Back to Journals » OncoTargets and Therapy » Volume 13
Hepsin Promotes Epithelial–Mesenchymal Transition and Cell Invasion Through the miR-222/PPP2R2A/AKT Axis in Prostate Cancer
Authors Li R, Li J, Yang H, Bai Y, Hu C, Wu H, Jiang H, Wang Q
Received 3 July 2020
Accepted for publication 22 October 2020
Published 24 November 2020 Volume 2020:13 Pages 12141—12149
DOI https://doi.org/10.2147/OTT.S268025
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjay Singh
Ruiqian Li, Jun Li, Hong Yang, Yu Bai, Chen Hu, Hongyi Wu, Haiyang Jiang, Qilin Wang
Department of Urology, Third Affiliated Hospital of Kunming Medical University, Kunming City, Yunnan Province, People’s Republic of China
Correspondence: Qilin Wang
Department of Urology, Third Affiliated Hospital of Kunming Medical University, Kunming City, Yunnan Province, People’s Republic of China
Tel +86 871-68179202
Email [email protected]
Purpose: To determine the role and underlying mechanism of hepsin in epithelial–mesenchymal transition (EMT) and cell invasion in prostate cancer.
Methods: The expression of hepsin in prostate cancer tissue samples and cell lines was measured by immunohistochemical staining and Western blotting. The EMT and cell invasion abilities of prostate cancer cells were detected by Western blot and transwell assays. RNA transfection was used to inhibit or overexpress related genes. The expression of miR-222 was detected by RT-qPCR. A dual‑luciferase reporter gene assay was performed to determine the target of miR-222.
Results: Hepsin expression was upregulated in prostate cancer tissue samples and cell lines. Inhibition of hepsin attenuated EMT and cell invasion and downregulated the expression of miR-222. Decreased miR-222 expression enhanced the level of PPP2R2A, which in turn attenuated the AKT signaling. Activation of miR-222 or AKT could block the inhibitory effects on EMT and cell invasion induced by hepsin deficiency.
Conclusion: Hepsin promotes EMT and cell invasion through the miR-222/PPP2R2A/AKT axis in prostate cancer.
Keywords: prostate cancer, hepsin, miR-222, PPP2R2A, AKT signaling
Introduction
Prostate cancer (PC) is one of the most common malignancies with high mortality among men worldwide.1,2 Most prostate cancer patients initially respond to androgen ablation. Prostate cancer can metastasize to the liver, bone and lungs. PC metastasis is responsible for the poor prognosis of PC and the majority of deaths related to this disease. Metastasis is a very complicated process and closely related to tumor environment, cell adhesion and cell invasion.3 Moreover, epithelial–mesenchymal transition (EMT) is proposed to be an important mechanism regulating the initial steps in cancer metastasis. Thus, it is important to investigate new targets involved in PC EMT.
Most prostate cancer patients initially respond to androgen ablation. Hepsin is a type II transmembrane serine protease (TTSP) and studies indicated that androgen is related to TTSP family.4 Hepsin is frequently overexpressed in PC5,6 and correlated positively with aggressiveness.7 The functions of hepsin in malignant biological behaviors, including autophagy,8 bone metastasis9 and tumor growth,10 of PC have been well studied. Hepsin is also involved in the diagnosis and prognosis of PC.11 Moreover, several studies have demonstrated a role for hepsin in PC metastasis.12,13 However, the mechanism underlying EMT in PC is largely unknown. Our previous results suggested that hepsin was overexpressed in PC and promoted EMT and invasion in PC.
Hepsin is required for the activation of hepatocyte growth factor (HGF) and subsequently promotes the expression of a specific receptor of HGF, MET.14 A study showed that derepressed hepsin proteolytically augmented HGF/MET signaling.15 Accumulating evidence indicates that microRNAs (miRNAs) are involved in the regulation of the biological functions of cancers, such as autophagy,16 DNA damage,17 pyroptosis18 and metastasis.19 It has been reported that miR-222 can be regulated by MET.20,21 Thus, we hypothesized that miR-222 may be modulated by hepsin. Moreover, miR-222 has been implicated in the development of cancer through the modulation of phosphatase 2A subunit B (PPP2R2A).22,23 PPP2R2A is considered a tumor suppressor and participates in the proliferation, invasion and EMT of cancer cells.24–26 Additionally, PPP2R2A is reported to modulate aggressive behaviors of cancer cells by blocking the AKT signaling.27 The AKT signaling is closely associated with EMT.28,29 In this study, we determined whether miR-222 and PPP2R2A are involved in hepsin-mediated PC EMT progression via the AKT signaling.
In the present study, we found that hepsin promotes EMT and cell invasion in PC cells. Then, we identified the regulatory mechanism involving hepsin in EMT through the miR-222/PPP2R2A/AKT pathway. Thus, our data suggest important roles for hepsin in PC EMT and invasion.
Materials and Methods
Cell Culture and Samples
Prostate epithelial cell line RWPE-1, and androgen-independent human prostate cancer PC3 and DU145 cell lines were obtained from the American Type Culture Collection (ATCC). PC3 cells were derived from metastatic bone and DU145 cells were derived from metastatic brain. Cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum and a 1% penicillin/streptomycin solution. All the cell lines were reauthenticated by short tandem repeat analysis. For AKT-activating treatment, cells were treated with the 10 μM AKT activator SC79 (SF2730, Beyotime, China).
This study has been approved by the Ethics Committee of Yunnan Provincial Oncology Hospital. The procedures related to human subjects were approved by Yunnan Provincial Oncology Hospital. All the patients provided informed consent for the use of their specimens before sampling. Twenty-six paired samples of prostate cancer tissue and benign tissue were obtained from the Oncology Hospital of Yunnan Province. Information of the CRC patients is summarized in Supplementary Table S1.
RNA Transfection
A miR-222 expression plasmid and negative mimics were purchased from Shanghai Genepharma. PC3 and DU145 cells were transfected with the miR-222 mimic or NC mimic using Lipofectamine 2000 (Invitrogen, USA). PC3 and DU145 cells were transfected with siRNA targeting hepsin (5ʹ-ggaucuuccaggccauaaatt-3ʹ and 5ʹ-uuuauggccuggaagaucct-3ʹ) (GenePharma, China). A nontargeting siRNA (5ʹ-uucuccgaacgugucacgutt-3ʹ and 5ʹ-acgugacacguucggagaatt-3ʹ) (GenePharma, China) was used as a negative control (siNC). The transfection efficiency was determined by RT-qPCR and Western blotting.
Immunohistochemistry (IHC) Staining
Prostate cancer and adjacent tissue samples were fixed with 4% paraformaldehyde and dehydrated with ethanol. The samples were embedded in molten paraffin. Paraffin-embedded tissue sections were deparaffinized and heated. Then, the tissue sections were incubated with antibodies and analyzed by using a DAB kit (DA1010, Solarbio, China).
Cell Invasion Assay
The invasive abilities of PC3 and DU145 cells were measured with transwell invasion assays. The upper chambers were coated with FBS-free medium-diluted Matrigel matrix (35234, BD, Biosciences, China). Cells (1x105) were added to the upper chamber of the transwell plate and incubated at 37°C for 24 h. Then, the cells remaining in the upper chamber were removed with cotton swabs. The cells in the lower chamber were fixed and stained with 0.3% crystal violet. The quantification of invasive cells was performed with Image-Pro Plus 6.0.
Dual-Luciferase Assay
Bioinformatics analysis was performed by TargetScan. The 3ʹ-UTR of PPP2R2A encompassing the target sequence for miR-222 was amplified and cloned into the pGL4.49 luciferase reporter plasmid (pGL4.49-PPP2R2A-wt). A mutant 3′-UTR of PPP2R2A was inserted into the pGL4.49 luciferase reporter plasmid (pGL4.49-PPP2R2A-mut). PC3 and DU145 cells were cotransfected with pGL4.49-PPP2R2A-wt or pGL4.49-PPP2R2A-mut and miR-222 mimics or NC mimics. Luciferase activity was measured by using the Dual-Luciferase Reporter Assay Kit (Promega, USA) according to the manufacturer’s instructions.
Western Blot Assay
Whole-cell lysates were prepared in RIPA lysis buffer (R0010, Solarbio, China) and quantified with the BCA Protein Assay Kit (PA115, TIANGEN BIOTECH, China). Cell lysates were separated by SDS-PAGE and then transferred to PVDF membranes (ISEQ00010, Millipore, USA). The membranes were blocked in 10% milk and incubated with primary and secondary antibodies. The following antibodies were used: anti-hepsin (1:2000, ab189246, Abcam, UK), anti-GAPDH (1:5000, ab9485, Abcam, UK), anti-PPP2R2A (1:1000, ab28370, Abcam, UK), anti-AKT (1:1000, ab18785, Abcam, UK), and anti-p-AKT (ser473) (1:1000, 9271, CST, USA), anti-N-cadherin (1:1000, ab18203, Abcam, UK), anti-MMP2 (1:1000, ab235167, Abcam, UK), anti-MMP9 (1:1000, ab38898, Abcam, UK). The intensity of Western blot bands was quantified by ImageJ.
Real-Time Quantitative PCR (RT-qPCR)
Total RNA was isolated with TRIzol reagent (Invitrogen, Thermo Fisher Scientific, USA). cDNA was synthesized with a PrimiScript RT reagent kit (RR047AA, Takara, Japan). RT-qPCR analysis was performed using the Bio-Rad CFX96 Real-Time PCR Detection System with SYBR Green PCR Master Mix (Bio-Rad). The primers used for RT-qPCR were as follows: miR-222 (5ʹ-cgcagctacatctggctactg-3ʹ and 5ʹ-gtgcagggtccgaggt-3ʹ) and U6 (5ʹ-cgcttcggcagcacatatac-3ʹ and 5ʹ-caggggccatgctaatctt-3ʹ).
Statistical Analysis
All experiments were independently performed with at least three biological repeats. All quantitative data are presented as the mean ± SEM, and differences were analyzed by Student’s t test or one-way ANOVA (*p<0.05, **p<0.01, and ***p<0.001).
Results
Hepsin Expression is Upregulated in Prostate Cancer Samples and Cells and Positively Associated with EMT and Cell Invasion in Prostate Cancer Cells
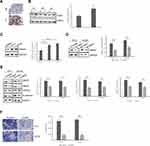
To determine hepsin expression in prostate cancer samples, we performed IHC staining and Western blot of prostate cancer and benign tissue samples. IHC staining showed that prostate cancer tissue (T) has increased hepsin staining compared with benign tissue (N). (Figure 1A). Western blot results indicated that hepsin expression was higher in prostate cancer tissue (Figure 1B). We next investigated the expression levels in the prostate cancer cell lines PC3 and DU145 and prostate epithelial cell line RWPE-1. As shown in Figure 1C, the expression levels in PC3 cells and DU145 cells were increased. Collectively, our results indicated that hepsin might serve as an oncogene in prostate cancer.
To determine the role of hepsin in prostate cancer cell EMT, we established PC3 and DU145 cells with hepsin knockdown (si-hepsin) (Figure 1D). Western blot assays showed that the knockdown of hepsin decreased expression of EMT markers including N-cadherin, MMP2 and MMP9 (Figure 1E). Moreover, hepsin silencing attenuated prostate cancer cell invasion (Figure 1F). These results suggested that hepsin inhibition impaired EMT, accounting for the inhibition of cell invasion.
Hepsin Promotes EMT by Regulating miR-222 Expression
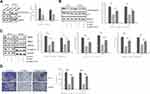
Studies have indicated that MET can be modulated by hepsin.15,30 Moreover, a study showed that miR-222 is a miRNA regulated by MET.31 miR-222 is involved in the development of prostate cancer.32,33 To determine whether miR-222 expression levels are altered in hepsin-deficient prostate cancer cells, we first analyzed miR-222 expression in hepsin-knockdown PC3 and DU145 cells by RT-qPCR. As shown in Figure 2A, hepsin knockdown decreased the expression of miR-122. We next transduced a miR-222 mimic into hepsin-knockdown PC3 and DU145 cells and found that the miR-122 mimic rescued N-cadherin, MMP2 and MMP9 expression in PC3 and DU145 cells (Figure 2A and B). Moreover, miR-222 mimic also reversed the hepsin-mediated inhibition of cell invasion (Figure 2C).
miR-222 Directly Targets the 3ʹ-UTR of PPP2R2A
To investigate the target gene of miR-222, we made predictions by using TargetScan. PPP2R2A is a target gene of miR-222 that plays a significant role in the development of cancers, including breast cancer,34 liver cancer35 and prostate cancer.36 To determine whether miR-222 targets PPP2R2A, we transfected PC3 and DU145 cells with vectors containing the full-length wild-type or mutant 3′-UTR of PPP2R2A and conducted a dual-luciferase reporter gene assay. As shown in Figure 3A and B, miR-222 decreased the luciferase activity of pGL4.49-PPP2R2A-wt but had minimal effects on the luciferase activity of pGL4.49-PPP2R2A-mut. We next treated PC3 and DU145 cells with an NC mimic or a miR-222 mimic and then performed Western blot assays. Western blot analysis showed that miR-222 mimic treatment suppressed PPP2R2A expression (Figure 3C). All these results indicate that miR-222 directly targets PPP2R2A.
Hepsin Promotes EMT and Cell Invasion in Prostate Cancer Cells via the PPP2R2A/AKT Signaling Pathway
Several studies have indicated that PPP2R2A inhibition can enhance AKT signaling37 and that AKT is responsible for the EMT process and cell invasion.38,39 Thus, we detected the AKT phosphorylation status in PC3 and DU145 cells upon hepsin knockdown. As shown in Figure 4A, the p-Akt (ser473)/Akt ratio was decreased in hepsin-knockdown PC3 and DU145 cells.
To check whether AKT signaling accounts for the EMT and cell invasion behaviors of prostate cancer cells, the AKT activator SC7940 was used to treat hepsin-knockdown PC3 and DU145 cells (Figure 4B). SC79 administration blocked the anti-EMT effects of hepsin knockdown (Figure 4C). Moreover, SC79 administration also promoted cell invasion in hepsin-knockdown PC3 and DU145 cells (Figure 4D).
Discussion
In this study, we report that hepsin promotes cell invasion and EMT by modulating the miR-222/PPP2R2A/AKT pathway in prostate cancer. Inhibition of hepsin in prostate cancer cells represses miR-222, which then induces the expression of PPP2R2A. Activated PPP2R2A is able to suppress the AKT pathway. As a consequence, cell invasion and EMT are attenuated by hepsin knockdown. Therefore, our studies reveal the role and underlying mechanism of hepsin in cell invasion and EMT in prostate cancer.
Hepsin is a type II transmembrane serine protease that is critical for the development of prostate cancer. Zhang et al reported that hepsin modulates cell cycle progression in prostate cancer.41 Willbold et al demonstrated that the proteolytic activity of hepsin is related to ER stress and autophagy in prostate cancer cells.8 An inhibitor of hepsin was reported to block prostate cancer bone metastasis.9 Here, we show that hepsin is overexpressed in prostate cancer and that hepsin knockdown inhibits cell invasion and EMT in prostate cancer.
Previous studies have suggested that hepsin is a type II transmembrane serine protease that proteolytically activates HGF. Moreover, the expression of MET, a specific receptor of HGF, can also be upregulated by hepsin.14,42 Accumulating evidence has demonstrated that the miR-222 level is increased by MET.20,21 Based on these data, we hypothesize that miR-222 can be modulated by hepsin. The functional roles of miR-222 in cell invasion and EMT have been well studied. For example, miR-222 has been shown to promote EMT by targeting adiponectin receptor 1 in breast cancer.43 Li et al demonstrated that miR-222 promotes invasion in gastric cancer cells.44 Here, we show that hepsin upregulates the expression of miR-222. In addition, miR-222 overexpression was shown to rescue the inhibitory effects on cell invasion and EMT mediated by hepsin knockdown.
Most miRNAs function by modulating the expression of their target genes. PPP2R2A is a target gene of miR-222 and is considered to be a tumor suppressor.24,45 Studies have indicated that PPP2R2A regulates cancer development via AKT pathways. Zhang et al reported that Pits2 promotes PPP2R2A-mediated AKT inhibition and represses pancreatic carcinogenesis. Zeng et al suggested that PPP2R2A is involved in bladder cancer chemoresistance via the AKT/mTOR axis.22 Our current data indicate that ectopic expression of miR-222 leads to the downregulation of PPP2R2A expression. The PPP2R2A-mediated AKT pathway is responsible for hepsin-induced cell invasion and EMT in prostate cancer.
Conclusion
Collectively, our results indicate that hepsin promotes cell invasion and EMT by increasing the expression of miR-222. As a consequence, upregulated miR-222 reduces PPP2R2A expression, which in turn activates the AKT pathway. Our study is the first to uncover the mechanism involving hepsin in prostate cancer cell invasion and EMT.
Funding
This work was supported by the Joint Special Fund of the Yunnan Science and Technology Department and Kunming Medical University Applied Basic Research, 2018FE001 (−257).
Disclosure
The authors declare that they have no competing interests.
References
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi:10.3322/caac.21262
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
3. Uygur B, Wu W. SLUG promotes prostate cancer cell migration and invasion via CXCR4/CXCL12 axis. Mol Cancer. 2011;10:139. doi:10.1186/1476-4598-10-139
4. Lucas J, True L, Hawley S, et al. The androgen-regulated type II serine protease TMPRSS2 is differentially expressed and mislocalized in prostate adenocarcinoma. J Pathol. 2008;215(2):118–125. doi:10.1002/path.2330
5. Stephan C, Yousef GM, Scorilas A. Hepsin is highly over expressed in and a new candidate for a prognostic indicator in prostate cancer. J Urol. 2004;171(1):187–191. doi:10.1097/01.ju.0000101622.74236.94
6. Kim HJ, Han JH, Chang IH, Kim W, Myung SC. Variants in the HEPSIN gene are associated with susceptibility to prostate cancer. Prostate Cancer Prostatic Dis. 2012;15(4):353–358. doi:10.1038/pcan.2012.17
7. Nandana S, Ellwood-Yen K, Sawyers C, et al. Hepsin cooperates with MYC in the progression of adenocarcinoma in a prostate cancer mouse model. Prostate. 2010;70(6):591–600. doi:10.1002/pros.21093
8. Willbold R, Wirth K, Martini T, Sültmann H, Bolenz C, Wittig R. Excess hepsin proteolytic activity limits oncogenic signaling and induces ER stress and autophagy in prostate cancer cells. Cell Death Dis. 2019;10(8):601. doi:10.1038/s41419-019-1830-8
9. Tang X, Mahajan SS, Nguyen LT. Targeted inhibition of cell-surface serine protease Hepsin blocks prostate cancer bone metastasis. Oncotarget. 2014;5(5):1352–1362. doi:10.18632/oncotarget.1817
10. Holt SK, Kwon EM, Lin DW, Ostrander EA, Stanford JL. Association of hepsin gene variants with prostate cancer risk and prognosis. Prostate. 2010;70(9):1012–1019. doi:10.1002/pros.21135
11. Pace G, Pomante R, Vicentini C. Hepsin in the diagnosis of prostate cancer. Ital J Urol Nephrol. 2012;64(2):143–148.
12. Tripathi M, Nandana S, Yamashita H, Ganesan R, Kirchhofer D, Quaranta V. Laminin-332 is a substrate for hepsin, a protease associated with prostate cancer progression. J Biol Chem. 2008;283(45):30576–30584. doi:10.1074/jbc.M802312200
13. Klezovitch O, Chevillet J, Mirosevich J, Roberts RL, Matusik RJ, Vasioukhin V. Hepsin promotes prostate cancer progression and metastasis. Cancer Cell. 2004;6(2):185–195. doi:10.1016/j.ccr.2004.07.008
14. Mukai S, Yamasaki K, Fujii M, et al. Dysregulation of type II transmembrane serine proteases and ligand-dependent activation of MET in urological cancers. Int J Mol Sci. 2020;21(8). doi:10.3390/ijms21082663
15. Tervonen TA, Belitškin D, Pant SM. Deregulated hepsin protease activity confers oncogenicity by concomitantly augmenting HGF/MET signalling and disrupting epithelial cohesion. Oncogene. 2016;35(14):1832–1846. doi:10.1038/onc.2015.248
16. microRNA-100 enhances autophagy and suppresses migration and invasion of renal cell carcinoma cells via disruption of NOX4-dependent mTOR pathway. Clin Transl Sci. 2020. doi:10.1111/cts.12798
17. Mairinger FD, Werner R, Flom E. miRNA regulation is important for DNA damage repair and recognition in malignant pleural mesothelioma. Virchows Archiv. 2017;470(6):627–637. doi:10.1007/s00428-017-2133-z
18. Wang L, Li K, Lin X. Metformin induces human esophageal carcinoma cell pyroptosis by targeting the miR-497/PELP1 axis. Cancer Lett. 2019;450:22–31. doi:10.1016/j.canlet.2019.02.014
19. Kim J, Yao F, Xiao Z, Sun Y, Ma L. MicroRNAs and metastasis: small RNAs play big roles. Cancer Metastasis Rev. 2018;37(1):5–15. doi:10.1007/s10555-017-9712-y
20. Yang Q, Wang P, Cui J, Wang W, Chen Y, Zhang T. Panax notoginseng saponins attenuate lung cancer growth in part through modulating the level of Met/miR-222 axis. J Ethnopharmacol. 2016;193:255–265. doi:10.1016/j.jep.2016.08.040
21. Acunzo M, Visone R, Romano G. miR-130a targets MET and induces TRAIL-sensitivity in NSCLC by downregulating miR-221 and 222. Oncogene. 2012;31(5):634–642. doi:10.1038/onc.2011.260
22. Zeng L-P, Hu Z-M, Li K, Xia K. miR-222 attenuates cisplatin-induced cell death by targeting the PPP2R2A/Akt/mTOR Axis in bladder cancer cells. J Cell Mol Med. 2016;20(3):559–567. doi:10.1111/jcmm.12760
23. Dong R, Zheng Y, Chen G, Zhao R, Zhou Z, Zheng S. miR-222 overexpression may contribute to liver fibrosis in biliary atresia by targeting PPP2R2A. J Pediatr Gastroenterol Nutr. 2015;60(1):84–90. doi:10.1097/mpg.0000000000000573
24. microRNA-665 is down-regulated in gastric cancer and inhibits proliferation, invasion, and EMT by targeting PPP2R2A. Cell Biochem Funct. 2020. doi:10.1002/cbf.3485
25. Koutsioumpa M, Chen H-W, O’Brien N. MKAD-21 suppresses the oncogenic activity of the miR-21/PPP2R2A/ERK molecular network in bladder cancer. Mol Cancer Ther. 2018;17(7):1430–1440. doi:10.1158/1535-7163.mct-17-1049
26. Liang W-L, Cao J, Xu B. miR-892a regulated PPP2R2A expression and promoted cell proliferation of human colorectal cancer cells. Biomed Pharm. 2015;72:119–124. doi:10.1016/j.biopha.2015.04.015
27. Wang Q, Li J, Wu W. Smad4-dependent suppressor pituitary homeobox 2 promotes PPP2R2A-mediated inhibition of Akt pathway in pancreatic cancer. Oncotarget. 2016;7(10):11208–11222. doi:10.18632/oncotarget.7158
28. Wei R, Xiao Y, Song Y, Yuan H, Luo J, Xu W. FAT4 regulates the EMT and autophagy in colorectal cancer cells in part via the PI3K-AKT signaling axis. J Exp Clin Cancer Res. 2019;38(1):112. doi:10.1186/s13046-019-1043-0
29. Luo J, Yao J-F, Deng X-F. 14, 15-EET induces breast cancer cell EMT and cisplatin resistance by up-regulating integrin αvβ3 and activating FAK/PI3K/AKT signaling. J Exp Clin Cancer Res. 2018;37(1):23. doi:10.1186/s13046-018-0694-6
30. Han Z, Harris PKW, Jones DE. Inhibitors of HGFA, matriptase, and hepsin serine proteases: a nonkinase strategy to block cell signaling in cancer. ACS Med Chem Lett. 2014;5(11):1219–1224. doi:10.1021/ml500254r
31. Yu J, Zhang W, Qian H, Tang H, Lin W, Lu B. SOCS1 regulates hepatic regenerative response and provides prognostic makers for acute obstructive cholangitis. Sci Rep. 2017;7(1):9482. doi:10.1038/s41598-017-09865-z
32. Walter BA, Valera VA, Pinto PA, Merino MJ. Comprehensive microRNA profiling of prostate cancer. J Cancer. 2013;4(5):350–357. doi:10.7150/jca.6394
33. Zhu J, Wang S, Zhang W. Screening key microRNAs for castration-resistant prostate cancer based on miRNA/mRNA functional synergistic network. Oncotarget. 2015;6(41):43819–43830. doi:10.18632/oncotarget.6102
34. Song Q, Song J, Wang Q. miR-548d-3p/TP53BP2 axis regulates the proliferation and apoptosis of breast cancer cells. Cancer Med. 2016;5(2):315–324. doi:10.1002/cam4.567
35. Liu K, Zhao X, Gu J, Wu J, Zhang H, Li Y. Effects of 12C6+ heavy ion beam irradiation on the p53 signaling pathway in HepG2 liver cancer cells. Acta Biochim Biophys Sin. 2017;49(11):989–998. doi:10.1093/abbs/gmx096
36. Pei N, Jie F, Luo J. Gene expression profiling associated with angiotensin II type 2 receptor-induced apoptosis in human prostate cancer cells. PLoS One. 2014;9(3):e92253. doi:10.1371/journal.pone.0092253
37. Wong QW-L, Ching A-K-K, Chan AW-H. MiR-222 overexpression confers cell migratory advantages in hepatocellular carcinoma through enhancing AKT signaling. Clin Cancer Res. 2010;16(3):867–875. doi:10.1158/1078-0432.ccr-09-1840
38. Jin Y, Xu K, Chen Q. Simvastatin inhibits the development of radioresistant esophageal cancer cells by increasing the radiosensitivity and reversing EMT process via the PTEN-PI3K/AKT pathway. Exp Cell Res. 2018;362(2):362–369. doi:10.1016/j.yexcr.2017.11.037
39. Wen W, Ding J, Sun W. Cyclin G1-mediated epithelial-mesenchymal transition via phosphoinositide 3-kinase/Akt signaling facilitates liver cancer progression. Hepatology. 2012;55(6):1787–1798. doi:10.1002/hep.25596
40. Zhu J-L, Wu -Y-Y, Wu D, Luo W-F, Zhang Z-Q, Liu C-F. SC79, a novel Akt activator, protects dopaminergic neuronal cells from MPP and rotenone. Mol Cell Biochem. 2019;461:81–89. doi:10.1007/s11010-019-03592-x
41. Zhang C, Zhang M, Wu Q, Peng J, Ruan Y, Gu J. Hepsin inhibits CDK11p58 IRES activity by suppressing unr expression and eIF-2α phosphorylation in prostate cancer. Cell Signal. 2015;27(4):789–797. doi:10.1016/j.cellsig.2014.12.020
42. Li S, Peng J, Wang H. Hepsin enhances liver metabolism and inhibits adipocyte browning in mice. Proc Natl Acad Sci U S A. 2020;117(22):12359–12367. doi:10.1073/pnas.1918445117
43. Hwang MS, Yu N, Stinson SY. miR-221/222 targets adiponectin receptor 1 to promote the epithelial-to-mesenchymal transition in breast cancer. PLoS One. 2013;8(6):e66502. doi:10.1371/journal.pone.0066502
44. Li N, Yu N, Wang J, et al. miR-222/VGLL4/YAP-TEAD1 regulatory loop promotes proliferation and invasion of gastric cancer cells. Am J Cancer Res. 2015;5(3):1158–1168.
45. Li Z, Tao Y, Wang X. Tumor-secreted exosomal miR-222 promotes tumor progression via regulating P27 expression and re-localization in pancreatic cancer. Cell Physiol Biochem. 2018;51(2):610–629. doi:10.1159/000495281
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.