Back to Journals » OncoTargets and Therapy » Volume 12
Hepatopulmonary syndrome after radiofrequency ablation of recurrent intrahepatic cholangiocarcinoma: a case report
Authors Wang Y, Ma K, Zhong A, Xiong Q, Chen J
Received 16 April 2015
Accepted for publication 25 June 2015
Published 2 April 2019 Volume 2019:12 Pages 2431—2438
DOI https://doi.org/10.2147/OTT.S86702
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jianmin Xu
Yu Wang, Kuansheng Ma, Ai Zhong, Qing Xiong, Jian Chen
Institute of Hepatobiliary Surgery, Southwestern Hospital, People’s Liberation Army Third Military Medical University, Chongqing, People’s Republic of China
Background: Radiofrequency ablation (RFA) is one of the definitive treatment modalities for liver cancer and has been increasingly used in the scenario of small-sized liver cancer. It is generally believed that RFA is minimally invasive and associated with a favorable safety profile in liver cancer patients. However, this interventional technique is subject to some morbidity in high-risk patients, such as those with complicating cirrhosis or a liver cancer located at a refractory segment.
Methods: Herein, we report the case of a middle-aged woman who developed acute liver failure with a complicating respiratory failure after RFA of recurrent intrahepatic cholangiocarcinoma.
Results: A diagnosis of hepatopulmonary syndrome was established. The patient was hospitalized in the intensive care unit for mechanical ventilation. Finally, the patient recovered from an eventful clinical course and survived free of recurrence until the last follow-up visit at 1 year after the discharge.
Conclusion: Our case report warns that hepatopulmonary syndrome, a less common morbidity secondary to liver cancer RFA, should require timely identification and appropriate management due to its life-threatening outcome.
Keywords: liver cancer, radiofrequency ablation, acute liver failure, hepatorenal syndrome, intensive care
Introduction
Liver cancer is the sixth leading cause of cancer-associated mortalities, with a worldwide prevalence, especially in eastern Asian population due to endemic hepatitis viral infection.1 Radiofrequency ablation (RFA) is one of the definitive interventional treatment modalities for liver cancer and is associated with a documented minimal invasiveness and a favorable safety profile.2 This interventional technique also exhibits an oncologic efficacy comparable to radical resection in hepatocellular carcinoma <3 cm.2
RFA is subject to some morbidity in high-risk patients, such as those with a complicating cirrhosis or a liver cancer located at a refractory segment. Previously reported major liver RFA-associated complications are as follows: intraperitoneal/hepatobiliary/gastrointestinal type, such as peritoneal bleeding/sepsis, tumor cell seeding, liver hematoma, hepatic arterial pseudoaneurysm, liver abscess with a complicating diaphragmatic abscess,3–5 liver failure,3–6 biliary tract injury,4–6 gastrointestinal bleeding,5,6 and biliary-enteric fistula;7 pulmonary/pleural type, such as pulmonary embolization4 and pleural effusion; renal type, such as hemolysis, hemoglobinuria, myoglobinuria, rhabdomyolysis, and acute renal failure;8 and other types, such as skin burn,3,9,10 hyperglycemia,5 cardiac infarction,3,6 cardiac arrest, and pericardial effusion.11
Hepatopulmonary syndrome (HPS) is characterized by an oxygenation defect induced by pulmonary vascular dilation in patients with end-stage liver disease.12 Approximately >20% of liver transplant candidates show HPS.13 When patients are diagnosed with HPS, their survival may be significantly decreased compared to other liver transplant candidates.14 However, the therapeutic methods for HPS, including pentoxifylline, methylene blue, and garlic, are not very effective.15,16 In fact, timely identification of HPS is an effective method and an appropriate management to decrease the life-threatening outcome.
Acute respiratory failure (ARF) alone rarely occurs secondary to RFA but may manifest as HPS in liver cancer patients experiencing acute liver failure (ALF) after RFA. Previous studies have shown that RFA is used in the setting of cholangiocarcinoma.17–19 Herein, we report the case of a middle-aged woman who developed ALF with a complicating ARF after RFA of recurrent intrahepatic cholangiocarcinoma (ICC).
Case report
The study was reviewed and approved by the Institutional Review Board and the ethics committee of Southwestern Hospital, People’s Liberation Army Third Military Medical University, Chongqing, People’s Republic of China. The patient or her legal representative provided the written informed consent prior to receiving any invasive diagnostic and therapeutic procedures. The patient had also approved participation in this study.
A 56-year-old woman was referred to our surgical unit with a left-sided liver mass on abdominal ultrasound scan during a routine health check in January 2014. The patient denied a previous history of chronic hepatitis, alcohol abuse, or blood transfusion but had transabdominal subtotal hysterectomy and concomitant unilateral ophorectomy for uterine leiomyoma and histologically confirmed benign ovarian cyst. Physical examination was unremarkable, and routine laboratory tests showed normal results with a serum alpha-fetoprotein level of 3.2 ng/mL (reference limits, 0–20 ng/mL). A more specific marker for cholangiocarcinoma, CA19-9, was also examined, with a serum level of 488.43 U/mL (reference limits, 0–37 U/mL).
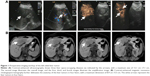
Contrast-enhanced upper abdominal ultrasonography revealed multiple liver tumors involving the left liver lobe and the left–right lobe junction, with the maximum dimensions of 12.1 cm ×7.1 cm (Figure 1A). Further magnetic resonance cholangiopancreatography was performed to delineate the anatomy of the left-sided liver tumor (Figure 1B) as contrast-enhanced computed tomography was contraindicated due to hypersensitivity to radiographic contrast medium. A diagnosis of left-sided liver cancer was suspected, and the liver function reserve in this patient was determined to be Child-Pugh class A. The patient voluntarily gave the written informed consent for elective laparotomy. Under general anesthesia, the patient underwent successful laparotomic left-sided hepatectomy, hilar lymphadenectomy, and concomitant cholecystectomy. Histology showed moderately differentiated ICC and chronic cholecystitis. The patient underwent an uneventful postoperative course.
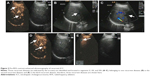
Follow-up upper abdominal ultrasonography on the eighth postoperative week identified three recurrent diseases located in the right lobe, involving Couinaud segments V, VII, and VIII, with sizes of 1.5 cm ×1.3 cm, 1.2 cm ×1.0 cm, and 0.9 cm ×1.0 cm, respectively (Figure 2A–E). The liver function reserve remained class A, and serum alpha-fetoprotein level was 2.9 ng/mL. After obtaining written informed consent from the patient, Doppler ultrasound-guided RFA was performed for these three recurrent diseases using LDRF-120S (Lead Electron Corporation, Mianyang, Sichuan, People’s Republic of China) under conscious sedation anesthesia. The puncture was made through the right subcostal margin, and a total of three sessions of RFA were given at an initial power of 78 W for a total duration of 16 minutes. Follow-up ultrasound scan on post-RFA day 3 identified a filling defect in the ablated area (Figure 3A–C) and a 1.0 cm ×0.8 cm hypoechogenic residual mass located in segment VIII (Figure 3D). Repeated liver and coagulation function tests showed no clinically significant abnormality. A second RFA was performed using the same protocol, at an initial power of 50 W for a total duration of 7 minutes.
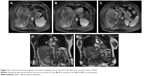
On the second day of the repeated RFA, the patient became febrile (39.2°C); hematology showed hemoglobin 80 g/L (110–160 g/L) (Figure not shown) and white cell count 10.4×109/L (4–10×109/L) (Figure not shown); liver biochemistry showed alanine transferase 5,304 IU/L (0–42 IU/L), asparate transferase 7,906 IU/L (0–42 IU/L) (Figure 4A), albumin 30.7 g/L (38–51 g/L) (Figure 4B), and total bilirubin 38.9 μmol/L (6–21 μmol/L) (Figure 4C); and coagulation function test showed prothrombin time 22.4 seconds and international normalized ratio 1.91. Emergency contrast-enhanced magnetic resonance imaging scan showed extensive liver necrosis (Figure 5A–E); therefore, a diagnosis of RFA-associated ALF was established. Symptomatic treatment included prophylactic antimicrobial therapy, blood transfusion, and nutritional liver support. Lactose enemas in combination with intravenous infusion of a mixture of aspartic acid, ornithine, and arginine were administered due to the presence of hyperammonemia (blood nitrogen 102 μmol/L [10–47 μmol/L]).
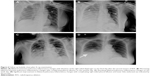
On the same day, the patient suddenly had respiratory distress and also became agitated and subsequently confused and her heart rate progressively increased with a deteriorating pulse oxygen saturation. Arterial blood gas analysis with the patient on nasal inhalation of oxygen at a flow rate of 4 L/min showed PO2 71 mmHg (75–100 mmHg), PCO2 50 mmHg (35–55 mmHg), and pH 7.32 (7.35–7.45). Chest X-ray showed bilateral pulmonary extensive inflammatory changes with complicating pleural effusion. Furthermore, we performed a contrast echocardiogram with agitated saline, which is a gold standard for HPS diagnosis. The contrasted echocardiogram also provided the diagnosis of HPS. Therefore, a diagnosis of HPS was added, considering the sequential occurrence of ALF and ARF (type 1, hypoxia without hypercapnia). Meanwhile, the possibility of acute respiratory distress syndrome or systemic inflammatory response syndrome as a cause of the respiratory pattern observed in the patient was differentiated and excluded.
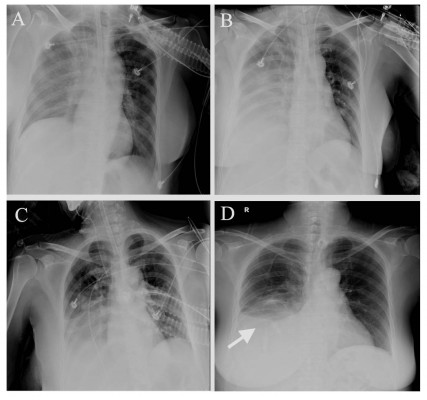
The patient was maintained on transnasal intubation and transferred to the respiratory care unit (RCU) for mechanical ventilation with tracheostomy. Repeated chest X-ray and ultrasound scan revealed progressively improving pulmonary sepsis and pleural effusion (Figure 6A–D), and the patient was weaned from the ventilator 2 weeks after hospitalization in the RCU. The liver function test also showed a steady improvement from the fifth week after RFA (Figure 4A–C). Finally, the patient was discharged from the hospital at the eleventh week after RFA and followed up at the outpatient clinic. The patient was in a generally good condition and has survived free of recurrence as of the last follow-up visit at 1 year after the discharge.
Discussion
Percutaneous RFA under ultrasonographic guidance can effectively ablate small-sized liver cancers, such as primary and recurrent hepatocellular carcinoma, using heat generated from high-frequency alternating current.20,21 Although this interventional technique is generally safe, RFA may be subject to some procedural morbidities, especially in high-risk patients, such as those with an underlying liver condition or an exophytic tumor located in segment V or VIII. In the former condition, the liver has a poor function reserve, while in the latter condition, the neighboring tissues or organs are at a high risk of iatrogenic injury.
ALF is a common complication after RFA for liver cancer and is associated with a high risk of short-term mortality.5 Risk factors predictive of post-RFA ALF are relatively less studied, while the underlying poor liver function reserve was one of the reported significant predictive factors.5 Although our patient had a well-compensated liver disease (noncomplicating ICC) and multiple small-sized tumors ablated within a short time, RFA was initiated and repeated within a short time after the primary surgery, which resulted in the extensive liver necrosis and a consequent hepatic decompensation. A delayed, staged RFA protocol might be required for patients with multiple liver tumors even at a small size, especially if located in the right lobe.
Chest- or lung-associated complications may occur after liver RFA and mainly manifest as pneumothorax and pleural effusion, especially when the liver tumor is located in proximity to the diaphragm.22 These respiratory complications are normally transient and mild and usually resolve after symptomatic treatment.23 ARF is rarely reported in liver cancer patients undergoing RFA. HPS is a relatively less common liver-associated critical condition occurring secondary to acute or chronic liver failure. The major pathogenesis of HPS is believed to result from pulmonary microvascular dilation and angiogenesis caused by a decreased clearance of vasodilators, such as nitric oxide, by the liver,24,25 namely, ventilation–perfusion mismatch. Our patient developed respiratory failure of sudden onset, mimicking acute respiratory distress syndrome, while she had a mild hypercapnia, suggesting the presence of carbon dioxide retention but excluding the possibility of alveolar gas exchange impairment. ARF in this patient could be explained as the result of multiple factors, including ARF, RFA-associated pulmonary and pleural injury, and systemic sepsis. If a confirmed diagnosis of HPS is desired, advanced critical care medicine techniques, such as transthoracic or transesophageal contrast echocardiography and technetium 99m-labeled macroaggregated albumin emission computed tomography scan, can be attempted if applicable.26 However, these techniques were less feasible in this critically ill patient.
HPS is associated with a high short-term mortality rate up to 45% in most severe cases.27 Liver transplantation is believed to be the definitive treatment modality for HPS, while oxygen therapy, including mechanical ventilation and extracorporeal membrane oxygenation,28 and medication with vasoconstricting somatostatin29 have also been attempted, but they exhibited a controversial therapeutic efficacy. In our case, the patient recovered from ALF with a complicating ARF after multimodal treatment, including liver function support, antisepsis, and oxygen therapy, involving an assigned medical team consisting of hepatobiliary surgeons, gastroenterologists, respiratory physicians, critical care physicians, radiologists, and clinical microbiologists. The nonrequirement of liver transplantation in this patient could be primarily attributed to a favorable function reserve before RFA and the absence of underlying liver condition associated with ICC, in contrast to hepatocellular carcinoma frequently with complicating chronic hepatitis and cirrhosis.30,31
Conclusion
To the best of our knowledge, the present case is the first report regarding HPS after RFA of recurrent ICC. ARF may occur in patients who had received RFA for multiple liver tumors even at a small size, and HPS should be timely identified in a patient experiencing ALF with a complicating ARF, as it is associated with serious or even life-threatening consequences. Multimodal treatment should be given to HPS patients, preferably in a well-facilitated RCU. Liver function support and even liver transplantation play an essential role in HPS management.
Acknowledgments
The authors wish to thank the Department of Medical Information, Southwestern Hospital, for the provision of medical data review. The authors would also like to thank Dr Harry H Qin, Institute of Liver Studies, King’s College Hospital, UK, for proofreading and editing the article.
Disclosure
The authors report no conflicts of interest in this work.
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. | ||
Feng K, Yan J, Li X, et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol. 2012;57:794–802. | ||
Wood TF, Rose DM, Chung M, Allegra DP, Foshag LJ, Bilchik AJ. Radiofrequency ablation of 231 unresectable hepatic tumors: indications, limitations, and complications. Ann Surg Oncol. 2000;7:593–600. | ||
Jansen MC, van Duijnhoven FH, van Hillegersberg R, et al. Adverse effects of radiofrequency ablation of liver tumours in the Netherlands. Br J Surg. 2005;92:1248–1254. | ||
Kong WT, Zhang WW, Qiu YD, et al. Major complications after radiofrequency ablation for liver tumors: analysis of 255 patients. World J Gastroenterol. 2009;15:2651–2656. | ||
Kasugai H, Osaki Y, Oka H, Kudo M, Seki T; Osaka Liver Cancer Study Group. Severe complications of radiofrequency ablation therapy for hepatocellular carcinoma: an analysis of 3,891 ablations in 2,614 patients. Oncology. 2007;72(suppl 1):72–75. | ||
Falco A, Orlando D, Sciarra R, Sergiacomo L. A case of biliary gastric fistula following percutaneous radiofrequency thermal ablation of hepatocellular carcinoma. World J Gastroenterol. 2007;13:804–805. | ||
Keltner JR, Donegan E, Hynson JM, Shapiro WA. Acute renal failure after radiofrequency liver ablation of metastatic carcinoid tumor. Anesth Analg. 2001;93:587–589. | ||
Yamagami T, Nakamura T, Kato T, Matsushima S, Iida S, Nishimura T. Skin injury after radiofrequency ablation for hepatic cancer. AJR Am J Roentgenol. 2002;178:905–907. | ||
Pompili M, Riccardi L, Garcovich M, Sollazzi L, Seccia A, Rapaccini G. Severe skin burn at needle entry point complicating radiofrequency ablation for hepatocellular carcinoma. Ultraschall Med. 2012;33:E359–E360. | ||
Zhang Z, Zhuang Z, Xu Z, et al. Post-operative pericardial effusion following treatment of small hepatocellular carcinoma with radiofrequency ablation: a case report. Oncol Lett. 2014;7:345–348. | ||
Rodriguez-Roisin R, Krowka MJ. Hepatopulmonary syndrome, a liver induced lung vascular disorder. N Engl J Med. 2008;358:2378–2387. | ||
Palma DT, Fallon MB. The hepatopulmonary syndrome. J Hepatol. 2006;45:617–625. | ||
Fallon MB, Krowka MJ, Brown RS. Pulmonary vascular complications of liver disease study group. Impact of hepatopulmonary syndrome on quality of life and survival in liver transplant candidates. Gastroenterology. 2008;135:1168–1175. | ||
Almeida JA, Riordan SM, Liu J. Deleterious effect of nitric oxide inhibition in chronic hepatopulmonary syndrome. Eur J Gastroenterol Hepatol. 2007;19:341–346. | ||
Tanikella R, Philips GM, Faulk DK, Kawut SM, Fallon MB. Pilot study of pentoxifylline in hepatopulmonary syndrome. Liver Transpl. 2008;14:1199–1203. | ||
Butros SR, Shenoy-Bhangle A, Mueller PR, Arellano RS. Radiofrequency ablation of intrahepatic cholangiocarcinoma: feasibility, local tumor control, and long-term outcome. Clin Imaging. 2014;38:490–494. | ||
Jiang K, Su M, Zhang W, et al. Complete radiofrequency ablation of hepatolithiasis-associated cholangiocarcinoma and successful management of post-ablation bronchobiliary fistula. Cell Biochem Biophys. 2014;68:555–559. | ||
Patel J, Rizk N, Kahaleh M. Role of photodynamic therapy and intraductal radiofrequency ablation in cholangiocarcinoma. Best Pract Res Clin Gastroenterol. 2015;29:309–318. | ||
Ikeda K, Osaki Y, Nakanishi N, et al. Recent progress in rediofrequency ablation therapy for hepatocellular carcinoma. Oncology. 2014;87:73–77. | ||
Cobianchi L, Ravetta V, Viera FT, et al. The challenge of extraabdominal desmoid tumor management in patients with Gardner’s syndrome: radiofrequency ablation, a promising option. World J Surg Oncol. 2014;12:361. | ||
Shibata T, Shibata T, Maetani Y, et al. Transthoracic percutaneous radiofrequency ablation for liver tumors in the hepatic dome. J Vasc Interv Radiol. 2004;15:1323–1327. | ||
Abbott DE, Sohn VY, Hanseman D, Curley SA. Cost-effectiveness of simultaneous resection and RFA versus-2 stage hepatectomy for bilobar colorectal liver metastases. J Surg Oncol. 2014;109:516–520. | ||
Rodríguez-Roisin R, Krowka MJ. Hepatopulmonary syndrome – a liver-induced lung vascular disorder. N Engl J Med. 2008;358:2378–2387. | ||
Zhang J, Fallon MB. Hepatopulmonary syndrome: update on pathogenesis and clinical features. Nat Rev Gastroenterol Hepatol. 2012;9:539–549. | ||
Aldenkortt F, Aldenkortt M, Caviezel L, Waeber JL, Weber A, Schiffer E. Portopulmonary hypertension and hepatopulmonary syndrome. World J Gastroenterol. 2014;20:8072–8081. | ||
Nayyar D, Man HS, Granton J, Gupta S. Defining and characterizing severe hypoxemia after liver transplantation in hepatopulmonary syndrome. Liver Transpl. 2014;20:182–190. | ||
Auzinger G, Willars C, Loveridge R, et al. Extracorporeal membrane oxygenation for refractory hypoxemia after liver transplantation in severe hepatopulmonary syndrome: a solution with pitfalls. Liver Transpl. 2014;20:1141–1144. | ||
Krowka MJ, Dickson ER, Cortese DA. Hepatopulmonary syndrome. Clinical observations and lack of therapeutic response to somatostatin analogue. Chest. 1993;104:515–521. | ||
Ieluzzi D, Covolo L, Donato F, Fattovich G. Progression to cirrhosis, hepatocellular carcinoma and liver-related mortality in chronic hepatitis B patients in Italy. Dig Liver Dis. 2014;46:427–432. | ||
Suh B, Park B, Shin DW, et al. High liver fibrosis index FIB-4 is highly predictive of hepatocellular carcinoma in chronic hepatitis B carriers. Hepatology. 2015;61:1261–1268. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.