuclear NF-κB p65 expression, we evaluated these clinicopathological factors by multivariate logistic analysis, which showed that residual disease ≥1 cm was associated with PR, SD, and PD and nuclear NF-Κb p65 over-expression was the risk factor for platinum resistance and refractory.
 |
Table 2 The Association Between the Clinicopathological Feature and Chemotherapy Response and Sensitivity in Ovarian Cancer Patients |
In ROC curves, nuclear expression of NF-κB p65 is capable of distinguishing chemotherapy response and platinum resistance and refractory (Area under the curves, AUC= 0.673, AUC=0.608, respectively). However, only platinum resistance and refractory were significantly predicted by nuclear NF-κB p65 expression (P = 0.021), which was in line with the multivariate logistic analysis (Figure 2, Table 3).
 |
Table 3 Multivariate Analyses of Clinicopathological Factors Respect to Chemotherapy Response and Sensitivity in Ovarian Cancer |
 |
Figure 2 The ROC curve calculation for nuclear NF-κB p65 positive expression, for chemotherapy resistance and platinum resistance and refractory disease. |
Survival Analysis of Prognostic Factors for Ovarian Cancer
Table 4 collectively reveals that histological grade, FIGO stage, LN metastasis, residual disease, response to chemotherapy, sensitivity to chemotherapy, as well as nuclear NF-κB expression are associated with overall survival (OS) as well as disease-free survival (DFS). In multivariate analysis, nuclear NF-κB p65 expression was an independent risk factor for OS, while sensitivity to chemotherapy was an independent factor of DFS (Table 5). The impact of nuclear NF-κB p65 expression on OS and DFS is shown in Figure 3.
 |
Table 4 Univariate Analyses of Prognostic Factors for OS and PFS in Ovarian Cancer |
 |
Table 5 Multivariate Analyses of Prognostic Factors for OS and PFS in Ovarian Cancer |
 |
Figure 3 The survival curves for the OS and DFS based on nuclear NF-κB p65 expression in ovarian cancer patients. |
NF-κB Expression in Ovarian Cancer Cell Lines
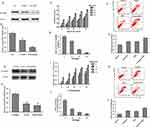
Considering the significant impact of nuclear NF-κB on clinical chemotherapy response and platinum resistance, it prompted us to further determine the NF-κB p65 expression in C13*OV2008, A2780cp and A2780s cells. Western blotting was performed to analyze the NF-κB p65 expression in C13*OV2008, A2780cp and A2780s cells, and in C13* and A2780cp cells treated with NF-κB inhibitor, PDTC, as shown in Figure 4A and G.
Protein level of NF-κB p65 in cisplatin-resistant cell lines (C13* and A2780cp) were significantly higher than in the cisplatin-sensitive cell lines (OV2008 and A2780s) (0.58 ± 0.04 vs 0.36 ± 0.03, 0.52 ± 0.03 vs 0.21 ± 0.06, P < 0.05). The increased expression can be suppressed by 30 µM of PDTC treatment (0.15 ± 0.06, 0.19 ± 0.05), which decreased the expression by 74.1% in comparison to the original C13* cells and 63.5% in comparison to the original A2780cp cells (P < 0.05), as shown in Figure 4B and H.
PDTC Changes the Chemosensitivity of Cisplatin-Resistant Cells
In order to test the effect of PDTC on Cisplatin-resistance, cisplatin-resistant cells, C13* cells and A2780cp were treated with PDTC (0 µM, 10 µM, 30 µM, 50 µM) and cisplatin (0, 1.25, 2.5, 5, 10 µg/mL) at a concentration gradient for 24 hours. MTT was used to assay cell viability. The proliferation inhibitory rate of cisplatin on C13* and A2780cp cells increased with graded concentrations of PDTC, which was shown in Figure 4C and D and I and J.
Apoptosis assay using annexin V staining showed that PDTC treatment for 48 hours can enhance cisplatin-induced apoptosis compared to cisplatin alone. The apoptosis rates of C13* cells were 15.1% ± 0.17%, 23.3% ± 0.25%, 19.9% ± 0.26%, and 29.5% ± 0.38% for the control, cisplatin only, PDTC only, and cisplatin + PDTC groups, respectively (Figure 4E and F). The apoptosis rates of A2780cp cells were 10.4% ± 0.21%, 20.5% ± 0.31%, 18.9% ± 0.15%, and 27.4% ± 0.26% for the control, cisplatin only, PDTC only, and cisplatin + PDTC groups, respectively (Figure 4K and L).
Discussion
The mortality rate of ovarian cancer ranks the first high in all gynecological malignancies.1 Majority of women were in stage III or IV disease at the first diagnosis. Although novel therapy regimens and targeted therapies were developed recently, there is only a marginal improvement in the prognosis of ovarian cancer patients.21
NF-κB acts as a moderator of genes which can regulate cell proliferation and cell survival. The proteins family of NF-κB has been demonstrated to be included in several crucial progressions such as cell apoptosis and cell cycle, thus, it plays an important role in cancer progression. In cancer, mutations both in NF-κB transcription factor encoding gene or NF-κB activity control gene (such as IκB gene) have been found. Additionally, many different tumor cells active NF-κB by secreting factors, resulting in defective co-ordination between malignant cells and the normal cells.22 NF-κB is constitutively active in different types of human tumors. Guo et al confirmed that NF-κB p65 in ovarian cancer is mostly nuclear and that its levels correlated positively with poor differentiation.11 In our study, over-expression of nucleus NF-κB p65 was also associated with advanced FIGO stage, residual disease ≥1 cm, low histological grade, chemoresistance, and platinum resistance and refractory disease, all of which are associated with a more aggressive ovarian cancer phenotype.
Chemoresistance is a major obstacle in therapy ovarian cancer.23 Therefore, identifying potential biomarkers for chemoresistance essential to mechanistically understand ovarian cancer. It was hypothesized that there might be pre-existing, resistant mutations that occurred before treatment, which accounting for a high frequency of platinum-resistant ovarian cancer at first relapse.24 Additionally, the interaction between the drug and tumor microenvironment may lead to a dysregulation of genes that are responsible for a variation in response to chemotherapy.17 Recent reports also have demonstrated that NF-κB also plays a crucial role in the developing of cancer cell chemoresistance. Many studies have revealed high NF-κB expression in drug-resistant cancer cells. A previous study25 found that the NF-κB expression in the drug-resistant cervical cancer cell (HeLa/B) was higher than drug-sensitive cells at baseline as well as after cisplatin induction. In ovarian cancer cell, blocking the activation of NF-κB by its inhibitor SN50 enhance the apoptosis and cisplatin sensitivity, so that increase the effect of chemotherapy.26 The study from Wang et al demonstrated that NF-κB was independently correlated with chemoresistance in ovarian cancer. In further, the survival rates of patients with NF-κB positive expression in ovarian cancer tissues were lower than NF-κB negative expression patients.12 In our study, the positive expression of nuclear NF-κB p65 in ovarian cancer tissues was correlated with chemotherapy response, platinum resistance and refractory disease, and poor prognosis. Then, we also noted abundant expression of NF-κB p65 level in cisplatin-resistant C13* and A2780cp cells, which significantly higher than that in cisplatin sensitive cells.
Mammals express 5 members of the NF-κB family: RelA (p65), RelB, c-Rel, p50/p105 (NF-κB1), and p52/p100 (NF-κB2), of which, the p50-p65 dimer usually binds directly to the inhibitor IκB, to form an inactive trimer, that is ubiquitously present in the cytoplasm. Upon stimulation with extracellular cues, NF-κB first dissociates from IκB, to exposing its nuclear localization sequence. Subsequently, the p50-p65 dimer undergoes a rapid nuclear translocation from the cytoplasm and binds its targeting DNA sequence, thus to mediate the transcription of the related genes.27 Thus, the expression of p65 can indicate the activity of NF-κB. The expression of nuclear NF-κB could be seemed as a marker of NF-κB activation. It was found that blocking NF-κB inhibits the proliferation of tumor cells and induces their death. Furthermore, NF-κB antagonist increases the sensitivity of resistant cells to chemotherapy, which was well substantiated in this study. We used the inhibitor of NF-κB, PDTC, we found cisplatin-resistant cells became more susceptible to cisplatin treatment upon the treatment of PDTC, an NF-κB inhibitor. Such a proliferation inhibitory rate of cisplatin in C13* and A2780cp cells are increased in a concentration-dependent manner. Defects in NF-κB also result in increased susceptibility to apoptosis.
Active NF-κB turns on the expression of genes that leads to continuous cell proliferation and protects the cells from different conditions that would otherwise cause them to die via increasing apoptosis.28 It has been demonstrated that canonical NF-κB is a Fas transcription activator, the alternative NF-κB is a Fas transcription repressor.29 Finally, we also found that PDTC treatment can enhance cisplatin-induced apoptosis. NF-κB promotes Fas-regulated apoptosis in tumor cells, and inhibition of NF-κB might dysregulate Fas-induced apoptosis to decrease host immune cell-mediated tumor suppression.
Our study had some limitations: (1) The study was based on a relatively small sample obtained via a cohort from a single center; (2) the results as well as the analyses which followed were based on clinical data and cell function, further investigation of underlying mechanisms may be necessary to elucidate the significance of these molecular processes.
Conclusion
NF-κB plays an important role in the clinicopathological features, chemoresistance, and prognosis of ovarian cancer. NF-κB inhibitor PDTC can enhance cisplatin sensitivity in platinum resistant ovarian cancer cells.
Abbreviations
CR, complete response; NF-κB, nuclear transcription factor-κB; LN, lymph node; FIGO, International Federation of Gynecology and Obstetrics; DFS, disease-free survival; RECIST, response evaluation criteria in solid tumors; PR, partial response; PD, progressive disease; SD, stable disease; PI, propidium iodide; ROC, receiver operating characteristic curve; OS, overall survival; AUC, area under curves; YSR, year survival rate.
Data Sharing Statement
The datasets used during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent
This research project was approved by the Ethics Committee of Tianjin Cancer Institute and Hospital. Ethics approval for cell lines: Use of the cell lines was approved by the ethics committee of the Ethics Committee of Tianjin Cancer Institute and Hospital. We confirmed that the cell lines were authenticated by STR profile.20
Written informed consents were obtained from each patient in accordance with the Declaration of Helsinki.
Written consents were obtained from each patient to publish the pathological tissue images as representative figures.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi:10.3322/caac.21442
2. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86. doi:10.1002/ijc.29210
3. Pujade-Lauraine E, Combe P. Recurrent ovarian cancer. Ann Oncol. 2016;27(Suppl 1):i63–i65. doi:10.1093/annonc/mdw079
4. McGuire WP, Markman M. Primary ovarian cancer chemotherapy: current standards of care. Br J Cancer. 2003;89(Suppl 3):S3–8. doi:10.1038/sj.bjc.6601494
5. Peng J, Hamanishi J, Matsumura N, et al. Chemotherapy induces programmed cell death-ligand 1 overexpression via the nuclear factor-kappaB to foster an immunosuppressive tumor microenvironment in ovarian cancer. Cancer Res. 2015;75:5034–5045. doi:10.1158/0008-5472.CAN-14-3098
6. Karin M, Cao Y, Greten FR, Li ZW. NF-κB in cancer: from innocent bystander to major culprit. Nat Rev Cancer. 2002;2:301–310. doi:10.1038/nrc780
7. Ghosh S, May MJ, Kopp EB. NF-κB and rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. doi:10.1146/annurev.immunol.16.1.225
8. Gilmore TD, Koedood M, Piffat KA, White DW. Rel/NF-kappaB/IkappaB proteins and cancer. Oncogene. 1996;13:1367–1378.
9. Lin YG, Kunnumakkara AB, Nair A, et al. Curcumin inhibits tumor growth and angiogenesis in ovarian carcinoma by targeting the nuclear factor-kappaB pathway. Clin Cancer Res. 2007;13:3423–3430. doi:10.1158/1078-0432.CCR-06-3072
10. Giopanou I, Bravou V, Papanastasopoulos P, et al. Metadherin, p50, and p65 expression in epithelial ovarian neoplasms: an immunohistochemical study. Biomed Res Int. 2014;2014:178410. doi:10.1155/2014/178410
11. Guo RX, Qiao YH, Zhou Y, Li LX, Shi HR, Chen KS. Increased staining for phosphorylated AKT and nuclear factor-κB p65 and their relationship with prognosis in epithelial ovarian cancer. Pathol Int. 2008;58:749–756. doi:10.1111/j.1440-1827.2008.02306.x
12. Wang L, Wang C, Jin S, Qu D, Ying H. Expression of NF-κB and PTEN in primary epithelial ovarian carcinoma and the correlation with chemoresistance. Int J Clin Exp Pathol. 2015;8:10953–10963.
13. Koti M, Gooding RJ, Nuin P, et al. Identification of the IGF1/PI3K/NF κB/ERK gene signalling networks associated with chemotherapy resistance and treatment response in high-grade serous epithelial ovarian cancer. BMC Cancer. 2013;13:549. doi:10.1186/1471-2407-13-549
14. Shuang T, Wang M, Zhou Y, Shi C. Over-expression of nuclear NF-κB1 and c-Rel correlates with chemoresistance and prognosis of serous epithelial ovarian cancer. Exp Mol Pathol. 2016;100:139–144. doi:10.1016/j.yexmp.2015.11.030
15. Wang Z, Li Y, Lv S, Tian Y. Inhibition of proliferation and invasiveness of ovarian cancer C13* cells by a poly(ADP-ribose) polymerase inhibitor and the role of nuclear factor-κB. J Int Med Res. 2013;41:1577–1585. doi:10.1177/0300060513480913
16. Chen Y, Zhang L, Pan Y, Ren X, Hao Q. Over-expression of semaphorin4D, hypoxia-inducible factor-1α; and vascular endothelial growth factor is related to poor prognosis in ovarian epithelial cancer. Int J Mol Sci. 2012;13:13264–13274. doi:10.3390/ijms131013264
17. Cooke SL, Brenton JD. Evolution of platinum resistance in high-grade serous ovarian cancer. Lancet Oncol. 2011;12:1169–1174. doi:10.1016/S1470-2045(11)70123-1
18. Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of national surgical adjuvant breast and bowel project protocols B-18 and B-27. J Clin Oncol. 2008;26:778–785. doi:10.1200/JCO.2007.15.0235
19. Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from national surgical adjuvant breast and bowel project B-18. J Natl Cancer Inst Monogr. 2001;2001:96–102. doi:10.1093/oxfordjournals.jncimonographs.a003469
20. Xing F, Sun C, Luo N, et al. Wogonin increases cisplatin sensitivity in ovarian cancer cells through inhibition of the phosphatidylinositol 3-kinase (PI3K)/Akt pathway. Med Sci Monit. 2019;25:6007–6014. doi:10.12659/MSM.913829
21. Ushijima K. Treatment for recurrent ovarian cancer-at first relapse. J Oncol. 2010;2010:497429. doi:10.1155/2010/497429
22. Taniguchi K, Karin M. NF-κB, inflammation, immunity and cancer: coming of age. Nat Rev Immunol. 2018;18:309–324. doi:10.1038/nri.2017.142
23. Gatti L, Zunino F. Overview of tumor cell chemoresistance mechanisms. Methods Mol Med. 2005;111:127–148. doi:10.1385/1-59259-889-7:127
24. Lambrechts S, Smeets D, Moisse M, et al. Genetic heterogeneity after first-line chemotherapy in high-grade serous ovarian cancer. Eur J Cancer. 2016;53:51–64. doi:10.1016/j.ejca.2015.11.001
25. Eichholtz-Wirth H, Sagan D. IkappaB/NF-kappaB mediated cisplatin resistance in HeLa cells after low-dose gamma-irradiation is associated with altered SODD expression. Apoptosis. 2000;5:255–263. doi:10.1023/A:1009656513307
26. Mabuchi S, Ohmichi M, Nishio Y, et al. Inhibition of NFκB increases the efficacy of cisplatin in in vitro and in vivo ovarian cancer models. J Biol Chem. 2004;279:23477–23485. doi:10.1074/jbc.M313709200
27. Siebenlist U, Franzoso G, Brown K. Structure, regulation and function of NF-kappaB. Annu Rev Cell Biol. 1994;10:405–455. doi:10.1146/annurev.cb.10.110194.002201
28. Takada Y, Kobayashi Y, Aggarwal BB. Evodiamine abolishes constitutive and inducible NF-κB activation by inhibiting IκBα kinase activation, thereby suppressing NF-κB-regulated antiapoptotic and metastatic gene expression, up-regulating apoptosis, and inhibiting invasion. J Biol Chem. 2005;280:17203–17212. doi:10.1074/jbc.M500077200
29. Liu F, Bardhan K, Yang D, et al. NF-κB directly regulates Fas transcription to modulate Fas-mediated apoptosis and tumor suppression. J Biol Chem. 2012;287:25530–25540. doi:10.1074/jbc.M112.356279
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.