Back to Journals » OncoTargets and Therapy » Volume 12
HIFU is safe, effective, and feasible in pancreatic cancer patients: a monocentric retrospective study among 523 patients
Authors Ning Z, Xie J, Chen Q, Zhang C, Xu L, Song L, Meng Z
Received 27 August 2018
Accepted for publication 27 November 2018
Published 1 February 2019 Volume 2019:12 Pages 1021—1029
DOI https://doi.org/10.2147/OTT.S185424
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Federico Perche
Zhouyu Ning, Jing Xie, Qiwen Chen, Chenyue Zhang, Litao Xu, Libin Song, Zhiqiang Meng
Department of Integrative Oncology, Fudan University Shanghai Cancer Center, Shanghai, China
Purpose: This study aimed to evaluate the clinical value of high-intensity focused ultrasound (HIFU) combined with gemcitabine (GEM) in treating unresectable pancreatic ductal adenocarcinoma (PDAC).
Patients and methods: A total of 523 unresectable PDAC patients were recruited from December 30, 2007 to January 30, 2015 at Fudan University Shanghai Cancer Center. Among them, 347 received HIFU combined with GEM (with regional intra-arterial chemotherapy [RIAC] or with systemic chemotherapy) and the remaining patients received GEM only. Postoperative complications were observed, and overall survival was recorded.
Results: The median overall survival of patients who received HIFU combined with GEM vs GEM alone was 7.4 vs 6.0 months (P=0.002); the 6-month, 10-month, 1-year, and 2-year survival rates for patients in these two groups were 66.3% vs 47.5% (P<0.0001), 31.12% vs 15.9% (P<0.0001), 21.32% vs 13.64% (P=0.033), and 2.89% vs 2.27% (P=0.78), respectively. In the combined therapy group, the most obvious survival benefits were obtained among patients who received HIFU plus RIAC and systemic chemotherapy (used in the intervals between RIAC treatments). There were no severe complications in patients undergoing HIFU treatment.
Conclusion: We demonstrated the survival benefit of HIFU among PDAC patients treated with GEM. The benefit was most obvious in PDAC patients treated with HIFU plus RIAC and systemic chemotherapy.
Keywords: PDAC, HIFU, multimodality therapy, gemcitabine, prognosis
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is a highly lethal cancer that is estimated to cause ~227,000 annual deaths worldwide.1,2 This phenomenon can be attributed to the absence of specific symptoms at early stages,2,3 and ~80% of patients have lost their chance of surgery upon the time of diagnosis, leading to a 5-year overall survival (OS) rate of <1%.4 Unresectable PDAC included unresectable locally advanced pancreatic cancer (LAPC) defined by the following: greater than 180° superior mesenteric artery or celiac encasement, aortic invasion, and unreconstructable superior mesenteric or portal vein involvement,5 metastatic PDAC, and some stage II patients with poorer physical status (Eastern Cooperative Oncology Group >2). For these patients, systemic administration of gemcitabine (GEM) has proven to be the mainstream first-line chemotherapy since 1997; other regimens such as FOLFIRINOX (leucovorin, fluorouracil, irinotecan, and oxaliplatin) and GEM plus nab-paclitaxel are effective regimens that represent the latest advancement in unresectable PDAC. However, patients experienced significantly increased toxicity compared to GEM alone.6,7 Among the randomized controlled trials comparing GEM/erlotinib with GEM alone,8 Moore et al reported the improvement in OS was only about 0.3 months by the addition of erlotinib to GEM in a total of 569 patients.9 Other than systemic chemotherapy, GEM-based regional intra-arterial chemotherapy (RIAC) has been proven to be effective for some metastatic and localized PDAC cancers.10,11 Despite ongoing efforts to establish a more favorable benefit–risk profile for patients receiving chemotherapy, the survival outcome to date is far from satisfactory.12,13
Besides chemotherapy, minimally invasive ablative therapies are another option for patients with unresectable PDAC who cannot undergo curative surgery and have a limited response to systemic chemotherapy. Since the late 1990s, high-intensity focused ultrasound (HIFU) has been used widely in China and Korea and is now recommended by the US National Institutes of Health (2013) as an alternative treatment for unresectable PDAC patients.3 HIFU effectively ablates pancreatic tumors by raising local tissue temperatures as high as 65°C, therefore destroying the tumor cells,1,3,5 breaking the stromal barrier of pancreatic cancer, and enhancing delivery of chemotherapy to pancreatic tumors.14 Some studies have reported that treatment with HIFU combined with chemotherapy has led to desirable results compared to chemotherapy alone.15,16 However, these studies were based on a relatively smaller sample size.
The present study aims to evaluate the potential clinical value of HIFU combined with GEM and examine the safety of HIFU in the treatment of unresectable PDAC.
Patients and methods
Patients
A total of 523 patients with unresectable PDAC were enrolled in our study from December 30, 2007 to January 30, 2015. Of these patients, 347 were treated with HIFU combined with GEM and a total of 176 patients received GEM monotherapy. All the patients were abstracted to an anonymized database and were analyzed retrospectively for baseline demographics, tumor characteristics, treatment details, and outcomes as documented in the medical records. The diagnosis of PDAC was histologically or cytologically confirmed. All patients were informed of the potential benefits and risks of HIFU therapy. This study was approved by the Ethics Committee of Fudan University Shanghai Cancer Center (Shanghai, China), and informed consent was obtained from every patient.
HIFU equipment and therapy
The ultrasound (US) therapeutic system was provided by Chongqing Haifu (HIFU) Tech (Chongqing, China). HIFU therapy was administered before chemotherapy. Patients were instructed to fast for 12 hours before HIFU therapy. The following parameters for the therapeutic US transducer were used: 1) frequency: 0.85 MHz (or 1.04 MHz); 2) focal length: 135.0 mm (or 151.0 mm); 3) diameter: 20 cm; 4) the scanning method: point-by-point method; 5) depth: the distance on the US channel from the center of the tumor to the skin (30–120 cm); and 6) input target energy: 200–400 J/spot. Real-time US was used to target the pancreatic tumor by moving the integrated probe, and the tumor was divided into slices with 5 mm separation by using US images. Blood pressure, pulse, respiration rate, and peripheral oxygenation were monitored during the HIFU treatment.
GEM therapy
GEM therapies were adopted on the next day after HIFU treatment in the combined therapy group, and GEM therapies were undertaken by the following approaches: either as combined therapy with HIFU or applied as monotherapy. For systemic chemotherapy, a dose of 1,000 mg/m2 GEM was administered by a 30-minute intravenous infusion on days 1 and 8 of a 4-week cycle. For RIAC, Seldinger technology was adopted. Angiography of the abdominal cavity artery and other target arteries of the cancer was used to determine the location, infringing range, and blood supply of the carcinoma. Next, a catheter was inserted into the target through the femoral artery. A dose of 1,000 mg/m2 GEM and a dose of 600 mg/m2 fluorouracil were administered for 30 minutes on day 1 of a 4-week cycle. For RIAC + systemic chemotherapy, RIAC was implemented on day 1 and a dose of 1,000 mg/m2 GEM was administered by a 30-minute intravenous infusion on day 8 of a 4-week cycle.
Safety assessment
The incidence of HIFU-related adverse events (AEs), including burns, abdominal pain, pancreatitis, jaundice, hemorrhage, gastrointestinal (GI) perforation, and intestinal necrosis, as reported in previous studies, was recorded. Serum amylase and urinary amylase levels were tested the next day after treatment.
Quality of life evaluation
Quality of life was assessed by the European Organization for Research and Treatment of Cancer quality of life questionnaire core 30. The European Organization for Research and Treatment of Cancer quality of life questionnaire core 30 encompasses 30 items. It incorporates five items which entail body function, role function, emotion function, and society function. Besides, it also evaluates the existence of fatigue, pain, nausea, and vomiting. Dyspnea, insomnia, loss of appetite, constipation, diarrhea, and economic difficulty are the aspects that need to be considered. The ultimate score ranges from 0 to 100. The function-associated score is in positive proportion to the well-being of the patient. The score associated with symptom and side effects is in adverse proportion to the patients’ well-being. Moreover, we assessed pain relief among these pancreatic patients. The degree of pain was evaluated by a numerical rating score, as depicted in the universally acknowledged brief pain inventory. The score ranges from 0 to 10; 0 refers to no pain at all and 10 refers to unbearable pain.
Patients’ follow-up
The OS was defined as the time from the date of pathologically confirmed diagnosis to the date of the last follow-up or the date of death. Censoring occurred if patients were still alive at the last follow-up or died of other causes.
Statistical analyses
All statistical analyses were conducted using SPSS 22.0, and data were expressed as the mean ± SD. Continuous variables were compared with the Student’s t-test or the Mann–Whitney U-test. Categorical variables were compared using chi-squared analysis or Fisher’s exact test, and exact 95% CIs were computed. OS was calculated according to Kaplan–Meier analysis, and P-values were evaluated by the log-rank test for censored survival data. P-values <0.05 were considered statistically significant.
Results
HIFU ablated the pancreatic tumor completely
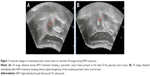
By scanning with a continuous HIFU beam and sweeping from the deep to the shallow regions of the tumor, the targeted regions in each slice of the tumor were completely ablated. US image obtained immediately after HIFU treatment showed obvious hyperechogenicity of the treated pancreatic tumor (Figure 1).
Patient characteristics
The patients’ characteristics are summarized in Tables 1 and 2. The average age in the combined therapy group and the GEM monotherapy group was 61.0 and 60.5 years, respectively. The male-to-female ratios were 1.94:1 and 1.38:1 in the two groups, respectively. Of the 347 combined therapy patients, 26 (7.5%) were at stage II, 75 (21.6%) were at stage III, and the remaining patients were at stage IV. A total of 73 (21%) patients had masses located in the head and neck of the pancreas, and 274 patients (79%) had masses in the body and tail of the pancreas. In the GEM monotherapy group, 22 (12.5%) patients had stage II disease, 31 (17.6%) had stage III disease, and 123 (69.9%) had stage IV disease. Seventy-nine (44.9%) patients had masses in the head and neck of the pancreas, and 97 (55.1%) in the body and tail of the pancreas. Chi-squared tests showed no differences between the two groups with respect to age, sex, and stage, but there was a difference with respect to tumor location. Of the 347 patients in the combined therapy group, there were 85 patients in the HIFU plus systemic chemotherapy group (group A), 128 patients in the HIFU plus RIAC group (group B), and 134 patients in the RIAC plus systemic chemotherapy group (group C).
  | Table 1 Baseline characteristics of patients in our study |
  | Table 2 Baseline characteristics of patients in the combined therapy group |
Among patients in the cohort of combined therapy, a total of 265 had liver metastasis and 42 had lung metastasis. Also, 56 patients had bone metastasis and the number of patients who had metastasis to peritoneum and lymph node was 36 and 23, respectively. In the gemcitabine-only group, the number of patients with metastasis to liver, lung, bone, peritoneum and lymph node was 93, 21, 34, 29, and 17, respectively.
OS benefits
The median OS of patients in the combined therapy group vs GEM monotherapy group was 7.4 vs 6.0 months (P=0.004; Figure 2). The 6-month, 10-month, 1-year, and 2-year survival rates for patients in these two groups were 66.3% vs 47.5% (P<0.0001), 31.12% vs 15.9% (P<0.0001), 21.32% vs 13.64% (P=0.033), and 2.89% vs 2.27% (P=0.78), respectively. Kaplan–Meier analysis was used to detect significant differences between pairs of groups (group B vs group A, P=0.022; group C vs group A, P=0.719; group C vs group B, P=0.002), as shown in Figure 3, and the median time was 7.9, 6.4, and 9.0 months in groups A, B, and C, respectively. Chi-squared tests showed that there were no differences among the three groups with respect to age, sex, and tumor site (P=0.984, 0.056, and 0.225, respectively) and stage in group C vs group B (P=0.769), but there were differences with respect to stage in group B vs group A (P=0.011) and group C vs group A (P=0.04).
  | Figure 2 The median OS in the combined therapy group (green line) and the gemcitabine monotherapy group (blue line): 7.4 vs 6.0 months, P=0.002. |
AEs and complications as well as quality of life evaluation
Overall, AEs occurred in 27 (7.78%) patients as shown in Table 3. Skin burns did occur in four patients, among whom one had deep second-degree burns. Vertebral injury, identified by MRI, occurred in two patients, although no symptoms were observed. Abdomen and waist pain occurred in two cases and gradually subsided within 1 week of a rectal indomethacin suppository. Obstructive jaundice concomitant with biliary tract infection developed in one case, in which a tumor was located on the head of the pancreas; percutaneous transhepatic cholangial drainage and anti-infective therapy were administered and showed good therapeutic efficacy. The serum amylase levels were increased in 13 cases (7.1%), 6 of which also showed abnormal urinary amylase levels the day after HIFU treatment. A total of 3 patients with pancreatic body cancer developed GI tract bleeding within 1 week of HIFU treatment. Since all of them were diagnosed by black stool and positivity for fecal occult blood, hemostatic treatment was given. Mild fever (<38.5°C) was observed in two cases; therefore, physical cooling was applied. There were no severe HIFU-related complications or AEs, such as severe skin burns or GI perforation, in any of the patients treated. Besides, we observed that the quality of life had improved and pain was reduced by HIFU treatment.
  | Table 3 Adverse events in the combined therapy group |
Tumor size was reduced in PDAC patients with HIFU treatment
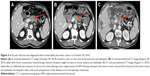
A slight increase in tumor volume (arrowhead) could be observed in some of the PDAC patients after some consecutive chemotherapy. However, these lesions showed a decrease in tumor size and the amount of soft tumor tissue (arrowhead) encasing the celiac artery after some sessions of HIFU therapy concurrent with some additional sessions of chemotherapy, as shown in Figure 4.
Discussion
Our data indicated that the median OS of patients in the HIFU combined with GEM group was prolonged by 1.4 months compared to that of the GEM monotherapy group. The OS was greatly improved in patients with HIFU plus RIAC and systemic chemotherapy (9.0 vs 6.0 months), which demonstrated that local therapies (HIFU and RIAC) combined with systemic chemotherapy may be the optimal treatment strategy. However, there was no statistical difference in the long-term survival (no less than 2 years) between these two groups.
By generating a high local concentration of acoustic energy in the focal spot, thereby inducing a rapid rise of temperature to over 65°C within a few seconds, HIFU treatment ablates tissues deep inside the body, which leads to potential necrosis, liquefaction, and fibrosis of the tissues in a targeted tissue without damaging adjacent vital anatomical structures.1 As recent clinical studies have demonstrated the tolerability and effectiveness of HIFU treatment, it has become an attractive alternative to standard treatment for unresectable PDAC, and the control of primary tumor by HIFU treatment may help prolong the survival of patients with unresectable PDAC.2,4,5,17
It is widely acknowledged that FOLFIRINOX or nab + GEM is the first-line chemotherapy recommended for pancreatic cancer patients. However, the effects of these chemotherapies are limited. Therefore, it is more of clinical value to evaluate the effect of HIFU combined with chemotherapy, such as FOLFIRINOX or nab + GEM. GEM + nab is widely applied among Chinese PDAC patients, whereas FOLFIRINOX is seldom used because few Chinese patients could tolerate this regimen. In this study, we enrolled patients subject to GEM monotherapy, considering their performance status (PS) and their disease stage.
GEM-based systemic chemotherapy remains the mainstay of treatment for PDAC due to its established efficacy in extending the survival of PDAC patients.18 The median OS of patients who received GEM alone and combinations of GEM with other antitumor drugs was about 6–10 months.19–26 Compared with systemic chemotherapy, RIAC generates high drug concentrations in the target areas while maintaining low systemic drug levels, thereby reducing the risk of systemic toxicity while improving the drug efficacy in the target tissue.27,28 Some studies have suggested that GEM-based RIAC has more clinical benefits by improving the median survival time of patients with metastatic pancreatic cancer and causing fewer systemic complications in comparison with systemic chemotherapy.29,30 Therefore, HIFU combined with RIAC and/or systemic chemotherapy should be a feasible and effective treatment strategy for unresectable PDAC and represents an ideal option for palliative therapy.
A recent clinical study examined HIFU in combination with systemic GEM therapy in patients with LAPC. The estimated median OS was 12.6 months (95% CI=10.2–15.0 months), and the OS rates at 1 and 2 years were 50.6% (95% CI=36.7%–64.5%) and 17.1% (95% CI=5.9%–28.3%), respectively.13 However, this study included only 39 patients with localized unresectable PDAC (stage III) and gemcitabine was used at days 1, 8, and 15. In our study, HIFU plus systemic chemotherapy (group A) showed a reduced survival benefit, with the estimated median OS time being 7.9 months (95% CI=6.7–9.1 months). We suggest that the difference could be ascribed to the difference in HIFU equipment applied in the treatment. Another possible explanation could be the inclusion of more stage IV patients (49/85, 57.6%), and lesser dosage of gemcitabine used in the present study. In order to evaluate the role of age and tumor location on the survival of these pancreatic patients, we detected their effects not only on the entire cohort of patients, but also on the combined group and the gemcitabine-only group. Results have shown that age was an independent prognostic factor in the subgroups of these PDAC patients. Primary tumor location was also a prognostic factor affecting OS. However, as a retrospective study, this is a deficiency which could not be totally avoided.
There are no similar studies reporting about HIFU combined with RIAC treatment strategy (group B) used in patients with unresectable PDAC, in whom the median OS time was 6.4 months, which did not show any survival benefit compared with group A. There data are in contrast to some studies which showed that GEM-based RIAC had superior clinical benefits in comparison with systemic chemotherapy alone,29,30 and we guess it may result from the higher rate of stage IV PDAC patients in group B than in group A (74.2% vs 57.6%). This may mean that HIFU combined with either GEM-based RIAC or systemic chemotherapy should have the same influence on survival benefit. Also, in future, prospective and well-designed randomized controlled studies (HIFU plus RIAC vs HIFU plus systemic chemotherapy) are warranted.
HIFU combined with RIAC and systemic chemotherapy strategy (group C) has been first reported in the present study, which showed significant survival benefits than the other two groups. The median OS of HIFU combined with GEM-based RIAC and systemic chemotherapy group was 9.0 months, and the 1-year OS rate was 23.1%, which was similar or slightly inferior to that reported in some recent studies about systemic chemotherapy31–35 in the treatment of unresectable PDAC and radio-chemotherapy in the treatment of LAPC and borderline resectable PDAC.36,37 What is more, group C showed survival advantage compared with group A, but there was no significant statistical difference. We assume it may result from the higher rate of stage IV PDAC patients in group C than in group A (76.1% vs 57.6%). The results suggest that pulsed systemic chemotherapy on the basis of HIFU plus RIAC could significantly increase patients’ survival, and it is expected to be the primary option for patients with unresectable PDAC.
Currently, the mechanism of the enhanced effect of HIFU on GEM treatment has not been fully elucidated. Some studies presumed that the mostly mechanistic rationale may be based on two aspects: 1) the hyperthermia caused by pulsed HIFU induces an increase in blood flow to pancreatic mass, which may increase the drug delivery and 2) HIFU can induce structural and molecular changes due to cavitation damage, shear stress, and micro-streaming, all of which may enhance drug extravasations and sensitize the cancer cells.38,39
In terms of safety, high-intensity US beams may produce burns in the tissues that lie between the transducer and the target area. Thermal injury could occur at either the shallower areas of the target lesion or due to unwanted deep penetration through the target area.40 No severe complications were observed in either group in our study, and they mostly occurred in tissues adjacent to the target lesion and lying in the travel path of the HIFU beams. Skin burns did occur in four patients. Among these patients, one developed deep second-degree burns and recovered automatically 4 weeks later. Vertebral injuries were observed in two cases, and bone-related AEs did not occur in these two patients. HIFU entails the risk of biliary perforation or biliary duct damage that can result in malignant biliary obstruction by thermal injury when cancers are located in the head of the pancreas. One patient with a lesion in this location suffered from biliary obstruction accompanied by acute infection of the biliary tract. After receiving percutaneous transhepatic cholangial drainage combined with anti-infection therapy, the patient showed complete recovery. GI bleeding was a rare occurrence following HIFU. In our study, a total of five patients experienced mild GI bleeding, and showed positive fecal occult blood testing (++ to +++) and no melena within a week after hemostatic therapy, and the patients were discharged from the hospital the next week. We speculate that GI bleeding was usually caused by tumor invasion to the adjacent digestive tract or by chemotherapy/radiotherapy which impairs noncancerous tissues, thereby increasing their sensitivity to HIFU scattering,40,41 and may be less directly related to HIFU treatment. Therefore, HIFU should be performed with caution in patients with pancreatic head cancer and body masses, as it is potentially detrimental to the invaded biliary and digestive tracts since these critical structures cannot be avoided when directing the travel path of HIFU beams.
In summary, HIFU in combination with GEM is a well-tolerated modality with promising activity in patients with unresectable PDAC. However, it cannot prolong the long-term survival (no less than 2 years) and more studies are needed to explore the association between HIFU and drug. Despite a lack of randomization and potential selective bias, the outcomes of HIFU combined with GEM in the present study are highly encouraging, and should be an impetus for further studies and hopefully be applied more in clinical practice.
Acknowledgment
The present study was supported by The National Natural Science Foundation of China (No 81774063).
Disclosure
The authors report no conflicts of interest in this work.
References
Lee ES, Lee JY, Kim H, et al. Pulsed high-intensity focused ultrasound enhances apoptosis of pancreatic cancer xenograft with gemcitabine. Ultrasound Med Biol. 2013;39(11):1991–2000. | ||
Wang Z, Ren ZG, Ma NY, et al. Intensity modulated radiotherapy for locally advanced and metastatic pancreatic cancer: a mono-institutional retrospective analysis. Radiat Oncol. 2015;10(1):14. | ||
Ge HY, Miao LY, Xiong LL, et al. High-intensity focused ultrasound treatment of late-stage pancreatic body carcinoma: optimal tumor depth for safe ablation. Ultrasound Med Biol. 2014;40(5):947–955. | ||
Zhou Y. High-intensity focused ultrasound treatment for advanced pancreatic cancer. Gastroenterol Res Pract. 2014;2014(5):1–11. | ||
Choti MA, Dixon E, Tyler D. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement by Callery et al. Ann Surg Oncol. 2009;16(7):1734–1735. | ||
Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–1825. | ||
von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–1703. | ||
Yang ZY, Yuan JQ, di MY, et al. Gemcitabine plus erlotinib for advanced pancreatic cancer: a systematic review with meta-analysis. PLoS One. 2013;8(3):e57528. | ||
Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a Phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25(15):1960–1966. | ||
Nakchbandi W, Müller H, Singer MV, Lohr JM, Nakchbandi IA. Prospective study on warfarin and regional chemotherapy in patients with pancreatic carcinoma. J Gastrointestin Liver Dis. 2008;17(3):285–290. | ||
Liu F, Tang Y, Sun J, et al. Regional intra-arterial vs. systemic chemotherapy for advanced pancreatic cancer: a systematic review and meta-analysis of randomized controlled trials. PLoS One. 2012;7(7):e40847. | ||
Smyth EN, Bapat B, Ball DE, André T, Kaye JA. Metastatic pancreatic adenocarcinoma treatment patterns, health care resource use, and outcomes in France and the United Kingdom between 2009 and 2012: a retrospective study. Clin Ther. 2015;37(6):1301–1316. | ||
Khanal N, Upadhyay S, Dahal S, Bhatt VR, Silberstein PT. Systemic therapy in stage IV pancreatic cancer: a population-based analysis using the national cancer data base. Ther Adv Med Oncol. 2015;7(4):198–205. | ||
Li T, Wang YN, Khokhlova TD, et al. Pulsed high-intensity focused ultrasound enhances delivery of doxorubicin in a preclinical model of pancreatic cancer. Cancer Res. 2015;75(18):3738–3746. | ||
Zhao H, Yang G, Wang D, et al. Concurrent gemcitabine and high-intensity focused ultrasound therapy in patients with locally advanced pancreatic cancer. Anticancer Drugs. 2010;21(4):447–452. | ||
Lee JY, Choi BI, Ryu JK, et al. Concurrent chemotherapy and pulsed high-intensity focused ultrasound therapy for the treatment of unresectable pancreatic cancer: initial experiences. Korean J Radiol. 2011;12(2):176–186. | ||
Vidal-Jove J, Perich E, del Castillo MA. Ultrasound guided high intensity focused ultrasound for malignant tumors: the Spanish experience of survival advantage in stage III and IV pancreatic cancer. Ultrason Sonochem. 2015;27:703–706. | ||
Ko AH, Ah K. Progress in the treatment of metastatic pancreatic cancer and the search for next opportunities. J Clin Oncol. 2015;33(16):1779–1786. | ||
Friess H, Langrehr JM, Oettle H, et al. A randomized multi-center Phase II trial of the angiogenesis inhibitor Cilengitide (EMD 121974) and gemcitabine compared with gemcitabine alone in advanced unresectable pancreatic cancer. BMC Cancer. 2006;6(1):285. | ||
Arshad A, Al-Leswas D, Stephenson J, Metcalfe M, Dennison A. Potential applications of fish oils rich in n-3 fatty acids in the palliative treatment of advanced pancreatic cancer. Br J Nutr. 2011;106(6):795–800. | ||
Bayraktar S, Rocha-Lima CM. Advanced or metastatic pancreatic cancer: molecular targeted therapies. Mt Sinai J Med. 2010;77(6):606–619. | ||
Gillen S, Schuster T, Meyer Zum Büschenfelde C, Friess H, Kleeff J. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010;7(4):e1000267. | ||
Cunningham D, Chau I, Stocken DD, et al. Phase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2009;27(33):5513–5518. | ||
Herrmann R, Bodoky G, Ruhstaller T, et al. Gemcitabine plus capecitabine compared with gemcitabine alone in advanced pancreatic cancer: a randomized, multicenter, Phase III trial of the Swiss Group for Clinical Cancer Research and the Central European Cooperative Oncology Group. J Clin Oncol. 2007;25(16):2212–2217. | ||
Park JK, Ryu JK, Lee JK, et al. Gemcitabine chemotherapy versus 5-fluorouracil-based concurrent chemoradiotherapy in locally advanced unresectable pancreatic cancer. Pancreas. 2006;33(4):397–402. | ||
Park B-B, Park JO, Lee HR, et al. A Phase II trial of gemcitabine plus capecitabine for patients with advanced pancreatic adenocarcinoma. Cancer Chemother Pharmacol. 2007;60(4):489–494. | ||
Yang M, Jq L, Tang ZQ. Study on pharmacokinetics of PLGA GEM sustained-release microspheres in nude mice with pancreatic cancer. Chin Pharm J. 2010;45:295–299. | ||
Tanaka T, Yamamoto K, Sho M, et al. Pharmacokinetic evaluation of pancreatic arterial infusion chemotherapy after unification of the blood supply in an animal model. J Vasc Interv Radiol. 2010;21(1):116–121. | ||
Aigner KR, Gailhofer S. Celiac axis infusion and microembolization for advanced stage III/IV pancreatic cancer – a Phase II study on 265 cases. Anticancer Res. 2005;25(6):4407–4412. | ||
Li Q, Wang MQ, Duan LX. Regional arterial infusion chemotherapy with lipid emulsion as a solvent for the treatment of advanced pancreatic cancer: a preliminary clinical study. J Interv Radiol. 2009;18:275–277. | ||
von Hoff DD, Ramanathan RK, Borad MJ, et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a Phase I/II trial. J Clin Oncol. 2011;29(34):4548–4554. | ||
Ramanathan RK, Goldstein D, Korn RL, et al. Positron emission tomography response evaluation from a randomized Phase III trial of weekly nab-paclitaxel plus gemcitabine versus gemcitabine alone for patients with metastatic adenocarcinoma of the pancreas. Ann Oncol. 2016;27(4):648–653. | ||
Goldstein D, El-Maraghi RH, Hammel P, et al. Nab-paclitaxel plus gemcitabine for metastatic pancreatic cancer: long-term survival from a Phase III trial. J Natl Cancer Inst. 2015;107(2):dju413. | ||
David G, Robert Hassan EM, Pascal H. Updated survival from a randomized Phase III trial (MPACT) of nab-paclitaxel plus gemcitabine versus gemcitabine alone for patients (pts) with metastatic adenocarcinoma of the pancreas. Presented at: Gastrointestinal Cancers Symposium; 2014; San Francisco, CA, USA. | ||
Borazanci E, Schroeder K, Jameson G. Reinitiating nab-paclitaxel plus gemcitabine in patients with advanced pancreatic cancer. Presented at: Gastrointestinal Cancers Symposium; 2014; San Francisco, CA, USA. | ||
Mellon EA, Hoffe SE, Springett GM, et al. Long-term outcomes of induction chemotherapy and neoadjuvant stereotactic body radiotherapy for borderline resectable and locally advanced pancreatic adenocarcinoma. Acta Oncol. 2015;54(7):979–985. | ||
Moningi S, Dholakia AS, Raman SP, et al. The role of stereotactic body radiation therapy for pancreatic cancer: a single-institution experience. Ann Surg Oncol. 2015;22(7):2352–2358. | ||
Kim Y-S, Rhim H, Choi MJ, Lim HK, Choi D. High-intensity focused ultrasound therapy: an overview for radiologists. Korean J Radiol. 2008;9(4):291–302. | ||
Poff JA, Allen CT, Traughber B, et al. Pulsed high-intensity focused ultrasound enhances apoptosis and growth inhibition of squamous cell carcinoma xenografts with proteasome inhibitor bortezomib. Radiology. 2008;248(2):485–491. | ||
Yu T, Luo J. Adverse events of extracorporeal ultrasound-guided high intensity focused ultrasound therapy. PLoS One. 2011;6(12):e26110. | ||
Lee KJ, Kim HM, Jung JW, et al. Gastrointestinal hemorrhage after concurrent chemoradiotherapy in locally advanced pancreatic cancer. Gut Liver. 2013;7(1):106–111. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.