Back to Journals » International Medical Case Reports Journal » Volume 15
Hepatobiliary and Pancreatic Duct Ascariasis: An Unusual Cause of Obstructive Jaundice and Severe Acute Cholangitis
Authors Temesgen R , Abebe H, Abera Y
Received 8 April 2022
Accepted for publication 3 June 2022
Published 11 June 2022 Volume 2022:15 Pages 281—286
DOI https://doi.org/10.2147/IMCRJ.S369442
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ronald Prineas
Rodas Temesgen,1 Haile Abebe,2 Yonas Abera3
1Arbaminch University, School of Medicine, Department of Internal Medicine, Arba Minch, Ethiopia; 2Arbaminch University, School of Medicine, Department of Radiology, Arba Minch, Ethiopia; 3Arbaminch University, School of Medicine, Department of Surgery, Arba Minch, Ethiopia
Correspondence: Rodas Temesgen, Arba Minch University, School of Medicine, Department of Internal Medicine, P.O.Box 21, Arba Minch, Ethiopia, Tel +251-9-87-12-74-42, Email [email protected]
Introduction: Acute cholangitis caused by hepatobiliary ascariasis is quite rare. Factors like a prior history of hepatobiliary surgery, pregnancy, and prolonged fasting increase the possibility of developing hepatobiliary ascariasis.
Case Presentation: We present a case of obstructive jaundice and severe acute cholangitis caused by massive hepatobiliary and pancreatic duct ascariasis in a 52-year-old male patient. The diagnosis was made based on clinical presentation, imaging, and other basic investigations. The treatment consisted of conservative medical treatment, without an anti-helminthic drug to avoid the death of the worm inside the bile duct with subsequent complications. Our patient declined surgical treatment and died three days after self-discharge.
Conclusion: Ascaris lumbricoides is a uncommon cause of acute cholangitis. It should be suspected in cases with typical clinical presentation and imaging evidence suggestive of hepatobiliary ascariasis. Endoscopic treatment is the treatment of choice in addition to anti-helminthic drugs and additional medical support. Conservative treatment alone is less likely to be successful.
Keywords: hepatobiliary ascariasis, acute cholangitis
Background
Ascaris is caused by Ascaris lumbricoides a nematode roundworm, which is among the most common gastrointestinal parasitic infection worldwide. It infects 1.2 billion people worldwide causing about 60,000 deaths per year1,2 The disease is most prevalent in tropical and subtropical developing countries3 Though it is an intestinal parasite, the worm can migrate to extraintestinal organs like the biliary system with an occasional invasion of the biliary tract leading to a variety of complications including cholecystitis, obstructive jaundice, and cholangitis. Hepatobiliary and pancreatic ascariasis are being diagnosed more often recently, with the development of diagnostic imaging techniques (ultrasound, computed tomography scan, etc.) Diagnosis of biliary Ascaris by ultrasound examination of the abdomen can show a mobile worm with an echogenic wall and central hypoechogenicity in the bile ducts and gall bladder with a sensitivity of up to 86%.4 Acute cholangitis is a hepatobiliary tract infection, with high mortality, unless treated early. Urgent biliary decompression is essential to reduce the elevated intra-biliary pressure and improve clinical outcome.5 Ascaris obstruction is a rare etiology of cholangitis6 This case report describes a case of hepatobiliary ascariasis (HBA) complicated by the development of acute cholangitis.
Case Presentation
Fifty-two-year-old man presented with intermittent right upper quadrant abdominal pain and swelling of 3 months duration. He also had nausea and vomiting. Ten days before his admission he had worsening of right upper quadrant abdominal pain, yellowish discoloration of the eye, fever, and vomiting. He had decreased urine amount starting 3 days before his admission. His past medical history is unremarkable; he was not on any medication and reported no allergies. His family history was unremarkable, he is a farmer. He reported not having alcohol and smoking history.
On initial observation, the patient was grossly jaundiced. His vital signs were remarkable for hypotension (with a blood pressure range of 90/70-80/40 mmHg) and temperature in the range of 36.5–38°C. Abdominal palpation revealed abdominal tenderness more on the right upper quadrant with no masses and positive shifting dullness. There was nothing to note on the rest of the examination.
Investigations
Blood tests were conducted which revealed a leucocytosis (17.5×109/L) with a neutrophilia of 85.8%, Hg 12.0 g/dl, Hct 32.2, MCV 94.1fl. Other results include increased creatinine (5.1mg/dl) blood urea nitrogen 118 mg/dl and a slight hypokalaemia (3.3 meq/l). Total bilirubin was elevated at 14.7 mg/dl; indirect bilirubin was raised as well (5 mg/dl) and direct bilirubin was raised markedly (9.7mg/dl). Liver enzymes were elevated (AST 153 U/L and ALT 124 U/L) and ALP was 750 U/L. The coagulation profile was deranged with a low albumin level. Serologic markers for hepatitis B and hepatitis C are negative. Serum amylase was normal; ultrasound showed a significantly dilated intrahepatic and extrahepatic biliary tree (Figure 1) with a common bile duct measuring 3.5 cm (Figure 2). The pancreatic duct is dilated, with an echogenic linear structure within the pancreatic duct up to the second part of the duodenum (Figures 3 and 4). The CBD measured 3.5 cm and the gallbladder was also distended with no stones seen (Figure 2). There were some ascites (Figure 5) and bowel Ascariasis (Figure 3).
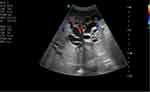 |
Figure 1 Colour Doppler shows dilated intrahepatic biliary tree more on left lobe of liver. |
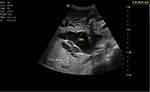 |
Figure 2 Dilated common bile duct (CBD) measuring 35 mm in diameter. |
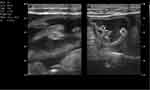 |
Figure 3 Multiple worm having echogenic wall and hypoechoic central part within bowel loops. |
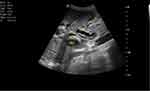 |
Figure 4 Dilated common bile duct (CBD) and pancreatic duct (PD) and linear echogenic wall and central hypoechoic worm within pancreatic duct. |
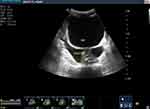 |
Figure 5 Ascites. |
The ultrasound report concluded that there was dilated biliary tree and pancreatic duct 2nd to ascariasis (Figure 4).
Due to the unavailability of ERCP in our setup, it was not performed for our patient.
Treatment
The patient was kept nil by mouth and was put on maintenance fluid. He was started on broad-spectrum antibiotics and analgesia. De-worming was postponed until surgery, or after spontaneous resolution of symptoms, to prevent the death of the worm in the duct. However, when the patient failed to improve, surgical exploration was considered and discussed with the patient. The patient declined surgical intervention; he stayed in the hospital for three more days on medical support. However, the patient was not showing any improvement. He opted for home care and declined any further medical care and was self-discharged against medical advice.
Outcome and Followup
Because the patient did not show up for follow-up or further treatment, the authors/treating physicians tried to contact the patient over the phone. The authors learned from the family that the patient died three days after self-discharge.
Discussion
Ascaris lumbricoides is a parasite infestation that causes biliary obstruction and can be complicated with acute cholangitis. Up to 1.2 billion world’s population is estimated to be infested by Ascariasis.6 The life cycle of A. lumbricoides begins by ingestion of A. lumbricoides ova, which then hatch in the small intestine. The larvae invade the small bowel mucosa, migrate through the systemic circulation to the lungs, ascend to the bronchial tree, and then are swallowed into the small intestine, where they mature into their adult form.7 Although adult ascarids are usually found in the small intestine, they can migrate to various organs such as the lungs, bladder, or biliary system. The disease can have different presentations, depending on the organs and systems affected. The spectrum of clinical diseases includes pulmonary, intestinal (including intestinal obstruction), appendicular, hepatobiliary, and pancreatic ascariasis.8 Some factors increase the possibility of HBA: a prior history of hepatobiliary surgery (cholecystectomy, choledocholithotomy, sphincteroplasty, endoscopic sphincterotomy) Pregnancy, the environment surrounding the worm; for instance, fever, prolonged fasting, etc.9,10 In our patient, even though he has a risk factor to develop ascariasis (low-income country, poor sanitary rural living conditions), no risk factor has been identified that predisposes him for HBA.
The clinical presentation of patients with HBA depends on the affected organ. Biliary colic is the most common presentation, presenting as dull aching pain located in the right upper quadrant, followed by acute cholangitis.11
The diagnosis of biliary ascariasis can be made based on clinical signs that are suggestive of biliary colic and imaging in the form of the ultrasound showing characteristic signs (eg, stripe sign, inner tube sign, or Spaghetti sign) or a cholangiogram demonstrating a filling defect in conjunction with clinical symptoms can be diagnostic.12 Ultrasonography is the imaging of choice, owing to its sensitivity and specificity to demonstrate both worms and their mobility in the biliary system over time.11 The findings reveal a typical longitudinal image of a hyperechoic tubular structure, without acoustic shadow or around hyperechoic structure, with a hypoechoic center (parallel echoic strip). CT and MR cholangiography (MRCP) will demonstrate the intraductal linear filling of the worm, described as ‘bull’s eye’ and ‘eye-glass’ signs. In the presence of coexisting stones or dead or decaying worms, CT, and MRCP had better visualization because they could reveal the duodenum and the ampullary orifice, while ultrasonography could not. ERCP plays a fundamental role in HBA, as its role is both diagnostic and therapeutic, used for direct visualization and extraction.12
Different parameter helps to evaluate the severity of HBA including organ failure, white cell count, high-grade fever, age, bilirubin level, and hypoalbuminemia. Our patient presented with severe acute cholangitis with renal failure and hepatic failure. This made our patient’s prognosis worse.
For our patient, the diagnosis was based on clinical findings and abdominal ultrasonography. Basic laboratory investigations, including complete blood count, liver function test, and stool examination, were obtained for diagnosis. Leucocytosis, elevation of serum bilirubin, transaminases, Alkaline phosphatase, deranged coagulation profile, low albumin, and acute renal failure were noted.
Treatment of biliary ascariasis includes: conservative treatment for acute cholangitis or cholecystitis, which included broad-spectrum antibiotics in acute cholangitis, analgesics, and intravenous fluids. Second, treatment of the helminth with oral anthelmintic, with a single dose of albendazole (400 mg) or a 3-day regimen of mebendazole (200 mg/day). Other medications including pyrantel pamoate, levamisole, piperazine citrate and thiabendazole can be used as well with an efficacy of 90–100%.13 Ideally, a stool sample should be repeated 2 weeks after starting the medication to confirm successful treatment. In biliary ascariasis, anti-helminth should be delayed until the worms spontaneously exit the biliary tree or addressed by endoscopy/surgery. This is because, if treatment is given while the worm is still present in the biliary tree, its death and subsequent carcass can lead to further complications. When conservative treatment fails endoscopic or open surgical management is indicated. ERCP for biliary drainage and decompression is the treatment of choice in biliary ascariasis. The surgical approach was usually indicated in patients with endoscopic failure, or who had coexisting obstructive jaundice and intestinal obstruction. The surgical procedures included choledochotomy and removal of worms and cholecystectomy.
In conclusion, A. lumbricoides is an unusual cause of acute cholangitis; ultrasonography is a quick and diagnostic modality in suspected patients, especially in low-income countries with limited diagnostic modalities like our setup. An endoscopic approach for bile duct clearance along with pharmacotherapy is the mainstay of treatment. The surgical approach is the alternative option in a setup where there is no endoscopy and in case of failure of endoscopic treatment. In biliary ascariasis, anti-helminth should be delayed until the worms spontaneously exit the biliary tree or treated by surgery or endoscopy to prevent its death and subsequent carcass which lead to further complications.
Ethical Clearance
Verbal consent was taken from the patient. Verbal consent was informed, witnessed and recorded. In addition, ethical clearance was obtained from Arba Minch General Hospital and Arba Minch University.
Acknowledgments
We would like to acknowledge the patient for providing us consent to share his history as a case report and Arba Minch general hospital and Arba Minch University for evaluating the case and giving us ethical clearance. Our last aknowledgement goes to Dr Melaku Almaw, a medical intern, for his contribution during this case report.
Funding
No specific grant was obtained for this case report from any funding agency.
Disclosure
The authors declare that there is no conflict of interest regarding the publication of this article.
References
1. Scott ME. Ascaris lumbricoides: a review of its epidemiology and relationship to other infections. Ann Nestlé. 2008;66:7–22.
2. Asaolu SO, Ofoezie IE. Ascaris SPP. In: Global Water Pathogen Project. E Lansing: Michigan State University, UNESCO; 2018.
3. Desita ZT. Biliary ascaris with obstructive jaundice in Ethiopia: a case report. Malays J Med Res. 2014;1:108–110.
4. Das AK. Hepatic and biliary ascariasis. J Glob Infect Dis. 2014;6(2):65–72. doi:10.4103/0974-777X.132042
5. Teng TZJ, Shelat VG. Enterococcus gallinarum causing acute cholangitis in an immunocompetent patient. Surg Infect. 2022;23(1):93–94. PMID: 34491854. doi: 10.1089/sur.2021.209.
6. Bethony J, Brooker S, Albonico M, et al. Soil- transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet. 2006;367:1521–1532. doi:10.1016/S0140-6736(06)68653-4
7. Jethwani U, Singh GJ, Sarangi P, et al.. Laproscopic management of wandering biliary ascariasis. Case Rep Surg. 2012;2012:1–4. doi:10.1155/2012/561563
8. Das AK. Hepatic and biliary ascariasis. J Glob Infect Dis. 2014; 6(2):65–72. doi:10.4103/0974-777X.132042
9. Khuroo MS, Rather AA, Khuroo NS, et al. Hepatobiliary and pancreatic ascariasis. World J Gastroenterol. 2016;22:7507–7517.
10. Das AK. Hepatic and biliary ascariasis. J Glob Infect Dis. 2014;62:65.
11. Saowaros V. Endoscopic retrograde cholangio-pancreatographic diagnosis and extraction of massive biliary ascariasis presented with acute pancreatitis: a case report. J Med Assoc Thai. 1999;82:515–519. PMID:10443103.
12. Ferreyra NP, Cerri GG. Ascariasis of the alimentary tract, liver, pancreas and biliary system: its diagnosis by ultrasonography. Hepatogastroenterology. 1998;45:932–937.
13. Wada K, Takada T, Kawarada Y, et al. Diagnostic criteria and severity assessment of acute cholangitis: Tokyo Guidelines. J Hepatobiliary Pancreat Surg. 2007;14(1):52–58. doi:10.1007/s00534-006-1156-7
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
