Back to Journals » Therapeutics and Clinical Risk Management » Volume 12
Herpetic esophagitis following bendamustine-containing regimen
Authors Yamane H , Monobe Y, Tanikawa T, Ochi N , Honda Y, Kawamoto H, Takigawa N
Received 13 October 2015
Accepted for publication 31 January 2016
Published 2 June 2016 Volume 2016:12 Pages 883—886
DOI https://doi.org/10.2147/TCRM.S98217
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Hiromichi Yamane,1 Yasumasa Monobe,2 Tomohiro Tanikawa,3 Nobuaki Ochi,1 Yoshihiro Honda,1 Hirofumi Kawamoto,3 Nagio Takigawa1
1Department of General Internal Medicine 4, 2Department of Pathology, 3Department of General Internal Medicine 2, Kawasaki Medical School, Kita-ku, Okayama, Japan
Abstract: A 76-year-old Japanese woman presented to our hospital with anorexia. Two years before, she was diagnosed with non-Hodgkin’s lymphoma and had received ten cycles of systemic chemotherapy. After salvage chemotherapy with bendamustine and rituximab (B–R), bone marrow suppression had lasted >3 months. Esophagogastroscopy revealed polynesic white protrusions in the mid-esophagus. These lesions were diagnosed as herpetic esophagitis. To the best of our knowledge, there is no other report in which herpetic esophagitis has been documented as an adverse event of B–R regimen. Because the complication could cause symptomatic gastrointestinal discomfort, physicians should be aware of this disease.
Keywords: bendamustine, herpetic esophagitis, immunodeficiency, non-Hodgkin’s lymphoma, rituximab
Introduction
Combination chemotherapy consisting of bendamustine and rituximab (B–R) has become one of the standard regimens for patients with indolent non-Hodgkin’s lymphoma (i-NHL). In several randomized clinical trials that established the efficacy and safety of the B–R regimen,1,2 the toxicity profile of the regimen was generally low grade with predominant hematologic toxicity. However, lymphopenia and infection with cytomegalovirus (CMV) were frequently observed in the B–R regimen compared to the standard regimen consisting of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone (R-CHOP).1 These results indicated that the B–R regimen might lead to immunodeficiency in some patients. Although herpetic esophagitis (HE) caused by herpes simplex virus is rarely reported in immunocompetent individuals, it is documented during periods of immunosuppression in patients with human immunodeficiency virus infection and so on. Therefore, HE is considered as an opportunistic disease.3 Here, we present the clinical course of a patient with an HE complication, following four cycles of B–R regimen for recurrent i-NHL. This case report was approved by the Ethics Committee of the Kawasaki Medical School and conformed to the Declaration of Helsinki (1975). Written informed consent was obtained from the patient’s proxies.
Case presentation
A 76-year-old Japanese woman presented to our hospital because of anorexia. She had a history of advanced right breast cancer, and a total right mastectomy with right axillary lymph node dissection was performed 25 years ago without additional chemotherapy or adjuvant radiation therapy. Seventeen months before presentation, she was diagnosed with a follicular lymphoma that transformed to a diffuse large B-cell lymphoma (t-FL). According to Ann Arbor classification, the tumor was clinically staged as IVA because the patient presented with multiple sites of nodal and extranodal involvement and diffuse skin involvement of thorax and multiple lymph nodes (neck, axilla, and mediastinum). The patient had received six cycles of R-CHOP for 5 months and was recorded as having complete remission once. Four months later, her disease relapsed with multiple mass lesions in the left breast. A fine-needle aspiration biopsy specimen from the tumor confirmed the recurrence of lymphoma; therefore, the patient received four cycles of B–R regimen every 3 weeks and achieved complete remission.
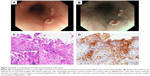
After completion of the B–R regimen, lymphopenia had continued over 3 months (Figure 1). During this time, the patient gradually lost her appetite and had gastrointestinal discomfort. To perform supportive care and to investigate the cause of anorexia, she was admitted again with peripheral blood lymphopenia of 385/μL. Esophagogastroscopy revealed polynesic white protrusions with shallow ulcers in the mid-esophagus (Figure 2A and B). These lesions were diagnosed as HE because several squamous cells had intranuclear inclusion bodies of Cowdry’s Type A (Figure 2C), and these cells were positively stained by anti-herpes simplex virus antibody (Figure 2D).
  | Figure 1 Clinical course of the case. |
Oral administration of valacyclovir (1,000 mg/d) was initiated as an antiviral treatment. Although appetite loss and gastrointestinal discomfort were relieved in 2 weeks, she developed severe interstitial pneumonia. Since CMV antigenemia test (C7-HRP) was positive (15 of 50,000 white blood cells), ganciclovir (500 mg/d) was administered intravenously. Unfortunately, she passed away because of CMV pneumonia without t-FL relapse.
Discussion
We reported a first case of HE as an adverse event of B–R regimen. Bendamustine is a water-soluble, bifunctional chemotherapeutic agent that also has potential antimetabolite properties and only partial cross-resistance with other alkylating agents.4 Designed in 1963 and reappraised in 1990s, the unique mechanism of action and favorable side-effect profile of this drug promise a major role in the management of lymphoproliferative disorders.4
However, an increase in viral infection is frequently noted as an adverse event of bendamustine-containing regimen. Gafter-Gvili et al5 reported a retrospective analysis of 234 patients treated with bendamustine-containing regimen in order to determine the incidence of various infections. They reported that 109 patients (46.6%) developed at least one infection and that 33.76% had severe infections. Seventy-six patients (41.5%) developed bacterial infections, nine patients (3.8%) had fungal ones, and 26 patients (11.5%) had viral ones. As discovered in the present case, reactivation of CMV has been reported in up to 20% of patients treated with bendamustine-containing regimen.6,7 Furthermore, a case report of Epstein–Barr virus-associated lymphoproliferative disorder, which was attributable to the immunosuppressive state, resulting from B–R regimen, has recently been published.8 These results indicate that bendamustine-containing regimen might lead to states of immunodeficiency compared to other regimens and that HE has the potential to easily develop in cases where bendamustine-containing regimen is used for patients with i-NHL. As the principal complaint in this case was a vague gastrointestinal discomfort and appetite loss, it was not initially obvious that HE was the cause of the symptoms. Usually, the main complaints of HE are reported as fever and appetite loss, with retrosternal chest pain due to esophageal stenosis secondary to mucosal edema, especially in cases of immunodeficiency.3,9,10 Although there were no stenotic lesions or edematous mucosa detected in the patient’s esophagus, her symptoms were relieved after treatment with valacyclovir. Thus, we suspected that HE was one of the causes of her gastrointestinal discomfort. However, the relationship between her symptoms and HE seemed to be unclear. Because she was in immunodeficient condition following heavy treatment for malignant lymphoma, her symptoms might be caused by systemic infection and/or latent disease progression of malignant lymphoma.
To the best of our knowledge, there is no other report in which HE has been documented as an adverse event of B–R regimen. Although an analysis of accumulated cases is required, this first report seems to be important because histological and endoscopic diagnosis was documented.
Conclusion
In conclusion, since HE may cause symptomatic gastrointestinal discomfort, physicians should be aware of this adverse event caused by chemotherapeutic regimens such as B–R.
Author contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in either drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that no conflicts of interest exist, including any financial and personal relationships with other people or organizations that could inappropriately influence this work.
References
Rummel MJ, Niederle N, Maschmeyer G, et al; Study group indolent Lymphomas (StiL). Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet. 2013;381:1203–1210. | ||
Flinn IW, Van Der Jagt R, Kahl BS, et al. Randomized trial of bendamustine-rituximab or R-CHOP/R-CVP in first-line treatment of indolent NHL or MCL: the BRIGHT study. Blood. 2014;123:2944–2952. | ||
Canalejo Castrillero E, García Durán F, Cabello N, García Martínez J. Herpes esophagitis in healthy adults and adolescents: report of 3 cases and review of the literature. Medicine (Baltimore). 2010;89: 204–210. | ||
Tageja N, Nagi J. Bendamustine: something old, something new. Cancer Chemother Pharmacol. 2010;66:413–423. | ||
Gafter-Gvili A, Ribakovsky E, Mizrahi N, et al. Infections associated with bendamustine containing regimens in hematological patients: a retrospective multi-center study. Leuk Lymphoma. 2015;25:1–7. | ||
Hosoda T, Yokoyama A, Yoneda M, et al. Bendamustine can severely impair T-cell immunity against cytomegalovirus. Leuk Lymphoma. 2013;54:1327–1328. | ||
Hasegawa T, Aisa Y, Shimazaki K, Nakazato T. Cytomegalovirus reactivation with bendamustine in patients with low-grade B-cell lymphoma. Ann Hematol. 2015;94:515–517. | ||
Muroi K, Sakata-Yanagimoto M, Sato T, et al. Late occurrence of Epstein-Barr virus associated lymphoproliferative disorder in a patient with follicular lymphoma treated with bendamustine and rituximab. Ann Hematol. 2015;94(12):2061–2062. | ||
Généreau T, Lortholary O, Bouchaud O, et al. Herpes simplex esophagitis in patients with AIDS: report of 34 cases. The Cooperative Study Group on Herpetic Esophagitis in HIV Infection. Clin Infect Dis. 1996;22:926–931. | ||
Jetté-Côté I, Ouellette D, Béliveau C, Mitchell A. Total dysphagia after short course of systemic corticotherapy: herpes simplex virus esophagitis. World J Gastroenterol. 2013;19:5178–5181. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.