Back to Journals » Cancer Management and Research » Volume 11
Heterogeneity of targeted lung lesion predicts platinum-based first-line therapy outcomes and overall survival for metastatic triple-negative breast cancer patients with lung metastasis: a “PET biopsy” method
Authors Xie Y, Gu B , Hu X , Zhang Y, Zhang J, Wang Z, Zhao Y, Gong C, Li Y, Yang Z, Wang B
Received 6 February 2019
Accepted for publication 5 May 2019
Published 2 July 2019 Volume 2019:11 Pages 6019—6027
DOI https://doi.org/10.2147/CMAR.S204364
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Chien-Feng Li
Yizhao Xie,1,2,* Bingxin Gu,2–5,* Xichun Hu,1,2 Yingjian Zhang,2–5 Jian Zhang,1,2 Zhonghua Wang,1,2 Yannan Zhao,1,2 Chengcheng Gong,1,2 Yi Li,1,2 Zhongyi Yang,2–5 Biyun Wang,1,2
1Department of Medical Oncology, Fudan University Shanghai Cancer Center, Shanghai, People’s Republic of China; 2Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, People’s Republic of China; 3Department of Nuclear Medicine, Fudan University Shanghai Cancer Center, Shanghai, People’s Republic of China; 4Center for Biomedical Imaging, Fudan University, Shanghai, People’s Republic of China; 5Shanghai Engineering Research Center of Molecular Imaging Probes, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Background: Platinum-based chemotherapy is widely used as first-line therapy for metastatic triple-negative breast cancer (mTNBC). Intratumor heterogeneity derived from fluorine-18 fluorodeoxyglucose (18,F-FDG) positron emission tomography/computed tomography (PET/CT) is a potential predictor of treatment outcomes and the prognosis of breast cancer. However, the presence of multiple lesions and complex calculation methods leads to difficulties in the clinical use of this parameter for metastatic breast cancer. The aim of this study is to provide a convenient and effective measurement of intratumor heterogeneity to predict treatment outcomes for mTNBC patients with lung metastasis.
Patients and methods: We enrolled mTNBC patients with lung metastasis who underwent 18,F-FDG PET/CT scans before first‐line therapy from three clinical trials (NCT01287624, NCT02341911 and NCT02546934). Apart from the regular FDG parameters, including standard uptake value (SUV), total lesion glycolysis (TLG) and metabolic tumor volume (MTV), we defined the lung index as the SUVmean divided by the difference between the SUVmax and SUVmean for the targeted lung lesion. The MTV was automatically exported from the manual delineation using software based on an adaptive threshold of SUV intensity >2.5 within the contouring margin. The TLG was calculated using the following formula: TLG=SUVmean×MTV. Progression‐free survival (PFS) and overall survival were estimated by the Kaplan–Meier method, and univariate and multivariate analyses were performed using the Cox proportional hazard model.
Results: The data from 31 patients were available for analysis. The median PFS of low-lung index (LI) patients was 8.1 months, which was significantly longer than that of high-LI patients (HR=3.3, 95% CI 1.5–7.3, p=0.003). Patients with low TLG had a significantly better PFS than those with high TLG (HR=2.6, 95% CI 1.2–5.8, p=0.014). Patients with low TLG had significantly longer overall survival than those with high TLG (31.2 months vs 13.9 months, HR=3.1, 95% CI 1.2–8.6, p=0.029). Multivariate analysis confirmed the predictive value of LI and TLG.
Conclusions: This study proposed a new “PET biopsy” method to evaluate the intratumor heterogeneity of mTNBC on pretreatment 18,F-FDG PET/CT scans and indicated the predictive value of LI and TLG for first-line platinum-based treatment outcomes and overall survival. These findings could help clinicians recognize patients who are likely to not only have a favorable response to platinum-based therapy but also a good prognosis.
Keywords: triple-negative breast cancer, chemotherapy, PET, tumor heterogeneity
Introduction
Breast cancer remains the most common malignant tumor and a major cause of death among women worldwide.1 In China, 278.9 thousand women were newly diagnosed with breast cancer, and 66 thousand women died of breast cancer in 2014, mostly from metastatic breast cancer (MBC).2 Triple-negative breast cancer (TNBC) is breast cancer with negative estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor-2 expression, and TNBC accounts for 15–20% of breast cancer.3 TNBC has a higher recurrence rate and worse prognosis than other breast cancer subtypes, and metastatic triple-negative breast cancer (mTNBC) only has a median overall survival (OS) of 1–1.5 years.4,5 Due to the limited number of therapeutic targets, chemotherapy remains the cornerstone for treating mTNBC. Platinum-based chemotherapy agents are widely used in the treatment of mTNBC.6,7 Since there are large differences in the treatment response, the demand for finding valuable predictive factors to identify mTNBC patients sensitive to platinum-based treatment is of vital importance.
Biomarkers have been proven to be good predictors for TNBC, both in early stage and metastatic stage disease.5,8 However, the biopsy procedure was not widely tolerated by patients, which resulted in its limited clinical use.
Intratumor heterogeneity is a potential predictor of treatment outcome and prognosis for different types of cancer, including esophageal cancer, lung cancer and breast cancer.9–11 Functional molecular imaging such as fluorine-18 fluorodeoxyglucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) provides a convenient and noninvasive method to evaluate tumor heterogeneity as well as conduct texture analysis. Several studies have explored the predictive value of tumor heterogeneity or PET/CT parameters of MBC; however, the methods to measure the tumor heterogeneity of MBC were different and complicated in these studies due to multiple tumor lesions and various breast cancer subtypes, which created difficulties in the clinical use of this parameter.11–13
In primary lung cancer, intratumor heterogeneity was a confirmed prognostic factor.10,14 Thus, we hypothesized that the heterogeneity of targeted lesions in the lungs of mTNBC patients with lung metastasis could also be a potential predictive and prognostic marker. In this study, we proposed a new “PET biopsy” method as a simple quantitative measurement to identify the tumor heterogeneity of mTNBC patients with lung metastasis and explored the predictive value of this parameter for first-line platinum-based therapy response as well as OS. We also evaluated the prognostic value of 18F-FDG PET/CT parameters in these patients.
Patients and methods
Patients
The patients were collected from three prospective clinical trials conducted at the Fudan University Shanghai Cancer Center. These studies evaluated first-line therapy for mTNBC with at least one treatment arm of gemcitabine plus platinum (GP), as follows: a Phase III study (GP arm, NCT01287624) and two ongoing Phase II trials (GP arm, NCT02341911 and NCT02546934). These three clinical trials all compared GP with other chemotherapy regimens (gemcitabine plus paclitaxel, gemcitabine plus carboplatin and platinum plus albumin paclitaxel) for first-line therapy in mTNBC patients. All patients who underwent a whole-body fluorodeoxyglucose (FDG) PET/CT scan within 30 days before first-line GP treatment and had confirmed lung metastatic lesions ≥8 mm were enrolled in this study. All patients who met these criteria were available for subsequent analysis. Since this study is an observational study, no intervention was applied to the patients. mTNBC was defined as unresectable, recurrent or MBC, including de novo stage IV breast cancer. ER, PR and HER-2 weredefined as ER<1%, PR<1%, and HER-2 immunohistochemistry (IHC) 0–1+ or immunohistochemistry (IHC) 2+ and negative fluorescence in situ hybridization, respectively. The tumor response was assessed after every two cycles of treatment until the disease progressed; after disease progression, the survival status was evaluated every 3 months. Medical and imaging data were retrospectively collected from the medical electronic database system.
This study was approved by the Fudan University Shanghai Cancer Center Ethics Committee and Institutional Review Boards for clinical investigation. All of the methods were performed in accordance with the Declaration of Helsinki and the relevant guidelines. All of the patients signed written informed consent forms before the study.
PET/CT scan
18F-FDG was automatically generated by a cyclotron (Siemens CTI RDS Eclipse ST, Knoxville, TN, USA), and the radiochemical purity was over 95%. All patients were required to fast for at least 6 hrs before the exam, and the blood glucose levels were under 10 mmol/L before the administration of 18F-FDG (dose: 7.4 MBq/kg). Before and after the injection, the patients laid down comfortably in a quiet and dimly lit room. All PET/CT scans were acquired on a Siemens biograph 16HR PET/CT scanner (Knoxville, TN, USA) approximately 60 mins after the injection. The PET/CT data acquisition protocol was as follows: CT scanning was first acquired from the proximal thighs to the head using a low-dose technique (120 kV, 80–250 mA, pitch 3.6, rotation time 0.5 ms). Immediately after the CT scan, a PET emission scan that covered the identical transverse field-of-view was obtained. We used a Gaussian-filter iterative reconstruction method to reconstruct the PET images. The coregistered images were displayed on a workstation.
Image analysis
Two board-certified experienced nuclear medicine physicians evaluated the images independently on a multimodality computer platform (Syngo, Siemens, Knoxville, TN, USA). The physicians discussed and reached a consensus in cases of discrepancy. Quantification of the tumor glucose metabolic activity was calculated using the standard uptake value (SUV) based on body weight. The targeted lesion of the lung metastasis was defined as the lesion with the largest diameter in the lung, and all analyses were performed on the targeted lesion. The maximum and mean SUV (SUVmax and SUVmean, respectively) for the targeted lesion were calculated by placing an individual ROI around the lesion on the coregistered and fused transaxial PET/CT images. The metabolic tumor volume (MTV) was automatically exported from the manual delineation using software based on an adaptive threshold of SUV intensity >2.5 within the contouring margin. The total lesion glycolysis (TLG) was calculated with the following formula: TLG=SUVmean×MTV. A new, simple measure of intratumor heterogeneity of lung metastasis known as the lung index (LI) was defined as the SUVmean divided by the difference between the SUVmax and SUVmean [(SUVmax- SUVmean)/SUVmean].
Statistical analysis
The quantitative data were presented as medians, and the categorical data were presented as counts (percentage). Since the study population was a small cohort, we selected the median as the cutoff value for the imaging parameters. The clinicopathological characteristics were summarized with descriptive statistics and compared between the two groups by the chi-square test. The treatment outcome was assessed by progression-free survival (PFS) and OS. PFS was measured from the date of GP initiation to the first documented disease progression or death. OS was defined as the time between the date of GP initiation and the date of death or last follow-up. Disease progression was determined by Response Evaluation Criteria in Solid Tumors version 1.1. PFS and OS were estimated using the Kaplan–Meier method and compared using the log-rank test. Hazard ratios with two-sided 95% CIs were calculated with unadjusted and adjusted Cox proportional hazards models for possible prognostic factors. A p-value <0.05 was considered statistically significant. All statistical analyses were managed using SPSS (IBM) version 23.0.
Results
Patient characteristics
A total of 31 patients who met the inclusion criteria were enrolled, and their data were analyzed in this study. The patient characteristics and disease characteristics at baseline are shown in Table 1. The median age was 47 years. All patients, except one de novo stage IV patient, underwent surgery and had metastatic disease. A total of 19.4% of the patients had 3 or more metastatic sites. In addition to lung lesions, 16.1% of patients had liver metastasis, and 22.6% of patients had bone metastasis.
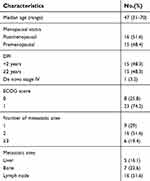 |
Table 1 Patients characteristics |
Predictive value of the baseline characteristics
With a median 35-month follow-up, 29 of the 31 patients experienced progressive disease, and 20 of the 31 patients died. The median PFS (mPFS) was 7.7 months (95% CI 6.8–8.6), and the median OS was 15.4 months (95% CI 10.1–20.8).
In terms of the relationship between the baseline characteristics and PFS, patients with liver metastasis had a significantly shorter PFS than those without liver disease (HR=3.6, 95% CI 1.2–10.7, p=0.02), even after balancing DFI, metastatic sites and FDG parameters in the multivariate analysis (p=0.039). Regarding OS, patients without liver metastasis had better survival outcomes than patients with liver metastasis (HR=4.0, 95% CI 1.1–15.3, p=0.038) in the univariate analysis but not in the multivariate analysis (p=0.12). Patients with one metastatic site were more likely to have a better outcome than those with 2 or more metastatic sites (HR=2.4, 95% CI 0.7–7.3, p=0.13), but this trend did not reach statistical significance. The detailed analysis is shown in Tables 2 and 3.
 |
Table 2 Summary of univariate and multivariate on PFS analysis |
 |
Table 3 Summary of univariate and multivariate on OS analysis |
Predictive value of the PET parameters
We evaluated the predictive value of intratumor heterogeneity and PET parameters. The cutoff point was determined by the median value of the LI (0.56), SUVmax (4.31), MTV (1.25 mL) and TLG (3.54 g). The median PFS of low-LI patients was 8.1 months, which was significantly longer than that of high-LI patients (5.2 months, HR=3.3, 95% CI 1.5–7.3, p=0.003, Figure1A). Patients with low TLG had a significantly better PFS than those with high TLG (mPFS 8.1 months vs 5.9 months, HR=2.6, 95% CI 1.2–5.8, p=0.014, Figure 1B). The SUVmax and MTV showed no significant value in predicting PFS with first-line therapy. In the multivariate analysis, LI was proven to be an independent predictor of PFS (p=0.03).
 |
Figure 1 Kaplan–Meier curves for progression-free survival by low and high level of LI (A) and TLG (B).Abbreviations: LI, lung index; TLG, total lesion glycolysis. |
With regard to OS,
 |
Figure 2 (A and B) Kaplan–Meier curves for overall survival by low and high level of LI (A) and TLG (B).Abbreviations: LI, lung index; TLG, total lesion glycolysis. |
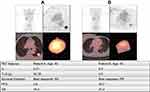 |
Figure 3 Examples of patient analysis.Abbreviations: LI, lung index; TLG, total lesion glycolysis; PFS, progression-free survival; OS, overall survival; PR, partial response; SD, stable disease . |
Discussion
Our study revealed a new method to determine the intratumor heterogeneity of MBC patients with lung lesions and investigated its potential predictive value for first-line treatment response and OS. We found that baseline LI and TLG values derived from 18F-FDG PET/CT could be potential predictors of treatment outcome for mTNBC. We defined this method as a “PET biopsy” given that heterogeneity is traditionally be derived from a pathological biopsy. Focusing on a targeted lesion on PET/CT allows for the analysis of PET parameters and intratumor heterogeneity to be simple and convenient for clinical use.
The CBCSG006 study enrolled 240 mTNBC patients and randomly assigned them to the GP and GT groups for first-line therapy.6 The results showed that the mPFS was 7.73 months in the cisplatin plus gemcitabine group and 6.47 months in the paclitaxel plus gemcitabine group (p=0.009). Based on this clinical trial, GP regimens have been widely used as first-line therapy for mTNBC.
Previous studies have evaluated biomarkers to predict the response to first-line platinum-based therapy for mTNBC and indicated that gBRCA1/2 mutations could predict the sensitivity of platinum-based therapy.5,15 However, considering the low rate of gBRCA mutation expression, which is only present in 8–12% of patients with TNBC, we tried to introduce a more general predictive marker. Tumor heterogeneity is an important feature of malignant cancer and is associated with cancer recurrence, metastasis, survival and drug resistance. 18F-FDG PET/CT is commonly used to determine tumor heterogeneity with the use of traditional parameters among different kinds of malignant tumors but is mainly applied for early or middle-stage disease.9,10,16 Because of the presence of multiple lesions, unbalanced baseline and treatment characteristics, complex methods to represent heterogeneity and a limited number of patients who undergo PET/CT, few studies have focused on mTNBC. We enrolled 31 patients from previous or ongoing clinical trials in our center to avoid bias on the baseline characteristics, treatment methods, disease evaluation and image interpretation.
Our results suggested that the new measurement method of tumor heterogeneity, LI, was a potential predictor of first-line therapy response for mTNBC with a cutoff value of 0.56, which corresponds to the outcomes reported by a previous study using a more complicated method.12,13 In addition, we also provided evidence that LI is an independent predictor of PFS after first-line therapy in the multivariate analysis. In this way, doctors could identify patients who are sensitive to platinum-based therapy. Moreover, we observed a trend that LI predicts OS, though this trend was not statistically significant (p=0.18). The reason behind the insignificance may lie in the limited sample size as well as the subsequent confounding treatments.
With regard to the traditional PET parameters, we found that patients with high TLG showed shorter first-line PFS (p=0.014) as well as OS (p=0.029) than those with low TLG at a cutoff value of 3.54 g. This phenomenon indicated that the combined features of FDG uptake per volume on the targeted lung lesion might be another potential predictor of treatment outcome in mTNBC. In contrast, the SUVmax and MTV did not appear to have prognostic value in this study, which was to some extent inconsistent with the results of previous studies on mTNBC.10,12 The reason could be that we analyzed a targeted lesion instead of analyzing all lesions of metastases as was done in prior research. Furthermore, one of the previous research studies did not evaluate TLG,12 and the another study had a large bias of confounding factors.10
The mechanism of tumor heterogeneity and the relationship between tumor heterogeneity and treatment outcome, however, remain uncertain. Several studies have explored and found that coding mutations, exogenous mutagens, DNA methylation, chromatin remodeling and posttranslational modification of histones can contribute to heterogeneity within tumors.17 Other studies indicated that the mechanisms of drug resistance and intratumor heterogeneity may be attributed to the heterogeneous therapeutic response within a tumor, the reduction of toxicity effects of the anticancer compound in the tumor, which could partly explain the poor outcomes of platinum-based therapy in our study, competitive selection during the evolution of cancer to a metastatic disease and genetic alterations that lead to increased de novo metastatic lesions.18,19 Until now, few studies have considered the reverse of tumor heterogeneity, which requires more effort.
Limitations existed in this study. First, our study was based on a small sample size from three clinical trials performed over different periods, and our results need to be confirmed by further prospective studies with larger cohorts. Second, heterogeneity of the targeted lung lesion may not represent the overall tumor heterogeneity conditions, and further studies are needed to determine the best method of evaluating, diagnosing and managing tumor heterogeneity.
Conclusion
This study proposed a new “PET biopsy” method to evaluate the intratumor heterogeneity of mTNBC on pretreatment 18F-FDG PET/CT scans and indicated the predictive value of LI and TLG for first-line platinum-based treatment outcome and OS. These findings could help clinicians recognize patients who are likely to have a favorable response to platinum-based therapy as well as a good prognosis.
Ethics approval
This study was approved by the Fudan University Shanghai Cancer Center Ethics Committee and Institutional Review Boards for clinical investigation. All of the methods were performed in accordance with the Declaration of Helsinki and the relevant guidelines. All of the patients signed written informed consent forms before the study.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available due to hospital policy but are available from the corresponding author upon reasonable request.
Acknowledgments
The authors would like to thank the patients, nurses and clinicians for their participation in this study. This work was supported by the Chinese Society of Clinical Oncology Youth Committee (CSCO YOUNG) through funding. This work was supported by grants from the National Natural Science Foundation of China (81874114). This work was supported by Shanghai Engineering Research Center of Molecular Imaging Probes (19DZ2282200) through funding.
Author contributions
Yizhao Xie collected all of the data, did the statistical analysis and wrote the manuscript. Bingxin Gu participated in the data analysis and revision of the manuscript. Xichun Hu, Yingjian Zhang, Jian Zhang and Zhonghua Wang provided the data. Chengcheng Gong, Yi Li and Yannan Zhao revised the manuscript. Biyun Wang and Zhongyi Yang designed, carried out the study and revised the manuscript. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Fitzmaurice C, Dicker D, Pain A, et al. The global burden of cancer 2013. JAMA Oncol. 2015;1(January 2014):505–527. doi:10.1001/jamaoncol.2015.0735
2. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China. CA Cancer J Clin. 2016;66(April):115–132. doi:10.3322/caac.21338
3. Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363(20):1938–1948. doi:10.1056/NEJMra1001389
4. Li X, Yang J, Peng L, Sahin AA, Huo L. Triple-negative breast cancer has worse overall survival and cause-specific survival than non-triple-negative breast cancer. Breast Cancer Res Treat. 2016;161(2):279–287
5. Zhang J, Lin Y, Sun XJ, et al. Biomarker assessment of the CBCSG006 trial: A randomized phase III trial of cisplatin plus gemcitabine compared with paclitaxel plus gemcitabine as first-line therapy for patients with metastatic triple-negative breast cancer. Ann Oncol. 2018;29(8):1741–1747. doi:10.1093/annonc/mdy209
6. Hu X-C, Zhang J, Xu B-H, et al. Cisplatin plus gemcitabine versus paclitaxel plus gemcitabine as first-line therapy for metastatic triple-negative breast cancer (CBCSG006): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2015;16(4):436–446. doi:10.1016/S1470-2045(15)70064-1
7. Staudacher L, Cottu PH, Diéras V, et al. Platinum-based chemotherapy in metastatic triple-negative breast cancer: the Institut Curie experience. Ann Oncol. 2011;22(4):848–856. doi:10.1093/annonc/mdq461
8. Stover DG, Coloff JL, Barry WT, Brugge JS, Winer EP, Selfors LM. The role of proliferation in determining response to neoadjuvant chemotherapy in breast cancer: a gene expression-based meta-analysis. Clin Cancer Res. 2016;22(24):6039–6050. doi:10.1158/1078-0432.CCR-16-0471
9. Tixier F, Le Rest CC, Hatt M, et al. Intratumor heterogeneity characterized by textural features on baseline 18F-FDG PET images predicts response to concomitant radiochemotherapy in esophageal cancer. J Nucl Med. 2011;52(3):369–378. doi:10.2967/jnumed.110.082404
10. Kang SR, Song HC, Byun BH, et al. Intratumoral metabolic heterogeneity for prediction of disease progression after concurrent chemoradiotherapy in patients with inoperable stage III non-small-cell lung cancer. Nucl Med Mol Imaging (2010). 2014;48(1):16–25. doi:10.1007/s13139-013-0231-7
11. Zhao Y, Liu C, Zhang Y, et al. Prognostic value of tumor heterogeneity on 18F-FDG PET/CT in HR+HER2− metastatic breast cancer patients receiving 500 mg fulvestrant: a retrospective study. Sci Rep. 2018;8(1):4–10. doi:10.1038/s41598-017-18445-0
12. Gong C, Ma G, Hu X, et al. Pretreatment 18 F‐FDG uptake heterogeneity predicts treatment outcome of first‐line chemotherapy in patients with metastatic triple‐negative breast cancer. Oncologist. 2018;23(10):1144–1152. doi:10.1634/theoncologist.2018-0001
13. Marinelli B, Espinet-Col C, Ulaner GA, et al. Prognostic value of FDG PET/CT-based metabolic tumor volumes in metastatic triple negative breast cancer patients. Am J Nucl Med Mol Imaging. 2016;6(2):120–127.
14. Cook GJR, Yip C, Siddique M, et al. Are pretreatment 18F-FDG PET tumor textural features in non-small cell lung cancer associated with response and survival after chemoradiotherapy? J Nucl Med. 2013;54(1):19–26. doi:10.2967/jnumed.112.107375
15. Tutt A, Tovey H, Cheang MCU, Kernaghan S, Kilburn L, Gazinska P. A randomised phase III trial of carboplatin compared with docetaxel in BRCA1/2 mutated and 2 pre-specified triple negative breast cancer “BRCAness” subgroups: the TNT trial. Nat Med. 2018;24(5):628–637. doi:10.1038/s41591-018-0009-7
16. Son SH, Kim DH, Hong CM, et al. Prognostic implication of intratumoral metabolic heterogeneity in invasive ductal carcinoma of the breast. BMC Cancer. 2014;14(1):585. doi:10.1186/1471-2407-14-585
17. McGranahan N, Swanton C. Clonal heterogeneity and tumor evolution: past, present, and the future. Cell. 2017;168(4):613–628. doi:10.1016/j.cell.2017.01.018
18. Ellsworth RE, Blackburn HL, Shriver CD, Soon-Shiong P, Ellsworth DL. Molecular heterogeneity in breast cancer: state of the science and implications for patient care. Semin Cell Dev Biol. 2017;64:65–72. doi:10.1016/j.semcdb.2016.08.025
19. Ramos P, Bentires-Alj M. Mechanism-based cancer therapy: resistance to therapy, therapy for resistance. Oncogene. 2015;34(28):3617–3626. doi:10.1038/onc.2014.314
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
