Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 14
High Expression of COX-2 Associated with the Depth of Invasion on Acral Melanoma by Increasing TGF-β1
Authors Gipsyianti N, Aziz A, Hernowo BS, Usman HA
Received 27 October 2020
Accepted for publication 26 December 2020
Published 3 March 2021 Volume 2021:14 Pages 209—216
DOI https://doi.org/10.2147/CCID.S285564
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Nastassa Gipsyianti, Afiati Aziz, Bethy S Hernowo, Hermin A Usman
Department of Pathology Anatomy, Faculty of Medicine, Universitas Padjadjaran/Dr. Hasan Sadikin General Hospital, Bandung, Indonesia
Correspondence: Hermin A Usman
Department of Pathology Anatomy, Faculty of Medicine, Universitas Padjadjaran/Dr. Hasan Sadikin General Hospital, Bandung, Indonesia
Email [email protected]
Introduction: Acral melanoma (AM) has a poor prognosis since it is easily metastatic and resistant to chemo and immunotherapy. Cyclooxygenase-2 (COX-2) is an enzyme that plays a role in the carcinogenesis process. The increased expression of COX-2 has an impact on increasing levels of Myeloid-Derived Suppressor Cell (MDSC), which is a key regulator of immune. The increase in MDSC produces Transforming Growth Factor β 1 (TGF-β 1), which will suppress Natural Killer (NK) cells and Dendritic Cells (DC) function so that tumor cells are spared from the immune systems and are easier to invade surrounding tissues.
Purpose: This study aimed to determine the role of COX-2 and TGF-β 1 on the depth of invasion on AM.
Materials and Methods: This study was a cross-sectional observational study on 40 paraffin blocks of AM cases during 2014– 2019 in the Department of Pathology Anatomy, Faculty of Medicine, Dr. Hasan Sadikin General Hospital, Bandung. The depth of invasion of all samples was measured by dotSlide imaging software and the immunohistochemical staining of COX-2 and TGF-β 1 was performed. The association between COX-2 and TGF-β 1 expression and AM depth of invasion were analyzed using Mann Whitney.
Results: The result showed a significant association between COX-2 and TGF-β 1 expression and depth of invasion on AM. COX-2 expression had a significant association with TGF-β 1 expression (0.0001). Through multivariate analysis, it was found that COX-2 had the greatest association with the depth of invasion (p=0.0001).
Conclusion: The findings showed that increasing expression of COX-2 in AM is associated with the depth of invasion by increasing TGF-β 1 and it might play important roles during the invasion process of AM.
Keywords: acral melanoma, COX-2, depth of invasion, TGF-β 1
Introduction
AM is a malignant melanoma (MM) subtype with a lower incidence than other types of melanoma in the Caucasian race. The incidence of acral melanoma was higher in Asian and African races. AM has a poor prognosis because it has radial and superficial growth patterns so that it easily metastasized.1,2 AM is melanoma occurring on the hands and feet (palms, soles, fingers, toes, and nail units). Unlike other MM, UV-radiation does not seem to play a significant part in the development of AM. Tumorigenesis in AM is a result of complex interactions among genetic changes in the tumor, the patient, and microenvironmental factors.3
MM is known to be resistant to chemotherapy or radiotherapy, so it can quickly invade and metastasize to other organs.4 One of the factors causing MM to become resistant to chemotherapy and radiotherapy that currently being investigated is tumor microenvironment (TME). TME is the environment around tumors, including surrounding blood vessels, immune cells, fibroblasts, signaling molecules, and extracellular matrix (ECM). Tumors can affect the microenvironment by releasing extracellular signals, promoting tumor angiogenesis and inducing peripheral immune tolerance.5 The main mediators of carcinogenesis in general that play a role in increasing COX-2 are Mitogen-Activated Protein Kinase (MAPK), Epidermal Growth Factor Receptor (EGFR) and Nuclear Factor Kappa β (NF-κβ). In MM, the most important mediator is NF-κβ. NF-κβ as a transcription factor that will also regulate the expression of anti-apoptotic, pro-proliferative, and pro-metastatic genes.6,7
NF-κβ immunoexpression is related to the depth of invasion of MM in the acral. NF-κB is an essential pathway for tumor cell survival processes. It is the transcription factor that will participate in regulating the expression of anti-apoptotic, pro-proliferation, and pro-metastatic genes. TME is said to have the role of inducing NF-κβ, although the full path is unknown. Several studies have also shown that the increased activity of NF-κβ will stimulate the expression of COX-2 inflammatory factors.8
COX-2 is a pleiotropic enzyme and produced by a variety of malignant tumor cells. COX-2 plays a role in the process of carcinogenesis and resistance of malignant tumor cells to chemotherapy and radiotherapy. The increase in the number of COX-2 has an impact on increasing MDSC, which is the critical immune regulator in malignancies. MDSC plays a role in the immune escape by activating TGF-β1 through the SMAD3 pathway. TGF-β1 supervises the functions of NK cells and DC that causing immune escape.9,10 Several studies about the roles of COX-2 and TGF-β1 on MM have been conducted, but specifically on AM are still limited.
This study analyzes the association between COX-2 and TGF-β1 immunoexpression and the depth of invasion in AM. The results of this study are expected to determine the pathogenesis of immune surveillance in acral melanoma to be the basis for the theory of anti-COX-2 as a supportive therapy.
Materials and Methods
A cross-sectional observational analysis design was implemented in the present study. The paraffin-embedded archival pathologic specimens from 40 patients with acral melanoma who underwent initial surgical resection between 2014 and 2019 in Dr. Hasan Sadikin General Hospital Bandung, Indonesia, which is fulfilled the inclusion and exclusion criteria. The inclusion criteria are cases of acral melanoma that have been biopsied excision or surgery and diagnosed histopathologically, limit of tumor cell invasion that can be assessed well from the surface to the deepest limit, and complete data of the patients. The exclusion criteria were the unknown location of paraffin block in the primary tumor and the depleted tumor mass paraffin block.
Ethics Statement
This study was reviewed and approved by the Research Ethics Committee of Hasan Sadikin Hospital (ethical clearance number: LB.02.01/X.6.5/18/2020) and was conducted in compliance with the Declaration of Helsinki. The patients informed consent was waived by ethics committee due to the research using bio-archive with un-linked patients' data. All data analyzed are anonymous, patient data are kept strictly confidential.
Clinicopathological Parameters
Clinicopathological parameters included in the analysis were age, sex, Clark level, ulceration, and degree of lymphocytic infiltration. The depth of invasion was measured using dotSlide software from epidermal surface to the deepest part of the invasion in the dermis.
Immunohistochemical Staining and Evaluation
Immunohistochemical staining on all samples was manually carried out by the label method streptavidin-biotin immunoperoxidase complex using the One-Step Neopoly Detection Kit (Biogear Scientific, BioVentures, Inc., Iowa, USA). The sample was cut 4μm then depolished by xylene and rehydrated by an alcohol solution—intake of antigens using decloaking equipment for 45–60 minutes with a temperature of 98°C.
The primary antibodies are COX-2 (mouse monoclonal antihuman cyclooxygenase-2 clone 3D4 (BZ-089830F-AM) Bioenzy) with 1:100 dilutions, overnight at 4°C and TGF-β1 (TGF-β1 (TGF-β1 anti-human mouse) (BZ-0867980F-AP) Bioenzy) with a dilution of 1:100.
Assessment of COX-2 and TGF-β1 immunoexpression in cytoplasm was assessed on a semi-quantitative score based on the intensity and distribution of positive cells. COX-2 and TGF-β1 are positive if the tumor cell cytoplasm is brown. The samples were scored according to following criteria: (a) percentage of immunoreactive cells, 0 = 0% 1 = <25%, 2 = 26–50%, 3 = 51 −75%, 4 = 76% −100% and (b) staining intensity, 0 = negative, 1 = weak, 2 = moderate, and 3 = strong. The final score generated used to score the slide was (a) x (b). Histoscore COX-2 negative= 1–3, positive 4–12, histoscore TGF-β1 negative= 0–5, positive 6–12. Each IHC score was independently assessed by two pathologists without prior knowledge of patient data.11,12
Data Analysis
This research was conducted as an analytic-observational cross-sectional design with statistical analysis of non-parametric Mann–Whitney test. P-value p≤0.05 was considered significant. The data obtained were recorded in a special form and then processed through SPSS program version 22.0 for Windows.
Results
Patient Characteristics
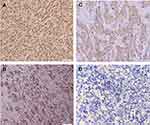
The mean age of the patients with AM was 63 years old (range of 29–86 years old) with 62.5% male and 37.5% female. The average depth of invasion in this study was 8.155 mm (range 2.554–24.791 mm). There were 40 cases of AM in this study including 2 cases of subungual melanoma, 5 cases of palm melanoma, and 33 cases of plant melanoma. The COX-2 immunoexpression with the weak, moderate, and strong intensities can be seen in Figure 1. The results of histoscore showed that the number of patients with the negative expression of COX-2 was 18 (45%) and the positive one was 22 (55%). The TGF-β1 immunoexpression with the weak, moderate, and strong intensities is presented in Figure 2. The results of histoscore showed that the number of patients with negative expression of TGF-β1 was 16 (40%) dan the positive one was 24 (60%). The number of patients with Clark Category II–III was 19 (47.5%) and IV–V was 21 (52.5%). The patients with the negative tumor-infiltrating lymphocyte (TIL) category were about 27 (67.5%), Brisk about 7 (17.5%) and Non-Brisk about 6 (15.0%). For patients with ulceration, the positive category was about 38 (95.0%) and the negative one was 2 (5%). Characteristics of subjects are presented in Table 1.
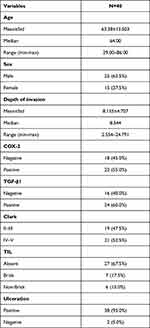 |
Table 1 Research Subject Characteristics |
Association of Depth of Invasion on Acral Melanoma with Patients’ Characteristics
The association between depth of invasion on AM and patients’ characteristics was analyzed. TIL has associations with depth of invasion on AM with a significant result (p=0.003). Also, there was no sample that has Clark Level I and Clark Level II–V. No significant association was seen between depth of invasion and other patients’ characteristics, such as age, sex, and ulceration (p>0.05). The association of Clinicopathological Characteristics and Depth of Invasion on Acral Melanoma are presented in Table 2.
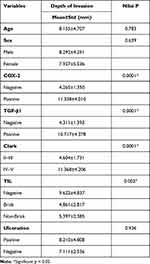 |
Table 2 Association of Clinicopathological Characteristics and Depth of Invasion on Acral Melanoma |
Table 2 shows that Clark Level and TILs have associations with depth of invasion on Acral Melanoma with significant statistic result (p=0.000). The association of pathological characteristics (COX-2 expression and TGF-β1expression) and depth of invasion on acral melanoma also has significant statistic result.
The association of COX-2 expression and depth of invasion has a positive association (p=0.000). When the COX-2 expression is positive, the invasion of tumor cells in acral melanoma becomes deeper.
Association of COX-2 with TGF-β1 on Acral Melanoma
The data in Table 3 show that there is association between COX-2 expression and TGF-β1. It shows that the statistical test result is significant (p = 0.001).
 |
Table 3 Association of COX-2 with TGF-β1 on Acral Melanoma |
Multivariate Analysis of Correlation Between COX-2 and TGF-β1 Imunoexpression and Depth of Invasion on Acral Melanoma
Multivariate analysis results in Table 4 show that the most influential factor on the depth of invasion on acral melanoma is COX-2 (p=0.0001). However, there is no significant result in Clark Level (0.111), ulceration (0.628), TILs (0.488), and TGF-β1 (p = 0.425).
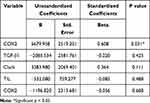 |
Table 4 Multivariate Analysis of Variables Related to Depth of Invasion Based on Binary Double Linear Regression Analysis |
Discussion
AM is less responsive to chemotherapy and radiation due to the tumor microenvironment (TME) mediated immune escape process. TME factor that plays a role in immune escape is COX-2.13 In this study, it was found that more than 50% of the samples expressed positive COX-2. COX-2 is the most important factor in the carcinogenesis process because it can affect various tumor cell growth pathways, proliferation, inhibit apoptosis, angiogenesis, invasion and metastasis. COX-2 is an enzyme that has a role in converting arachidonic acid into PGE, which is the main metabolite in the carcinogenesis process.14
The findings of this study showed that there was a significant correlation between COX-2 immunoexpression and the depth of invasion (p=0.0001) so that the higher COX-2 immunoexpression, the deeper the invasion of tumor cells into the stroma. This result is the same as the results of the study of Jafarian et al which stated that there was a correlation between COX-2 immunoexpression and the depth of MM invasion (p<0.001),4 and the study of Jana et al that presented increased COX-2 immunoexpression as an indicator of poor prognosis in breast carcinoma because it was related to progressive invasion and metastasis.15 The increase in COX-2 in the immune pathway in cancer cells affects immunoediting that plays a role in invasion which consists of elimination, balance and immune escape phases, where tumor cells are difficult to recognize by the immune system and form a microenvironment tumor environment that supports tumor cells to proliferate and invade surrounding tissues.14
COX-2 causes acral melanoma to avoid immune processes in the body through the activity of the COX-2-PGE2-Prostaglandin E receptor signaling pathway so that it suppresses the function of cells that play a major role in immunity such as DC, NK cells, T cells.7 NK is a part of lymphocytes that participate in innate immunity. PGE2 inhibits the potential for NK cells to migrate, exerts cytotoxic effects, and secretes interferons. PGE inhibits all major receptors for NK cells, namely NKR, NKG2D, CD16 and natural cytotoxicity receptors (NCR: NKp30, NKp44, NKp46) thereby reducing their ability as immune surveillance.7,16
COX-2 becomes a key immunomodulator of DC cells by increasing IL-10 production. IL-10 can decrease DC cell function by inhibiting the ability of DC cells to present antigens and activate T cells. The increase in COX-2 in cancer cells also plays a role in increasing the accumulation of MDSC. This is in accordance with the research of Obermajer and Kalinski that there is a relationship between increased COX-2 and MDSC levels in malignant ovarian tumors.17,18
The increase in COX-2 immunoexpression in malignancies is due to the large number of signals contributing to the COX-2 activation process, such as the MAPK. EGFR, and NF-κβ pathways, which are the central modulators in the process.19 There is an association between increased NF-κβ immunoexpression and the depth of invasion of AM. Several studies have shown that the increased activity of NF-kB will stimulate the expression of COX-2 inflammatory factors.8,19
The result of this study is consistent with Usman et al’s research which states that there is an association between NF-κβ and CD8 and the depth of invasion of AM. An increase in NF-κβ which is a transcription factor plays a role in stimulating COX-2 expression so that the amount increases.8
The study of Goulet et al found shallow levels of COX-2 expression in early-stage melanoma but obtained very high levels of COX-2 expression in melanomas with higher stages and metastasis.24 Significant results were obtained in the association between COX-2 and tumor stage in MM that the higher the MM stage, the stronger COX-2 expression was obtained.24
Accumulation COX-2 in a malignant tumor cell activates the SMAD3 pathway, which is the main pathway in oncogenic TGF-β1. Meanwhile, the accumulation of MDSCs produces the increased TGF-β1 through the activation of the SMAD3 pathway, which is the main canonical pathway producing TGF-β1.20
The result showed a significant association between TGF-β1 immunoexpression and depth of invasion (p = 0.0001) so that the higher the TGF-β1 immunoexpression, the deeper the invasion. These results are consistent with the results of Lv et al’s research which states that there is a correlation between TGF-β1 immunoexpression and invasion of breast carcinoma.21 It says that the increase in TGF-β1 was associated with a poor prognosis related to staging and metastasis in carcinoma of the breast. TGF-β1 influences invasion due to its function as an immunosuppressor against tumor cells to escape tumor cells to immune function.18,22 TGF-β1 suppresses immune cell function by controlling CD8 + T cells, inhibiting T cell proliferation, regulating CD4 + and CD8 + cell suppression, suppressing NK cell functions, DC cells, and increasing Treg so that tumor cells can survive in the body and form a suitable environment to invade the surrounding tissues.21
The results of the study showed that there was an association between COX-2 expression and TGF-β1 expression, as is supported by data in Table 3. Based on the results in Table 3, there is a significant association between COX-2 and TGF-β1 immunoexpression (p=0.0001). These results are in line with the research of Mao et al that states an increase in COX-2 is associated with an increase in TGF-β1. Increased COX-2 induces MDSC immunosuppressive function by producing TGF-β1 via the MAPK/ERK activation pathway against EP2/4 receptors.18
COX-2 influences the increase in MDSC by activating the MAPK/ERK pathway to EP2/EP4 receptors. MDSC infiltrates the tumor microenvironment and stops the immune response by inhibiting T cells and NK cells through increased TGF-β1 production in tumor cells. MDSC also accelerates angiogenesis, tumor development, and metastasis.17,18
Inhibiting the increase in COX2/PGE2 can block the MDSC induction, thus TGF-β1 production is reduced and possibly restore immune function against cancer cells.18 The increase in COX-2 affects the increased MDSC accumulation, which will induce TGF-β1 through the MAPK/ERK pathway. TGF-β1 has a function as an immunosuppressor by inhibiting the function of CD8, NK cells, and DC cells. It causes the tumor cells to escape the body’s immune factors and invade the surrounding area.18
Previous study shows COX-2 is currently the target of new therapies in melanoma because TME factors play a huge role in its pathogenesis, especially in immune factors. COX-2 is said to be the most critical factor in this process. It can influence immunosuppression by inducing MDSC and TGF-β1.10,18,23 Therefore, based on the results of this study, the use of anti-COX-2 is one of the strong considerations as supportive therapy for AM. Multivariate analysis data (Table 4) revealed that COX-2 has a major role in invasion of AM. Its expression simultaneously effect the depth of invasion along with increase of TGF-β1. The limitation of this study is that there was no examination of COX-2 and TGF-β1 expression by the PCR method to strengthen the results of the study. Therefore, the authors suggest the further research to be carried out using PCR.
Conclusion
The expression of COX-2 is associated with depth of invasion on AM by increasing of TGF-β1. Thus, positive COX-2 immunoexpression can be a prediction factor of AM aggressiveness which is related to increasing risk of metastasis. It proves that the immune surveillance has a role in the invasion of AM.
Acknowledgment
The author would like to thank the staff of Pathology Anatomy Installation of Faculty of Medicine Universitas Padjadjaran/Dr. Hasan Sadikin General Hospital for the support during the work of this research. Funding for this research was partly obtained from an internal grant from Universitas Padjadjaran 2020 (No.1427/UN 6.3.1/LT/2020).
Disclosure
The authors report no conflicts of interest for this work.
References
1. Tas F, Erturk K. Acral lentiginous melanoma is associated with certain poor prognostic histopathological factors but may not be correlated with nodal involvement, recurrence, and a worse survival. Pathobiology. 2018;85(4):227–231. doi:10.1159/000488457
2. Wang Y, Zhao Y, Ma S. Racial differences in six major subtypes of melanoma: descriptive epidemiology. BMC Cancer. 2016;16(1):691. doi:10.1186/s12885-016-2747-6
3. Rapisuwon S, Qin Y, Roszik J, Carapeto F, Patel S, Carvajal R. Systemic Therapy for Mucosal, Acral and Uveal Melanoma. In: Balch CM, Atkins MB, Garbe C, editors. In: Cutaneous Melanoma. Springer International Publishing; 2020:1301–1335.
4. Jafarian A, Roshan N, Gharib M, et al. Evaluation of cyclooxygenase-2 expression in association with clinical-pathological factors in malignant melanoma. Iran J Pathol. 2019;14(2):96. doi:10.30699/ijp.14.2.96
5. Seip K. Tumor–microenvironment interactions in malignant melanoma. Impact on metastatic phenotype and drug resistance. Department of Tumor Biology, Institute for Cancer Research, Oslo University Hospital, The Norwegian Radium Hospital, Oslo, Norway; 2017: 11–22
6. Davis LE, Shalin SC, Tackett AJ. Current state of melanoma diagnosis and treatment. Cancer Biol Ther. 2019;20(11):1366–1379. doi:10.1080/15384047.2019.1640032
7. Liu B, Qu L, Yan S. Cyclooxygenase-2 promotes tumor growth and suppresses tumor immunity. Cancer Cell Int. 2015;15(1):106. doi:10.1186/s12935-015-0260-7
8. Usman HA, Hernowo BS, Tobing MDL, Hindritiani R. The major role of NF-κB in the depth of invasion on acral melanoma by decreasing CD8+ T cells. J Pathol Transl Med. 2018;52(3):164. doi:10.4132/jptm.2018.04.04
9. Zhang Y, Kirane A, Huang H, et al. Cyclooxygenase-2 inhibition potentiates the efficacy of vascular endothelial growth factor blockade and promotes an immune stimulatory microenvironment in preclinical models of pancreatic cancer. Mol Cancer Res. 2019;17(2):348–355. doi:10.1158/1541-7786
10. Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182(8):4499–4506. doi:10.4049/jimmunol.0802740
11. Muthu AKM, Phaik-Leng Cheah M, Man Fong Chew MBBSM, Yen-Fa Toh MDM. Cyclooxygenase-2 (COX2) expression in adenocarcinoma surpasses that of squamous cell carcinoma in the uterine cervix. Malays J Pathol. 2017;39(3):251–255. doi:10.1016/j.ijrobp.2004.04.030
12. Zhong W-Q, Chen G, Zhang W, et al. Epithelial-mesenchymal transition in keratocystic odontogenic tumor: possible role in locally aggressive behavior. Biomed Res Int. 2015;2015. doi:10.1155/2015/168089
13. Lv J, Dai B, Kong Y, Shen X, Kong J. Acral melanoma in Chinese: a clinicopathological and prognostic study of 142 cases. Sci Rep. 2016;6(1):1–9. doi:10.1038/srep31432
14. Hashemi Goradel N, Najafi M, Salehi E, Farhood B, Mortezaee K. Cyclooxygenase-2 in cancer: a review Hashemi. J Cell Physiol. 2019;234(5):5683–5699. doi:10.1002/jcp.27411
15. Jana D, Sarkar DK, Ganguly S, et al. Role of cyclooxygenase 2 (COX-2) in prognosis of breast cancer. Indian J Surg Oncol. 2014;5(1):59–65. doi:10.1007/s13193-014-0290-y
16. Mittal D, Gubin MM, Schreiber RD, Smyth MJ. New insights into cancer immunoediting and its three component phases–elimination, equilibrium and escape. Curr Opin Immunol. 2014;27:16–25. doi:10.1016/j.coi.2014.01.004
17. Obermajer N, Kalinski P. Key role of the positive feedback between PGE2 and COX2 in the biology of myeloid-derived suppressor cells. Oncoimmunology. 2012;1(5):762–764. doi:10.4161/onci.19681
18. Mao Y, Sarhan D, Steven A, Seliger B, Kiessling R, Lundqvist A. Inhibition of tumor-derived prostaglandin-e2 blocks the induction of myeloid-derived suppressor cells and recovers natural killer cell activity. Clin Cancer Res. 2014;20(15):4096–4106. doi:10.1158/1078-0432.CCR-14-0635
19. Ghasemi M, Afshar P, Sheidaei S, Moeini Y, Larijani LV. The role of immunohistochemistry expression of COX-2 in differentiating pigmented benign and malignant skin neoplasms. Med J Islam Repub Iran. 2019;33:75. doi:10.34171/mjiri.33.75
20. Tian M, Schiemann WP. PGE2 receptor EP2 mediates the antagonistic effect of COX-2 on TGF-β signaling during mammary tumorigenesis. FASEB J. 2010;24(4):1105–1116. doi:10.1096/fj.09-141341
21. Lv Z-D, Kong B, Li J-G, et al. Transforming growth factor-β 1 enhances the invasiveness of breast cancer cells by inducing a Smad2-dependent epithelial-to-mesenchymal transition. Oncol Rep. 2013;29(1):219–225. doi:10.3892/or.2012.2111
22. Yu X, Buttgereit A, Lelios I, et al. The cytokine TGF-β promotes the development and homeostasis of alveolar macrophages. Immunity. 2017;47(5):903–912. doi:10.1016/j.immuni.2017
23. Jayaraman P, Parikh F, Newton JM, et al. TGF-β1 programmed myeloid-derived suppressor cells (MDSC) acquire immune-stimulating and tumor killing activity capable of rejecting established tumors in combination with radiotherapy. Oncoimmunology. 2018;7(10):e1490853. doi:10.1080/2162402X.2018.1490853
24. Goulet AC, Einsphar JG, Alberts DS, et al. Analysis of cyclooxygenase 2 (COX-2) expression during malignant melanoma progression. Cancer Biology and Therapy. 2003;2(6):713–718.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.