Back to Journals » Journal of Hepatocellular Carcinoma » Volume 6
Hepatitis B And Hepatitis C Viral Infections And Associated Factors Among Patients With Diabetes Visiting Gondar Referral Teaching Hospital, Northwest Ethiopia: A Comparative Cross-Sectional Study
Authors Million Y , Teklu T , Alemu S , Ferede A , Belachew T , Desta K
Received 9 July 2019
Accepted for publication 17 September 2019
Published 8 October 2019 Volume 2019:6 Pages 143—150
DOI https://doi.org/10.2147/JHC.S222609
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ahmed Kaseb
Yihenew Million,1 Takele Teklu,2 Shitaye Alemu,3 Aster Ferede,4 Teshome Belachew,1 Kassu Desta5
1Department of Medical Microbiology, School of Biomedical and Laboratory Sciences, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia; 2Department of Immunology and Molecular Biology, School of Biomedical and Laboratory Sciences, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia; 3Department of Chronic Diseases, School of Medicine, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia; 4Department of Public Health, College of Health Sciences, Debre Markos University, Debre Markos, Ethiopia; 5Department of Medical Microbiology, School of Allied Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia
Correspondence: Yihenew Million
Department of Medical Microbiology, School of Biomedical and Laboratory Sciences, College of Medicine and Health Sciences, University of Gondar, PO Box 196, Gondar, Ethiopia
Tel +2515888522141
Email [email protected]
Background: The liver is the major site of Hepatitis B virus and Hepatitis C virus replications. Patients with diabetes tend to be at an increased risk for developing various forms of liver diseases. The infection of the liver can cause or exacerbate diabetes. On the other hand, diabetes can cause or intensify the severity of liver infection. This comparative cross-sectional study was conducted with the aim to determine the prevalence of Hepatitis B and Hepatitis C virus infections and associated factors among patients with diabetes visiting the University of Gondar referral teaching hospital, northwest Ethiopia.
Results: Out of the 610 participants (305 patients with diabetes, 305 people with no diabetes) of the study, 65 (10.7%) were positive for Hepatitis infections, of whom 44 (14.4%) and 21 (6.9%) were positive for at least one of the viruses in patients with diabetes and people with no diabetes, respectively. Out of the diabetic and non-diabetic groups of the study, 26 (8.5%) and 14 (4.6%) (95% CI, 0.96–4.02) were positive for Hepatitis B virus, respectively, while 23 (7.5%) and 7 (2.3%) (95% CI, 1.46–8.68) of the diabetes and non-diabetic groups were positive for Hepatitis C virus, respectively. History of blood transfusion (95% CI, 1.36–12.71) and unprotected sex (95% CI, 1.25–10.15) were significantly associated with Hepatitis B virus infection, while the type of diabetes (95% CI, 1.25–10.89) was associated with anti-Hepatitis C virus positivity.
Conclusion: Positivity for Hepatitis C virus was significantly associated with Type II diabetes. Blood transfusion and unprotected sex were risk factors for Hepatitis B virus infections. Further studies that elaborate temporal associations and find out explanations for the relationship between diabetes and Hepatitis C viral infections are of paramount importance.
Keywords: HBV, HCV, DM, co-infection
Background
In adult, Hepatitis B Virus (HBV) infection usually resolves and develops protective immunity, but Hepatitis C Virus (HCV) infection tends to progress into a chronic infection in most causalities. The incidence of liver cirrhosis or hepatic cell carcinoma (HCC) is high among individuals with chronic HBV and/or HCV infections.1,2 Moreover, HCV has been displayed to produce extrahepatic manifestations.3 The World Health Organization (WHO) estimated that about 350 million people are infected with chronic HBV, and 170 million suffer from chronic HCV infection worldwide.4–6
Diabetes mellitus is a disease condition that encompasses a group of metabolic disorders regarded as hyperglycemia following irregularities in the secretion or action of insulin or both According to American Diabetes Association (ADA), there are three ways to diagnose diabetes are possible: Symptoms of diabetes plus casual plasma glucose concentration greater than or equal to 200 mg/dL (11.1 mmol/), Fasting Plasma Glucose (FPG) greater than or equal to 126 mg/dL (7.0 mmol/L) and 2 hrs post-load glucose greater than or equal to 200 mg/dL (11.1 mmol/L) during an oral glucose tolerance test (OGTT).7 It has been perceived to be rigorously linked with advanced age, obesity, and lack of exercise. On the other hand, studies have shown that hepatitis might contribute to the development of diabetes.8,9 Conversely, a study highlighted that diabetes patients are at high risk for the infection because they are subjected to more frequent medical interventions.10
The liver contributes a significant part in glucose metabolism. Hence, efficient liver function is vital to retain glucose homeostasis. The International Diabetes Federation (IDF) estimated that 415 million adults had diabetes and that the digit will rise to 642 million by 2040. Thus, diabetes has grown into a serious public health problem.11
The seroprevalence of hepatitis B and hepatitis C has been studied among people with a low sensitive method, rapid test kit, in a non-comparative way and on a small sample which was difficult to reach a conclusion about hepatitis infections among diabetic and non-diabetic groups, especially in our study area. We thus determined the prevalence of HCV and HBV infection among diabetic and non-diabetic people.
Methods
A comparative cross-sectional study was conducted from October 2016 to February 2017 at the University of Gondar referral teaching hospital, northwest Ethiopia. The hospital had a range of specialties, 400 beds, over 400 staff and provides referral services to about five million people in the region. The diabetic clinic at the University of Gondar referral teaching hospital was established in 1985 and has been giving services to 5022 registered DM patients and 3024 of them were type II victims.
Diabetes mellitus patients who were on treatment and visiting the diabetic clinic for check-ups were systematically recruited. Voluntary blood donors at the University of Gondar referral teaching hospital with FBS < 100 mg/dL or RBS <126 mg/dL were considered as a comparative group.
The sample size was determined by using the double population proportion formula, considering 80% power and a 95% confidence interval. Systematically selected 305 diabetic patients and 305 age-sex wise matched volunteer blood donors were conveniently selected and included. The study was carried out according to the declaration of Helsinki for human experimentation and ethically cleared by the Departmental Research and Ethics Review Committee (DRERC) of Addis Ababa University, College of Health Sciences, School of Allied Health Sciences, and Department of Laboratory Sciences. After explaining the aim of the study and the possible need for blood tests, written informed consent was obtained from participants. Two senior nurses collected the socio-demographic and other associated data by using a structured and pretested questionnaire.
A senior laboratory technologist collected 5 mL of venous blood sample using sterile disposable vacutainer tube left for 30 mins to facilitate clotting, and the clotted blood was centrifuged to separate the serum from the blood. The serum was divided into two aliquots. One of the aliquots was used for anti-HCV antibody screening as per the manufacturer’s instructions (Anti-HCV cassette, Linear Chemicals SL, Barcelona, Spain). The other aliquots were used for the serologic status of HbSAg. ELISA was used to measure both hepatitis B surface antigen (HBsAg) and anti-HCV. SPSS version 20 software was used to enter, clean and analyze the data collected. Our study used Student’s t-test for continuous variables and Chi-square for the categorical types. A multivariate logistic regression model was fitted taking two or more of the independent variables into consideration to simultaneously determine the value of the dependent variable for each subject.
Results
Socio-Demographic Characteristics
Diabetic patients were enrolled systematically while they were on follow-ups either for glucose monitoring or treatments, and clients who had medical check-up or diagnosed with disease conditions other than DM were recruited as a comparative group. Thirteen participants who had random blood glucose of greater than 160 mg/dL were excluded from the study. In this study, a total of 610 participants (305 diabetic, 305 non-diabetic) were enrolled. The mean age was 37.7 and 36.3 years among diabetic patients and non-diabetic groups, respectively. The majority, 56.4% of the diabetic and 59.7% participants, were male. Among individuals who were tested with HBV and HCV serology, no significant differences were observed in terms of age and sex between the groups (p=0.056 and p=0.412, respectively). The mean duration of diabetes was 6.38 ± 5.29 (range 1–22) years, and the mean random blood sugar for the comparative group was 89.43 ± 9.05 mg/dL, “Table 1”.
Prevalence Of HBV And HCV Infection
Out of the total 610 participants, 65 (10.7%) were found positive for hepatitis infections, of whom 44 (14.43%) and 21 (6.89%) study participants were positive for at least one of the viruses among diabetes and non-diabetic groups, respectively. The prevalence of HBV infection in diabetic and non-diabetic groups was found 26 (8.5%) and 14 (4.6%) (COR=1.94; 95% CI = 0.99–3.79; P-value = 0.053). The burden of sero-positivity against anti-HCV antibody in diabetic and non-diabetic groups was found to be 23/305 (7.5%) and 7/305 (2.3%), respectively, and the difference was statistically significant (COR= 3.47; 95% CI = 1.47–8.23; P-value = 0.005). Co-infection was observed among 5 (1.64%) of the diabetic group, while none was detected among the non-diabetic group, “Table 2”.
 |
Table 2 Burden Of Hepatitis B And Hepatitis C Virus Infection Among Diabetes And Control Groups At University Of Gondar Referral Teaching Hospital (October 2016–February 2017) |
Associated Factors For HBV And HCV Infections
With respect to clinical practices, hospital admission (P-value < 0.001), surgical procedures (P-value < 0.001) and Venous or body piercing (P-value = 0.02) showed statistically significant differences between diabetic and non-diabetic groups. In the bi-variable analysis, only blood transfusion (COR= 3.05, 95% CI; 1.12:8.29) and unprotected sex (COR= 2.75 95% CI; 1.15:6.56) were significantly associated with HBsAg sero-positivity, “Table 3”. The odds of detecting HBsAg among participants with history of blood transfusion, unprotected sex and circumcision were 4.15, 3.56 and 4.58 times greater than with those who had no such history, respectively, “Table 4”. The odds of detecting anti-HCV antibody were 3.69 times greater in T2DM than T1DM (AOR=3.69, 95% CI; 1.25–10.89), “Table 5”.
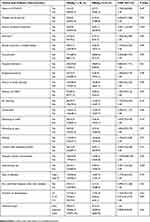 |
Table 3 Bi-Variable Analysis Of Factors For HBsAg Sero-Positivity Among Diabetes Patients At University Of Gondar Referral Teaching Hospital (October 2016–February 2017) |
 |
Table 4 Multi-Variable Analysis Of Factors For HBsAg Sero-Positivity Among Diabetes Patients At University Of Gondar Referral Teaching Hospital (October 2016–February 2017) |
 |
Table 5 Multi-Variable Analysis Of Factors For Anti-HCV Sero-Positivity Among Diabetes Patients At University Of Gondar Referral Teaching Hospital (October 2016–February 2017) |
Discussion
This study included 610 (305 diabetic patients, 305 comparative group) participants grouped according to their blood glucose values. We compared the prevalence of HCV and HBV infection among the participants, and 10.7% of them were found positive for hepatitis infections, whereas 14.43% and 6.89% were sero-positive for at least one of the viruses among diabetic and comparative groups, respectively. Hospital admission, surgical procedures, and venous or body piercing were among significant differences observed among the groups. History of blood transfusion, unprotected sex, and circumcision were the possible factors which were significantly associated with HBV infections. T2DM was significantly associated with anti-HCV positivity. Therefore, diabetic patients were more likely to get HCV infection. This might be related to either the disease itself or to the fact that patients who were infected with HCV might have developed diabetes owing to extrahepatic effects of the virus.
A study conducted in the USA from 1999 to 2010 using the enzyme-linked immunoassay and chemiluminescent immunoassay for the detection of HBc indicated a prevalence of 8.2% (95% CI = 6.8–9.8) HBc sero-positivity for those with diabetes,12 which is in line with the result of the present study. But a study in China reported a higher prevalence of (13.5%) HBV infection among diabetic patients,13 as compared to a prevalence of 8.5% in this study. This might be due to cultural variations, socio-demographic, and epidemiological factors.
The magnitude of HBV infection (8.5%) in our work is higher than that of a study in Ghana,14 Turkey, a study using a third-generation commercial chemiluminescence assay,26 and in Ethiopia, a comparative cross-sectional study using rapid diagnostic test in 2014,15 which indicated a prevalence of 5.5%, 3.8%, and 3.7%, respectively. The prevalence of HBV infection we detected turned out to be higher than that of Woldiya general hospital might be attributed to larger sample size and a higher sensitive diagnostic technique (ELISA) we employed.
The prevalence (7.5%) of HCV infection noted in this study was close to the result of (9.9%) a case–control study at Jimma referral teaching hospital, Ethiopia, screened by a rapid antibody test.16 Our result was slightly lower than that of Nigeria, 2009 (11%),17 India, 2016 (11%),18 Alger, 2012 (12.9%),19 and Pakistan, 2010 (13.7%),20 whereas it was much lower than reports from Iraq, 2015 (15%),21 DHQ teaching hospital, 2014 (27.38%)22 and Egypt, 2007 (32%).23 On the other hand, a higher burden (7.5%) of HCV infection we found was higher compared to that of Sudan, 2014 (1.7%),24 Saudi, 2016 (1.9%),25 and Turkey, 2015 (3.8%).26
In our study, the likelihood of detecting anti-HCV antibody was 3.69 times greater in T2DM than T1DM (AOR= 3.69, 95% CI; 1.25:10.89), which was in line with that of tertiary care hospital, India, which showed all were T2DM.18 Also, HCV infection was detected in one-third of patients with T2DM in DHQ teaching hospital, Pakistan.22 Thus, a higher burden of HCV infection in T2DM can explain the fact that HCV infection contributes to the insulin receptor and insulin signaling defects, leading to T2DM.27,28
In the present study, blood transfusion, unprotected sex, and circumcision were significant predictors of HBV infection. But in other studies, history of invasive procedures and chronic liver disease, duration of diabetes mellitus, poor diabetic regulation, and insulin treatment usage were associated with HBV infection.15 Even though this study did not detect any significant epidemiological factors of HCV infection, other studies showed factors associated with HCV infections among DM patients. These included sharing materials, elevated transaminases, exposure to blood or its products, disease duration, tattooing, blood transfusion, and hospitalization more than two times.25
Conclusion
The difference in the prevalence of sero-positivity against anti-HCV antibody in diabetic and control groups was statistically significant. The association between Hepatitis C virus infection and type II diabetes in the region was statistically significant. Hence, the absence of a single significant predictor for anti-HCV positivity in diabetes group indicates that HCV might have a role in the pathogenesis of diabetes. History of blood transfusion and unprotected sex were significantly associated with HBV infection.
Abbreviations
Ab, Antibody; Ag, Antigen; ALP, Alkaline Phosphatase; AST, Aspartate Transaminase; CDC, Center for Disease Control; CHB, Chronic Hepatitis B Infection; CLD, Chronic Liver Disease; DM, Diabetic Mellitus; DNA, Deoxy Ribose nucleic acid; EIA, Enzyme Immunoassay; ELISA, Enzyme-Linked Immune Sorbent assay; HAV, Hepatitis A virus; HBc, Hepatitis B core; HBIG, Hepatitis B immune Globulin; HBsAg, Hepatitis B surface Antigen; HBV, Hepatitis B Virus; HCC, Hepatocellular Carcinoma; HCV, Hepatitis C Virus; OPD, Out Patient Department; RNA, Ribose Nucleic Acid; SOPS, Standard Operating Procedures; T1DM, Type 1 Diabetic Mellitus; T2DM, Type 2 Diabetic Mellitus; TMB, 3, 3ʹ, 5, 5ʹ-Tetramethylbenzidine.
Ethics Approval And Consent To Participate
Ethical clearance was obtained from the Department Research and Ethics Review Committee (DRERC) of Addis Ababa University, College of Health Sciences, School of Allied Health Sciences, Department of Laboratory Sciences with a protocol number of DRERC/224/16/MLS and the study was conducted in accordance with the declaration of Helsinki. Then, permission was obtained from the University of Gondar referral teaching hospital to access data from the study population. All eligible participants were informed that participation was voluntary and personal information obtained was coded to maintain confidentiality.
Availability Of Data And Material
The datasets used and/or analyzed during the study are available from the corresponding author on reasonable request.
Acknowledgments
We thank all the study participants, data collectors and supervisors for their participation. This manuscript was presented at the Department of Medical Laboratory Sciences, School of Allied Health Sciences, Addis Ababa University for a partial fulfilment of the requirements for MSc degree.
Author Contributions
YM designed and implemented the study, collected data, undertook statistical analysis, performed data interpretation, and drafting the manuscript. TT, SA, TB, AF and KD participated in data analysis and data interpretation and reviewed the manuscript. All authors contributed toward the data analysis, drafting, and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Sorrell MF, Belongia EA, Costa J, et al. National Institutes of Health consensus development conference statement: management of hepatitis B. Hepatology. 2009;49(5 Suppl):S4–S12. doi:10.1002/hep.22946
2. Eke AC, Eke UA, Okafor CI, Ezebialu IU, Ogbuagu C. Prevalence, correlates and pattern of hepatitis B surface antigen in a low resource setting. Virol J. 2011;8(1):12. doi:10.1186/1743-422X-8-12
3. Zignego A, Ferri C, Pileri S, Caini P, Bianchi F. Extrahepatic manifestations of Hepatitis C Virus infection: a general overview and guidelines for a clinical approach. Dig Liver Dis. 2007;39(1):2–17. doi:10.1016/j.dld.2006.06.008
4. Ayele AG, Gebre-Selassie S. Prevalence and risk factors of hepatitis B and hepatitis C virus infections among patients with chronic liver diseases in public hospitals in Addis Ababa, Ethiopia. ISRN Trop Med. 2013;2013:1–7. doi:10.1155/2013/563821
5. Lavanchy D, Kane M. Global epidemiology of hepatitis B virus infection. In: Yun-Fan L, Fabien Z, editors. Hepatitis B Virus in Human Diseases. New York City: Springer; 2016:187–203.
6. Basnayake S, Easterbrook P. Wide variation in estimates of global prevalence and burden of chronic hepatitis B and C infection cited in published literature. J Viral Hepat. 2016;23(7):545–559. doi:10.1111/jvh.12519
7. Association AD. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(Supplement 1):S81–S90. doi:10.2337/dc14-S081
8. Naing C, Mak JW, Ahmed SI, Maung M. Relationship between hepatitis C virus infection and type 2 diabetes mellitus: meta-analysis. World J Gastroenterol. 2012;18(14):1642–1651. doi:10.3748/wjg.v18.i14.1642
9. Reilly ML, Schillie SF, Smith E, et al. Increased risk of acute hepatitis B among adults with diagnosed diabetes mellitus. J Diabetes Sci Technol. 2012;6(4):858–866. doi:10.1177/193229681200600417
10. Kowdley KV, Wang CC, Welch S, Roberts H, Brosgart CL. Prevalence of chronic hepatitis B among foreign‐born persons living in the United States by country of origin. Hepatology. 2012;56(2):422–433. doi:10.1002/hep.24804
11. Organization WH. Global Report on Diabetes. World Health Organization; 2016.
12. Schillie S, Xing J, Murphy T, Hu D. Prevalence of hepatitis B virus infection among persons with diagnosed diabetes mellitus in the United States, 1999–2010. J Viral Hepat. 2012;19(9):674–676. doi:10.1111/j.1365-2893.2012.01616.x
13. Lu J, Hou X, Tu H, et al. Chronic hepatitis B virus infection status is more prevalent in patients with type 2 diabetes. J Diabetes Investig. 2017;8(4):619–625. doi:10.1111/jdi.12609
14. Ephraim R, Nsiah P, Osakunor D, Adoba P, Sakyi S, Anto E. Seroprevalence of hepatitis B and C viral infections among type 2 diabetics: a cross-sectional study in the Cape Coast Metropolis. Ann Med Health Sci Res. 2014;4(5):719–722. doi:10.4103/2141-9248.141529
15. Mekonnen D, Gebre-Selassie S, Fantaw S, Hunegnaw A, Mihret A. Prevalence of hepatitis B virus in patients with diabetes mellitus: a comparative cross sectional study at Woldiya General Hospital, Ethiopia. Pan Afr Med J. 2014;17(1). doi:10.11604/pamj.2014.17.40.2465
16. Ali S, Abera S, Mihret A, Abebe T. Association of hepatitis C virus infection with type II diabetes in Ethiopia: a hospital-based case-control study. Interdiscip Perspect Infect Dis. 2012;2012:1–7. doi:10.1155/2012/354656
17. Ndako JA, Echeonwu GO, Shidali NN, et al. Occurrence of hepatitis C virus infection in type 2 diabetic patients attending Plateau state specialist hospital Jos Nigeria. Virol J. 2009;6(1):98. doi:10.1186/1743-422X-6-19
18. Ramana BV, Sreedhar BKV, Chaudhury A. Prevalence of hepatitis C virus infection in type 2 diabetic patients at a tertiary care hospital. Mintage J Pharm Med Sci. 2013;2(4):23–25.
19. Greca LF, Pinto L, Rados D, Canani L, Gross JL. Clinical features of patients with type 2 diabetes mellitus and hepatitis C infection. Braz J Med Biol Res. 2012;45(3):284–290. doi:10.1590/s0100-879x2012007500013
20. Jadoon NA, Shahzad MA, Yaqoob R, Hussain M, Ali N. Seroprevalence of hepatitis C in type 2 diabetes: evidence for a positive association. Virol J. 2010;7(1):304. doi:10.1186/1743-422X-7-304
21. Aboud RS. The relationship between hepatitis C virus infection and diabetes mellitus type 2. J Pharm Chem Biol Sci. 2015;3:73–78.
22. Khan N, Khan N, Hussain J, Ullah H, Khan H. Frequency of Hepatitis C in type 2 diabetic patients. Gomal J Med Sci. 2014;12(2).
23. El-Bendary MM, Abdel Aziz MY, El-Arman MM. Hepatitis C virus infection and diabetic microvascular complications “Arabic abstrcts”. J Taibah Univ Med Sci. 2007;1(2):A3.
24. Madny AG, Adam AA. Seroprevalence of hepatitis C virus among type 2 diabetes mellitus patients in blue nile state, Sudan. Am J Res Commun N. 2014;2:141–147.
25. Ba-Essa EM, Mobarak EI, Elrahman SA. Frequency and factors associated with hepatitis C virus infection among patients with diabetes, Dammam, KSA. J Egypt Public Health Assoc. 2016;91(2):53–58. doi:10.1097/01.EPX.0000483164.17996.b8
26. Korkmaz H, Kesli R, Pamuk BO, Ipekci SH, Terzi Y, Kebapcilar L. Assessment of evidence for positive association and seroprevalence of hepatitis B and C in diabetic patients in a developing country. J Investig Med. 2015;63(2):251–257. doi:10.1097/JIM.0000000000000126
27. Antonelli A, Ferrari SM, Giuggioli D, et al. Hepatitis C virus infection and type 1 and type 2 diabetes mellitus. World J Diabetes. 2014;5(5):586–600. doi:10.4239/wjd.v5.i5.586
28. Gao T-T, Qin Z-L, Ren H, Zhao P, Qi Z-T. Inhibition of IRS-1 by hepatitis C virus infection leads to insulin resistance in a PTEN-dependent manner. Virol J. 2015;12(1):12. doi:10.1186/s12985-015-0241-4
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

