Back to Journals » OncoTargets and Therapy » Volume 12
High expression of GMⅡ is associated with poor prognosis of gastric cancer patients
Authors Zhu S , Li Y, Xiao W, Yang Y
Received 29 January 2019
Accepted for publication 10 April 2019
Published 4 June 2019 Volume 2019:12 Pages 4379—4389
DOI https://doi.org/10.2147/OTT.S203345
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Leo Jen-Liang Su
Shasha Zhu,1,* Yao Li,2,* Wei Xiao,1 Yaying Yang1
1Department of Pathology, Molecular Medicine and Cancer Research Center, Chongqing Medical University, Chongqing 400016, People’s Republic of China; 2Yongchuan Hospital of Chongqing Medical University, Chongqing 402160, People’s Republic of China
*These authors contributed equally to this work
Background: Being an important N-glycosylation enzyme in eukaryotic cells, Golgi α-mannosidaseⅡ (GMⅡ) has been suggested to function as a target for cancer treatment based on the inhibitory effect on cancer growth and metastasis by the swainsonine, an inhibitor of GMⅡ. This study aims to investigate GMⅡ expression and its prognostic value in primary gastric cancer.
Methods: The GMⅡ expression was examined by using the quantitative PCR and Western blotting in 37 paired gastric cancer and noncancerous tissues. We analyzed the relationship between its expression and the clinicopathological parameters by immunohistochemistry in 185 paraffin-embedded gastric cancer tissue specimens. Furthermore, we detected the GMⅡ expression in cultured gastric cancer cell lines and the normal gastric cell line and observed the changes of proliferative and invasive capacities of gastric cell lines after GMⅡ scilencing and overexpressing in vitro.
Results: The GMⅡ mRNA (P<0.0001) and protein (P<0.01) expression of 37 tumor tissues were increased compared with those of the matched adjacent normal tissues. Human gastric cancer cell lines also showed higher GMⅡ expression (P<0.001) compared with normal gastric cell lines. The immunohistochemical analysis revealed that GMⅡ was an independent predictor of the overall survival of patients. In addition, GMⅡ overexpression in the normal gastric cell line GES-1 significantly promoted the cell proliferation and invasion, while GMⅡ knockdown in gastric cancer cell line BGC-823 significantly inhibited the cell proliferation and invasion.
Conclusion: GMⅡ may become an indicator for monitoring the prognosis of primary gastric cancer and it may provide a new direction for precise treatment.
Keywords: Golgi а-mannosidaseⅡ, primary gastric cancer, prognosis, proliferation, invasion
Introduction
Primary gastric cancer is a malignant tumor with poor prognosis. According to global cancer statistics in 2018, the incidence and mortality of gastric cancer ranked the fifth and the third, respectively, in the global tumor ranking.1,2 Therefore, it is particularly important to further explore the molecular mechanism of gastric cancer occurrence and progression and to accurately identify more valuable prognostic markers and therapeutic targets, so as to effectively improve the diagnosis, treatment and prognosis of this disease.
Tumor cells generally undergo activation, rapid division and proliferation, adherence to other cell types and cellular substrates, invasion of tissues and often accompanied by altered glycosylation. Thus, changes in glycosylation are recognized as one of the characteristics of cancer cells.3–6 Glycosylation is a process involving the synergy of endogenous combinations of hundreds of specific glycosyltransferases and glycosidases that catalyze the adjunction of saccharide conformations to different locations of extended glycans in a stepwise manner.7 Thus, the altered expression of enzymes participated in the glycosylation can result in the changes of glycosylation.
There are two types of glycosylation in glycoproteins: N-glycosylation and O-glycosylation. Golgi α -mannosidaseⅡ (GMⅡ), a type Ⅱ transmembrane protein of golgi apparatus, 125 kDa in size, plays an important role in N-glycosylation in eukaryotic cells.8 In preliminary clinical trials, some researchers found that the swainsonine (SW) can reduce tumor growth and metastasis in cancer patients,9 and they speculated that the effect of SW on the tumor progress maybe resulting from the inhibition of GMⅡ which is related to the N-glycan structure of tumor cell surface.10 Furthermore, it was also demonstrated that many types of cancers such as colorectal carcinoma,melanoma and breast carcinoma showed the reduced growth rate in the tumor-bearing mice when GMⅡ was inhibited by SW.11–13 Thus, GMII is considered to be a target for inhibiting the development of cancer cells.14–16
As far as we know, reports on the prognostic value of GM II in primary gastric cancer have not been found. In the experiments, we analyzed the expression of GMII in gastric cancer and adjacent normal tissues by real-time quantitative reverse transcription (qRT-PCR), Western blotting and immunohistochemistry (IHC). Meanwhile, our study is the first to try to explore the correlation between GMII expression and clinicopathological features and to evaluate its prognostic value in the survival of gastric cancer patients after operation. In addition, the proliferation and invasion of GMII-knockdown gastric cancer cells and GMII-overexpressing normal gastric mucosal cells, respectively, were examined to evaluate the functional role of GMII in primary gastric cancer.
Materials and methods
Human tissue samples
Thirty-seven pairs of cancerous and adjacent noncancerous stomach tissue (more than 5 cm away from cancer tissue) were collected in the First Affiliated Hospital of Chongqing Medical University. Another 185 paraffin-embedded primary gastric cancer samples were collected from the Department of Pathology, the First Affiliated Hospital of Chongqing Medical University. There were no preoperative radiotherapy or chemotherapy for these patients. The histological type and grade of gastric cancers were judged by two pathological doctors according to the WHO standard. Postoperative follow-up was performed every 3 months in the first 2 years and every 6 months after 2 years. The period of follow-up is 6–93 months and the median is 46.5 months. The study was conducted in accordance with the Declaration of Helsinki and the protocol was approved by Ethics Committee of Chongqing Medical University (Chongqing, China) and written informed consent was obtained from each patient involved in the study.
Cell culture
Gastric cancer cell lines SGC-7901, MKN-28, BGC-823 and immortalized gastric mucosal epithelial cell line GES-1 purchased by the Procell Life Science &Technology (Procell, China) were cultured in RPMI-1640 medium (Hyclon, USA) with 10% FBS, 100 units/mL penicillin and 100 μg/mL streptomycin at 37°C, a humidified 5% CO2 atmosphere.
Real-time quantitative reverse transcription PCR analysis
Total RNA was obtained from cells and tissues by TRIzol (Invitrogen, USA). The reverse transcription reaction was performed at 37°C for 15 mins and then at 85°C for 5 s. Quantifcation of GMⅡ and β-actin was examined with the SYBR® PrimeScript™ RT-PCR Kit (Takara, Japan). The primers we used were as followes: GMⅡ:(F) 5ʹ-CTTCTCAGGGGACCTGCTCT-3ʹ,(R) 5ʹ-TCAGCATGGTCTCTGCATTG-3ʹ;β-actin: (F) 5ʹ-CCTGGCACCCAGCACAAT-3ʹ;(R) 5ʹ-GGGCCGGACTCGTCATAC-3ʹ. All experimental steps and procedures were followed by the manufacturer’s instructions and were repeated three times separately.
Immunohistochemistry analysis
IHC staining was performed by using UltraSensitiveTMS-P (KIT-9710,MXB Boitechnologist), all steps including deparaffinization, hydration, antigen retrieval, serum blocking, primary antibody incubation and secondary antibody incubation were in accordance with the manufacturer’s protocol. The anti-GMⅡ primary antibody (Santa Cruz, USA) was at a dilution 1:400 and incubated the sections at 4°C overnight. It was stained with 3,3ʹ-diaminobenzidine tetrahydrochloride and hematoxylin.
GMⅡ expression was assessed by staining intensity and staining percentage. The staining percentage was scored as 0 (0–9%), 1 (10–25%), 2 (26–50%) or 3 (51–100%). The staining intensity was determined as 0 (negative), 1 (weak), 2 (moderate) or 3 (strong) under microscopy. The final score was obtained by multiplying the two scores and ranked from 0 to 9. “-”,“+”,“++” and “+++” were corresponding to scores 0, 1–3, 4–6 and 7–9. According to GMⅡ expression levels, there were two groups of gastric cancer: low GMⅡexpression group (“-” and “+”) and high GMⅡ expression group (“++” and “+++”). The IHC staining score was performed by two double-blinded independent observers who were unaware of all the clinical parameters.
Western blotting analysis
Total protein was collected from cells and tissues by RIPA lysis buffer (Beyotime, China) and dissociated by PAGE and then transferred onto PVDF membranes (Millipore, USA). The band was blocked with 5% skim milk for 60 mins, then followed by incubating with anti-GMII primary antibody (1:800, Santa Cruz, USA) and anti-β-actin primary antibody (1:1000, Beijing Biosynthesis, China) separately for 4°C overnight, and next incubated with the secondary antibody (1:1000, Santa Cruz, USA) for 2 hrs at homeothermy. The protein bands were exposed by enhanced chemiluminescence. The data was analyzed by Quantity One software.
RNA interference and cell transfection
Short hairpin oligoes (shRNAs) targeting GMⅡ were synthesized by GenePharma Company (Shanghai, China), as follows (GMⅡ-shRNA-1:5-CACCGCGTTTGCTAGCTGAGAATAATTCAAGAGATTATTCTCAGCTAGCAAACGCTTTTTTG-3ʹ; GMⅡ-shRNA-2:5ʹ-CACCGCAGACTGTCTGTTTGCTTCATTCAAGAGATGAAGCAAACAGACAGTCTGCTTTTTTG-3ʹ; GMⅡ-shRNA-3:5ʹ-CACCGCTGGGCTATTGATCCCTTTGTTCAAGAGACAAAGGGATCAATAGCCCAGCTTTTTTG-3ʹ; GMⅡ-shRNA-NC:5ʹ-CACCGTTCTCCGAACGTGTCACGTCAAGAGATTACGTGACACGTTCGGAGAATTTTTTG-3ʹ). The annealed double-stranded oligonucleotides were ligated into the pGPU6/GFP/Neo vectors and the constructs were transfected transiently into BGC-823 applying Lipofectamine 2000 (Invitrogen, USA) according to the protocol. A eukaryotic overexpression plasmid vector pReceiver-M03 with the full-length of human GMⅡ cDNA was obtained from the GeneCopoeia Inc. (Maryland, USA). Negative control was made of empty vector. The overexpression plasmid and empty vector were transfected transiently into GES-1 cells 48 hrs later, and GMⅡ protein expression was detected by Western blotting to screen the best silencing construct for further experiments.
Cell counting kit-8 (CCK-8) assay
Cells (100 µL, 4×103 cells per well) were added in 96-well plates and cultured in cell incubator overnight. Subsequently, 10 µL of CCK-8 reagent (Dojindo, Japan) per well was added, and the plate was placed in the cell incubator for another 2 hrs. The optical density was measured at 450 nm. The assay was set up at various times (0 hr, 24 hrs, 48 hrs, 72 hrs) to detect the cell growth curve.
Cell cycle detection
Each experimental group was subjected to trypsin digestion in the logarithmic growth phase, and then made into a 1×106/ml cell suspension, which was washed twice with PBS and fixed with 70% pre-cooled ethanol at 4°C for 24 hrs. Finally, the cells were sent to detect the distribution of cell cycle and the proliferation index (PI) is calculated by flow cytometry. PI = (S + G2/M)/(G0/G1+ S + G2/M) ×100%.
Invasion assay
Matrigel (50 µL/well, at a 1:8 dilutions in serum-free RPMI 1640) was placed into transwell polycarbonate chambers (8-µm pore size; Corning). Transfected cells were trypsinized and washed 3 times with RPMI 1640, followed by resuspension in serum-free medium. RPMI 1640, 600 µL, with 10% FBS was pre-added to the bottom chambers, and the cell suspensions (200 µL,1×104/well) were added into the top chambers and then cultured for 24 hrs. The upper chamber cells were scraped off, followed by fixing down chamber cells with methanol and staining them with crystal violet (0.1%). Cell counting was performed in 5 randomly selected regions using an optical microscope. Experiments repeated 3 times separately and the average was calculated.
Statistical analysis
Student’s t-test was used for the comparison between two groups. The relationship between GMⅡ expression and various clinicopathological parameters was analyzed by χ2 test. Survival curves were determined by the Kaplan–Meier method and compared by the log-rank test. To analyze the effects of GMII expression and clinicopathological variables on survival, univariate and multivariate analyses, both were performed by using the Cox proportional hazards regression model. We used GraphPad Prism 6.0 and SPSS 19.0 for data analysis. P<0.05 was considered statistically significant.
Results
GMⅡ mRNA expression analyzed by real-time quantitative RT-PCR
To compare the mRNA expression of GMII in gastric cancer tissues and normal gastric mucosa, we performed RT-PCR experiments and found that GMII mRNA was detected in both tissues. However, the expression level of GMII mRNA in gastric cancer tissues was quite more than that in normal one (P<0.0001, Figure 1).
GMⅡ protein expression analyzed by Western blotting analysis
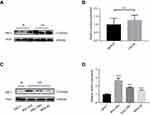
Matching with qRT-PCR results, Western blot results also showed that protein expression of GMII was much higher in 37 gastric tumor tissues than that in matched noncancerous tissues (P=0.014, Figure 2A and B). Similarly, the expression of GMII protein was significantly increased in the gastric cancer cell lines SGC-7901, MKN-28, especially BGC-823, in comparison with the normal gastric cell line GES-1 (Figure 2C and D).
Immunohistochemical analysis of GMⅡ expression and its relevance with the clinicopathological parameters
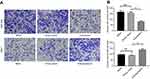
Similarly, we found that almost all of the cases (n=185) showed the positive staining of GMⅡ which was localized to the cytoplasm, especially in cancerous tissues, significantly higher expression of GMⅡ was observed compared with the normal gastric mucosal tissues. Overall, 97 of 185 (53.30%) cancerous tissues showed the high GMⅡ expression, whereas only 68 of 185 (36.76%) noncancerous tissues showed the high GMⅡ expression (Figure 3). It is a significant difference between them (P=0.0016). Table 1 summarizes the correlation between GMII expression and various clinicopathological features. The results indicated that high expression of GMII was significantly correlated to tumor infiltration depth (P<0.0001) and distant metastasis (P=0.0002), rather than age, sex, tumor size, differentiation and local lymph node metastasis.
 | Table 1 Correlation between GMⅡ expression and clinicopathological parameters of 185 gastric cancer cases |
GMⅡ expression and clinical outcome
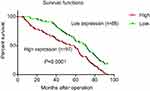
The clinical data analyses show the 5-year overall survival of patients with high GMⅡ expression (31.96%) was significantly reduced than that of patients with low GMⅡ expression (54.55%) (P<0.0001, log-rank test, Figure 4). Univariate Cox regression analyses showed that depth of tumor infiltration, local lymph node metastasis, distant metastasis, tumor differentiation and GMⅡ expression were significantly interrelated with overall survival (Table 2). In addition, the multivariate Cox regression analysis suggested that tumor infiltration (P=0.0005), distant metastasis (P<0.0001) and GMⅡ expression (P<0.0001) may be independent forecast indicators of the overall survival of patients with gastric adenocarcinoma (Table 2).
 | Table 2 Univariate and multivariate analyses of overall survival of gastric cancer patients |
Knockdown and overexpression of GMⅡ on cell lines
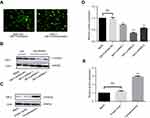
We silenced the GMⅡ expression in BGC-823 cell line with shRNA vectors targeting for GMⅡ gene and transfected the GMⅡ overexpressing vector into GES-1 cells, respectively. Successful transfections showed the green fluorescence under a fluorescence microscope (Figure 5A). The GMⅡ expression was detected by Western blotting in transfected cells. We selected the best silencing vector based on Western blot results and obtained stable transfected cells (Figure 5B and D).
Effect of knockdown and overexpression of GMⅡ on cell proliferation and cell cycle
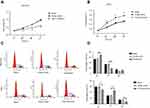
We carried out the CCK-8 assay to detect the effect of knockdown and overexpression of GMⅡ on cell proliferation. It is observed that the GES-1 cell proliferation after GMⅡ overexpressing was evidently increased than that of GES-1 control cells, while BGC-823 cell proliferation after GMⅡ silencing evidently decreased than that of BGC-823 control cells (Figure 6A and B). The difference between them was significant (P<0.05). Furthermore, we took flow cytometry to identify the role of GMⅡ on the cell cycle. Compared with the empty vector group, GMII silencing significantly raised the propagation of cells in the G1 phase and reduced the propagation of cells in the S phase, while the overexpression of GMII was just the opposite (Figure 6C and D).
Effect of knockdown and overexpression of GMⅡ on invasion
To assess the function of GMII in cell invasion, we examined the invasive ability of GMII-silenced BGC-823 cells and GMII-overexpressed GES-1 cells. It is found out that the average number of migrated GMII-silenced BGC-823 was 82.43±3.22, which was much lower than that of negative vector transfection (158.9±5.13) and blank control (166±7.38). The average number of migrated GMII-overexpressed GES-1 was 138.9±5.51, which was much higher than that of empty vector transfection (91.12±1.12) and blank control (96.55±1.03). The difference between them was significant (P<0.05) (Figure 7A and B).
Discussion
As we all know, the enzymatic process which produces glycosidic linkages of saccharides to other saccharides, lipids or proteins is called glycosylation.6,17 We have learned in previous studies that changes in protein glycosylation patterns, such as neoexpression, underexpression or overexpression of glycans, have been observed in a variety of tumors, and these changes have been considered as a hallmark of cancer.18,19 Glycosylation modifications often cause uncontrolled proliferation and invasion behavior of cancer cells by changes in expression, metabolism, functions, properties, stability and/or cellular localization of glycoproteins.14,20,21
As the most highly studied form of protein glycosylation in eukaryotic organisms,N-glycosylation is proved to play a crucial role during tumor progression in more and more evidence, and they participate in several cellular mechanisms, such as metabolism, signaling, growth, cell–cell adhesion, cell–matrix interaction, invasionand metastasis.22,23 Studies have shown that N-glycosylation can be induced by oligosaccharide precursors linked by the synthesis of dolichol lipids and then the pruning of glycan precursors in the endoplasmic reticulum and Golgi determines the correct folding and secretion of glycoproteins.24,25 Three glucose residues and one mannose are removed by glucosidase and endoplasmic reticulum mannosidase, respectively, followed by transferring the glycoprotein into the Golgi, and the N-glycan contains eight mannose residues called high mannose glycans. Usually, there are few high mannose glycans reaching the cell surface, since most mannose residues are cut by Golgi mannosidase, which is a prerequisite for the formation of complex or hybrid glycans.
GMⅡ plays a central role in the Golgi processing pathway, and it is highly specific for the single GlcNAc attached to the Man α1,3-Man arm of the substrate GlcNAcMan5GlcNAc2-Asn-X in a β1,2 locations and clips two mannose residues from the branched GlcNAcMan5GlcNAc2 mannose to form the core GlcNAcMan3GlcNAc2 glycosyl structure, an essential precursor for the further addition of N-acetyl-glucosamine units. On the basis of the preliminary experimental data, it could be clearly showed that the α-mannosidase inhibitor swainsonine (SW) has a growth and metastasis suppressor function in many types of cancers including gastric cancer.9,26 And because of the higher efficacy of inhibition of GMⅡ than any other types of α-mannosidase by SW, the researchers speculated that the GMⅡ makes an important role in cancer progression.
However, nowadays, no reports about the prognostic significance of GMⅡ in gastric adenocarcinoma existed. In the study, we found that GMⅡ expression in gastric cancer tissues was obviously increased than that in the corresponding noncancerous tissue and it is related to the depth of tumor infiltration, distant metastasis and reversely correlated with the overall survival rate of patients after resection. Therefore, our data prompted that GMⅡ should have the prognostic values in the gastric carcinoma patients.
GMII trims the mannose residue and contributes to the formation of the core GlcNAcMan3GlcNAc2 glycosyl structure, which is a necessary basis for the further adjunction of N-acetyl-glucosamine, and so we took it for granted that the high expression of GMII leads to abnormal N-glycan formation of the glycoproteins. Recently, a study reported that swainsonine can decrease the synthesis of β1,6-branched N-glycans by inhibiting GMII, which means that GMII should participate in the β1,6-branched N-glycan formation.27 In the last years, some studies have pointed that alterations of β1,6-branched of N-glycans were related to proliferation potential, tissue invasion and metastasis, which were recognized as hallmarks of cancer progression.28 For example, the E-cadherin modification increased by β1,6-branched N-glycans can induce E-cadherin-mediated cell–cell adhesion instability,29 the β1,6-branched chain on the N-glycan of the tumor α5β1 integrin β1 subunit can down-regulate the formation of focal contact, thereby increasing tumor motility and invasiveness through ECM,30 and the abnormal glycosylation of tissue inhibitor of metalloproteinase-1 (TIMP-1) with increased β1,6 branching structure can increase the malignant behavior of tumors and promote tumor progression, and the aberrant glycosylation of TIMP-1 with increased β1,6 branching structure can promote the malignant behavior and accelerates the tumor progression.31 Since GMⅡ is a crucial factor for β1,6-branched of N-glycans, it should take part in the β1,6-branched of N-glycans-associated tumor malignant behavior, and if its expression in the tumor is down-regulated, maybe prevent the tumor progression. So, GMⅡ may be a therapeutic target in tumors.
For further exploring the function of GMⅡ in gastric carcinoma, we silenced its expression in cell lines BGC-823 which shows the high expression of GMⅡ, and by detecting the changes of proliferation and invasion capacities of the tumor cells, it is discovered that the cell proliferation and invasion decreased significantly. At the same time, we up-regulated the GMⅡ expression in GES-1 which shows the relatively low expression of GMⅡ, and we found that after the up-regulation of GMⅡ expression in GES-1, its proliferation and invasion capacities increased significantly.
Conclusion
Our study shows that high GMII expression may promote the growth and invasion of gastric cancer cells. When GMII is down-regulated, its growth and invasion are significantly inhibited. However, the specific molecular mechanism of GMII regulation of gastric cancer is still unclear, and so further research is still needed. We believe that a better understanding of the role of GMII in malignant tumors may provide us with a new direction in the treatment of gastric adenocarcinoma. GMII might serve as a valuable prognostic marker and potential target for gene therapy in the treatment of primary gastric cancer.
Acknowledgment
This work was supported by the Science and Technology Research Program of Chongqing Municipal Education Commission (Grant No: KJ1702016, for Yaying Yang).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Luo G, Zhang Y, Guo P, Wang L, Huang Y, Li K. Global patterns and trends in stomach cancer incidence: age, period and birth cohort analysis. Int J Cancer. 2017;141(7):1333–1344. doi:10.1002/ijc.30835
3. Häuselmann I, Borsig L. Altered tumor-cell glycosylation promotes metastasis. Front Oncol. 2014;4:28. doi:10.3389/fonc.2014.00028
4. Kannagi R, Sakuma K, Cai BH, Yu SY. Tumor-Associated Glycans and Their Functional Roles in the Multistep Process of Human Cancer Progression. Japan: Springer; 2015.
5. Ferreira IG, Pucci M, Venturi G, et al. Glycosylation as a main regulator of growth and death factor receptors signaling. Int J Mol Sci. 2018;19(2):580. doi:10.3390/ijms19020580
6. Pinho SS, Reis CA. Glycosylation in cancer: mechanisms and clinical implications. Nat Rev Cancer. 2015;15(9):540–555. doi:10.1038/nrc3982
7. Oliveira-Ferrer L, Legler K, Milde-Langosch K. Role of protein glycosylation in cancer metastasis. Semin Cancer Biol. 2017;44:141–152. doi:10.1016/j.semcancer.2017.03.002
8. Herscovics A. Importance of glycosidases in mammalian glycoprotein biosynthesis. Biochim Biophys Acta. 1999;1473(1):96–107.
9. Sun JY, Zhu MZ, Wang SW, Miao S, Xie YH, Wang JB. Inhibition of the growth of human gastric carcinoma in vivo and in vitro by swainsonine. Phytomedicine. 2007;14(5):353–359. doi:10.1016/j.phymed.2006.08.003
10. Kuntz DA, Nakayama S, Shea K, et al. Structural investigation of the binding of 5-substituted swainsonine analogues to Golgi alpha-mannosidase II. Chembiochem. 2010;11(5):673–680. doi:10.1002/cbic.200900750
11. Fiaux H, Kuntz DA, Hoffman D, et al. Functionalized pyrrolidine inhibitors of human type II alpha-mannosidases as anti-cancer agents: optimizing the fit to the active site. Bioorg Med Chem. 2008;16(15):7337–7346. doi:10.1016/j.bmc.2008.06.021
12. Dennis JW, Koch K, Beckner D. Inhibition of human HT29 colon carcinoma growth in vitro and in vivo by swainsonine and human interferon-alpha 2. J Natl Cancer Inst. 1989;81(13):1028–1033.
13. Dennis JW, Koch K, Yousefi S, VanderElst I. Growth inhibition of human melanoma tumor xenografts in athymic nude mice by swainsonine. Cancer Res. 1990;50(6):1867.
14. Gerber-Lemaire S, Juillerat-Jeanneret L. Studies toward new anti-cancer strategies based on alpha-mannosidase inhibition. Chimia. 2010;64(9):634. doi:10.2533/chimia.2010.634
15. Elsen JMHV, Kuntz DA, Rose DR. Structure of Golgi α‐mannosidase II: a target for inhibition of growth and metastasis of cancer cells. Embo J. 2014;20(12):3008–3017. doi:10.1093/emboj/20.12.3008
16. Rose DR. Structure, mechanism and inhibition of Golgi α-mannosidase II. Curr Opin Struc Biol. 2012;22(5):558–562. doi:10.1016/j.sbi.2012.06.005
17. Rodrigues JG, Balmaña M, Macedo JA, et al. Glycosylation in cancer: selected roles in tumour progression, immune modulation and metastasis. Cell Immunol. 2018;333:46–57. doi:10.1016/j.cellimm.2018.03.007
18. Munkley J, Elliott DJ. Hallmarks of glycosylation in cancer. Oncotarget. 2016;7(23):35478. doi:10.18632/oncotarget.v7i23
19. Ferreira JA, Magalhaes A, Gomes J, et al. Protein glycosylation in gastric and colorectal cancers: toward cancer detection and targeted therapeutics. Cancer Lett. 2017;387:32–45. doi:10.1016/j.canlet.2016.01.044
20. Rambaruth NDS, Dwek MV. Cell surface glycan–lectin interactions in tumor metastasis. Acta Histochem. 2011;113(6):591–600. doi:10.1016/j.acthis.2011.03.001
21. Glavey SV, Huynh D, Reagan MR, et al. The cancer glycome: carbohydrates as mediators of metastasis. Blood Rev. 2015;29(4):269–279. doi:10.1016/j.blre.2015.01.003
22. Lange T, Samatov TR, Tonevitsky AG, Schumacher U. Importance of altered glycoprotein-bound N- and O-glycans for epithelial-to-mesenchymal transition and adhesion of cancer cells. Carbohyd Res. 2014;389:39–45. doi:10.1016/j.carres.2014.01.010
23. Arnold JN, Saldova RU, Rudd PM. Evaluation of the serum N-linked glycome for the diagnosis of cancer and chronic inflammation. Proteomics. 2010;8(16):3284–3293. doi:10.1002/pmic.200800163
24. Stanley P, Taniguchi N, Aebi M. N-Glycans. 2015:99–111. doi:10.1101/glycobiology.3e.009.
25. Aebi M. N-linked protein glycosylation in the ER. Biochim Biophys Acta Mol Cell Res. 2013;1833(11):2430–2437. doi:10.1016/j.bbamcr.2013.04.001
26. Sun JY, Yang H, Miao S, et al. Suppressive effects of swainsonine on C6 glioma cell in vitro and in vivo. Phytomedicine. 2009;16(11):1070–1074. doi:10.1016/j.phymed.2009.02.012
27. Dong Z, Zuber C, Pierce M, Stanley P, Roth J. Reduction in Golgi apparatus dimension in the absence of a residential protein, N-acetylglucosaminyltransferase V. Histochem Cell Biol. 2014;141(2):153–164. doi:10.1007/s00418-013-1146-1
28. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi:10.1016/j.cell.2011.02.013
29. Pinho SS, Osório H, Nita-Lazar M, et al. Role of E-cadherin N-glycosylation profile in a mammary tumor model. Biochem Bioph Res Co. 2009;379(4):1091–1096. doi:10.1016/j.bbrc.2009.01.024
30. Pinho SS, Seruca R, Gärtner F, et al. Modulation of E-cadherin function and dysfunction by N-glycosylation. Cell Mol Life Sci. 2011;68(6):1011–1020. doi:10.1007/s00018-010-0595-0
31. Kim YS, Ahn YH, Song KJ, et al. Overexpression and beta-1,6-N-acetylglucosaminylation-initiated aberrant glycosylation of TIMP-1: a “double whammy” strategy in colon cancer progression. J Biol Chem. 2012;287(39):32467–32478. doi:10.1074/jbc.M112.370064
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.