Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 15
Hepatic Fibrosis Assessed Using Fibrosis-4 Index Is Predictive of All-Cause Mortality in Patients with Chronic Obstructive Pulmonary Disease
Authors Yong SH, Leem AY, Kim YS , Park MS , Chang J , Kim SU , Jung JY
Received 18 December 2019
Accepted for publication 30 March 2020
Published 17 April 2020 Volume 2020:15 Pages 831—839
DOI https://doi.org/10.2147/COPD.S242863
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Seung Hyun Yong,1 Ah Young Leem,1 Young Sam Kim,1 Moo Suk Park,1 Joon Chang,1 Seung Up Kim,2,3 Ji Ye Jung1
1Division of Pulmonology, Department of Internal Medicine, Severance Hospital, Yonsei University College of Medicine, Seoul, Republic of Korea; 2Department of Internal Medicine, Institute of Gastroenterology, Yonsei University College of Medicine, Seoul, Republic of Korea; 3Yonsei Liver Center, Severance Hospital, Seoul, Republic of Korea
Correspondence: Seung Up Kim
Department of Internal Medicine, Yonsei University College of Medicine, 50-1 Yonsei-ro, Seodaemun-gu, Seoul 03722, Republic of Korea
Email [email protected]
Ji Ye Jung
Division of Pulmonology, Department of Internal Medicine, Yonsei University College of Medicine, 50-1 Yonsei-ro, Seodaemun-gu, Seoul 03722, Korea
Email [email protected]
Background: Various comorbidities influence the prognosis of patients with chronic obstructive pulmonary disease (COPD). We investigated if liver fibrosis assessed using fibrosis-4 index (FIB-4) is associated with all-cause mortality in patients with COPD.
Methods: We included 756 patients diagnosed with COPD between 2006 and 2010. Medical records were retrospectively reviewed until 2018. FIB-4 was calculated using the following equation: [age (years) × aspartate aminotransferase (IU/L)/(platelet count (109/L) × √alanine aminotransferase (IU/L))].
Results: From a total of 756 patients, 582 (76.9%) patients were categorized into survivor and 174 (23.1%) into non-survivor groups. The non-survivor group was significantly older with a higher proportion of male, smoker and lower FEV1/FVC ratio than the survivor group (all P< 0.05). Various comorbidities were more frequently observed in the non-survivor group (P< 0.05). In addition, the non-survivor group had significantly higher FIB-4 than the survivor group (1.8 vs 1.4, P< 0.001). In multivariate analysis, older age (hazard ratio [HR]=1.05), underlying malignancy (HR=2.94), coronary artery occlusive disease (HR=1.58), higher FIB-4 (HR=1.15), and higher GOLD stage (HR=1.26) were significantly associated with the increased risk of all-cause mortality (P< 0.05), whereas body mass index (HR=0.95) was independently protective for all-cause mortality (all P< 0.05). The high FIB-4 (> 1.57) group showed a significantly lower cumulative survival rate than the low FIB-4 (≤ 1.05) group (P=0.031, Log-rank test). In multivariate regression analysis, higher FIB-4 independently predicted the risk of acute exacerbation (odds ratio=1.08, P=0.034).
Conclusion: Higher fibrotic burden assessed using FIB-4 was independently predictive of the increased risk of all-cause mortality and acute exacerbation in patients with COPD.
Keywords: chronic obstructive pulmonary disease, exacerbation, FIB-4, liver fibrosis, mortality
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by chronic airflow limitation and an increased inflammatory response of the airways.1 However, beyond the airways, COPD is a complex and heterogeneous respiratory disease involving multi-organ inflammation. Although the mechanisms are yet to be investigated in detail, chronic inflammation in COPD and comorbidities accelerate the natural progression of one another.1 Therefore, systemic comorbidities of COPD are important challenges in reducing future risks of mortality and exacerbation, which are the main treatment goals in COPD.
A recent study investigated the prevalence and severity of non-alcoholic fatty liver disease (NAFLD), defined by a noninvasive surrogate for the diagnosis of fatty liver, in patients with COPD and showed a close association between NAFLD and COPD.2 The prevalence of simple steatosis, non-alcoholic steatohepatitis, and fibrotic liver were 41%, 37%, and 61%, respectively, in patients with COPD. Because NAFLD is primarily linked with metabolic comorbidities of COPD and systemic inflammation,2 the authors insisted that NAFLD should be included among COPD comorbidities, and hypothesized that COPD progression in NAFLD might be attributable to oxidative stress and systemic inflammation. Moreover, several studies reported a significant association between NAFLD and decreased lung function in the general population.3–7 However, detailed underlying mechanisms still remain unresolved.
Fibrosis-4 index (FIB-4), a noninvasive, simple-to-use equation, which includes laboratory and clinical variables such as aspartate aminotransferase (AST), alanine aminotransferase (ALT), platelet count, and age as constituent variables, was proposed to evaluate the degree of liver fibrosis in patients with chronic liver diseases.8,9 FIB-4 has proven to be a significant prognostic predictor for the development of hepatocellular carcinoma in patients with chronic liver diseases.10–12 Besides evaluating chronic liver diseases, FIB-4 has been used to assess liver fibrosis and its relation with other systemic inflammatory diseases or conditions, including arterial stiffness/abnormal central hemodynamics in heart failure, cerebral white matter hyperintensity, rheumatoid arthritis, and polyangiitis.13–17
Accordingly, we investigated whether liver fibrosis assessed using FIB-4 is associated with all-cause mortality in patients with COPD, based on the concept that liver fibrosis might be a sequela of hepatic or systemic inflammation.
Methods
Study Subjects
We extracted the details of 1,282 patients who were diagnosed COPD based on the 10th revision of the International Statistical Classification of diseases and Related Health Problems (ICD-10) codes and medications prescribed from January 2006 to December 2010 in Severance Hospital, South Korea.
The inclusion criteria were, (1) age ≥ 40 years; (2) ICD-10 codes for COPD or emphysema (J43.0x-J44.x, with the exception of J43.0 as primary or secondary (within the fifth position) diagnosis); and (3) use of more than one of the following COPD medications at least twice per year, long-acting muscarinic antagonist, long-acting beta-2 agonist (LABA), inhaled corticosteroid + LABA, short-acting muscarinic antagonist (SAMA), short-acting beta-2 agonist (SABA), SAMA + SABA, phosphodiesterase-4 inhibitor, systemic beta-agonist, or methylxanthine.
However, 84 patients without pulmonary function information, 57 patients without obstructive pulmonary function, 312 patients with a history of asthma, and 73 patients with chronic liver disease were excluded as they failed to fulfill inclusion criteria (Figure 1). Finally, 756 patients with COPD were enrolled and their medical records were retrospectively reviewed until 2018. Index date from when the patients were followed up was defined as the date of the first COPD medication prescribed to the patients.
The study protocol was consistent with the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Institutional Review Board (IRB) of Severance University. The requirement for an informed consent was waived on the acknowledgement of IRB of Severance University, due to the reason that data given to researchers are anonymized with no identifying information available. Study data are available upon request, so any interested researchers can access and share the data by submitting a request and receiving the permission from the corresponding author and IRB of Severance University. The data will be provided anonymously.
Exacerbation
COPD exacerbation was defined as a ≥ 2-day aggravation of one of the following three symptoms (cough, sputum, and dyspnea) resulting in an unscheduled hospital visit, a requirement of antibiotics and/or oral corticosteroids, or hospitalization for additional treatment.
Assessment of Liver Fibrosis
FIB-4 was calculated using the following equation: age (years) × aspartate aminotransferase (AST) (IU/L)/[platelet count (109/L) × √alanine aminotransferase (ALT) (IU/L)]. The study population was divided into three risk groups, low (Q1; (≤1.05)), intermediate (Q2-3), and high (Q4; (>1.57)) FIB-4 groups.
Statistical Analysis
Categorical variables between the two groups were analyzed using the χ2 test and were expressed as number and percentage. Continuous variables between the two groups were compared using Student’s t-test and were expressed as mean ± standard deviation. The predictive role of FIB-4 for all-cause mortality was described as hazard ratio (HR) using the multivariate Cox hazards model analysis and Kaplan-Meier curve. The risk of exacerbation is described as the odds ratio (OR) with 95% confidence interval (CI) using generalized estimating equations for longitudinal data of exacerbation. P-value of <0.05 was considered to be statistically significant. All statistical analyses were conducted using SPSS software version 25 (SPSS, Chicago, IL).
Results
Baseline Characteristics
The baseline characteristics of the enrolled 756 patients with COPD at the time of diagnosis are shown in Table 1. The mean age was 67.9 years with a predominance of male patients (85.1%). Among the underlying comorbidities, hypertension was the most common (33.6%), followed by malignancy (20.1%) and diabetes mellitus (17.3%). Most patients with COPD were ever smokers (82.5%). The initial pulmonary function test results showed a mean FEV1 of 1.39 L (57% of predicted), mean FVC of 2.48 L (69.9% of predicted), and mean FEV1/FVC ratio of 56.1%. The mean FIB-4 was 1.53.
 |
Table 1 Baseline Characteristics at the Time of COPD Diagnosis (n=756) |
Comparison Between Survivors and Non-Survivors
During the study period (mean follow-up, 6.1 years), 174 (23.1%) patients died, whereas 582 (76.9%) were alive. When the baseline characteristics were compared (Table 2), non-survivors were significantly older (mean 71.3 years vs 66.9 years, P<0.001) and had a higher proportion of males (91.4% vs 83.2%, P=0.007) and those with smoking history (89.7% vs 80.3%, P=0.004) than survivors. Various comorbidities including malignancy, cerebral vascular disease, coronary artery occlusive disease (CAOD), congestive heart failure, chronic kidney disease, diabetes mellitus, and hypertension were more frequently observed in non-survivors than in survivors (all P<0.05). Among pulmonary function test results, FEV1 (mean 1.32 L vs 1.41 L, P=0.044), FVC (mean 2.36 L vs 2.52 L, P=0.013), and FVC % predicted (67.1% vs 70.8%, P=0.017) were significantly lower in non-survivors than in survivors. The FIB-4 was significantly higher in non-survivors than in survivors (mean 1.8 vs 1.4, P<0.001).
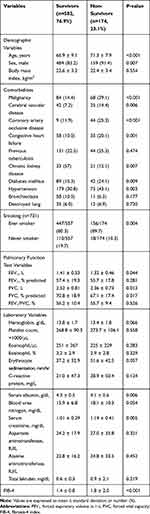 |
Table 2 Comparison Between Survivors and Non-Survivors |
Multivariate Cox Hazard Analysis for All-Cause Mortality
Multivariate Cox hazard analysis to identify the independent predictors of all-cause mortality is shown in Table 3. Older age (HR=1.05, 95% CI 1.03–1.07), underlying malignancy (HR=2.94, 95% CI 2.15–4.02), CAOD (HR=1.58, 95% CI 1.10–2.26), higher GOLD stage (HR=1.26, 95% CI 1.02–1.56), and higher FIB-4 (HR=1.15, 95% CI 1.03–1.27) were significantly associated with the increased risk of all-cause mortality (all P<0.05), whereas higher body mass index (HR=0.95, 95% CI 0.89–0.99) was independently protective for all-cause mortality (all P<0.05).
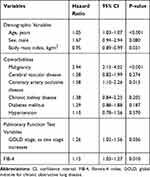 |
Table 3 Multivariate Cox Hazard Model to Identify Independent Predictors of All-Cause Mortality |
The study population was stratified into three risk groups (low [Q1], intermediate [Q2-3], and high [Q4] FIB-4 groups). The high FIB-4 group (>1.57) had a significantly lower cumulative survival rate than the low FIB-4 group (≤1.05) (P=0.031, Log-rank test) (Figure 2).
Multivariate Regression Analysis for the Relationship Between FIB-4 and Acute Exacerbation
The number of patients who had ever experienced acute exacerbation during the follow-up duration was 468 (61.9%), and the mean number of acute exacerbations of COPD per year was 0.42 in this study. On multivariate regression analyses (Table 4), higher GOLD stage (OR=1.19, 95% CI 1.07–1.34) was independently associated with the increased risk of acute exacerbation, whereas higher FIB-4 (HR=1.05, 95% CI 1.01–1.15) was independently associated with the increased risk of acute exacerbation (all P<0.05).
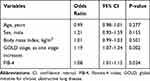 |
Table 4 Multivariate Regression Analysis for the Relationship Between FIB-4 and Acute Exacerbation |
Discussion
Based on the hypothesis that liver fibrosis, a sequela of hepatic or systemic inflammation, may act as an unfavorable prognostic factor in patients with COPD, we investigated the relationship between fibrotic burden in liver and COPD. Towards this, we used FIB-4 and various clinical outcomes, including all-cause mortality and acute exacerbations in patients with COPD. Finally, we found that higher FIB-4 independently predicted the higher risk of all-cause mortality and acute exacerbations. In addition, the higher FIB-4 group showed a significantly lower cumulative survival than the low FIB-4 group. The cutoff of FIB-4 to defined higher FIB-4 group in this study was 1.57, which is quite similar to the established cutoff of 1.45 indicating the presence of significant liver fibrosis (≥ stage 2 fibrosis).8
COPD is a complex respiratory disease with systemic inflammation frequently accompanying various comorbidities such as cardiovascular disease, lung cancer, metabolic syndrome, depression, and dysfunctional skeletal myopathy.18,19 All these comorbidities impact on prognosis, quality of life, and physical activities of patients with COPD, and play a major role in the classification of the various phenotypes of COPD. Interest regarding liver diseases in patients with COPD has been increasing recently. NAFLD is highly prevalent and is considered as one of the contributors of cardio-metabolic comorbidities in patients with COPD.20 According to a recent meta-analysis, NAFLD is strongly associated with reduced pulmonary function. Reduced lung volumes at baseline were also associated with increased NAFLD incidence, especially in Asians.6 However, only a few studies have investigated how fibrotic burden in the liver impacts the outcomes of patients with COPD.18,21
Divo et al investigated various comorbidities and their influence on the risk of mortality in patients with COPD.18 Although the prevalence of liver cirrhosis was lower than that of cardiovascular and metabolic diseases, liver cirrhosis was significantly associated with the increased risk for death.18 Contrarily, our current study recruited only patients with a milder fibrotic burden in the liver after excluding those with known liver disease. Nevertheless, subtle or subclinical fibrosis progression in the liver, reflected by a higher FIB-4, was independently associated with a higher risk of mortality and acute exacerbation. This might mean that fibrotic burden in the liver might have a prognostic implication in patients with COPD. Indeed, in a recent longitudinal study, it was evident that liver fibrosis, not just liver steatosis, was independently associated with long-term overall mortality in patients with NAFLD.22 This issue is further supported by Viglino et al who reported a three-fold higher risk of developing the first cardiovascular event and death in patients with COPD with co-existing liver fibrosis—assessed using another non-invasive patented surrogate, FibroTest®—when compared to those without liver fibrosis.21,23
Several comorbidities are associated with increased risk of acute exacerbation in patients with COPD, such as gastro-esophageal reflux disease, anxiety, depression, pulmonary embolism, pulmonary hypertension, and cardiovascular disease.1 In addition to previously known comorbidities, higher FIB-4 was independently associated with the increased risk of acute exacerbation in our current study. In contrast, liver disease did not impact the rate of acute exacerbations in the study by Viglino et al, with only a severe level of exacerbation noted in emergency admission, hospitalization, and the intensive care unit.21 The authors demonstrated a tendency of an increase in the risk of acute exacerbation-related hospitalization in those with liver fibrosis (P=0.06). We included both moderate and severe exacerbation cases; however, increased FIB-4 was not associated with each type of exacerbation (data not shown). Moreover, airflow limitation was less severe among the participants of the study by Viglino et al, resulting in a lower proportion of patients who had experienced at least one exacerbation in contrast to that in our study (45.9% vs 61.9%).
There are several common pathophysiologic mechanisms shared between COPD and liver fibrosis. First, COPD and liver fibrosis are characterized by similar inflammatory processes involving tumor necrosis factor-alpha, transforming growth factor-beta, leptin, and adiponectin.24–29 These pro-inflammatory mediators promote tissue damage and remodeling in the lung and contribute to the progression of liver fibrosis. In addition, chronic intermittent hypoxia induces liver fibrosis through several pathways. In the study on rodent models of sleep apnea, Toll-like receptor 4-mediated mitogen-activated protein kinase and nuclear factor-kB signaling were related to increased liver fibrosis.30 Moreover, hepatocyte hypoxia-inducible factor-1 induced liver fibrosis in a mouse model of NAFLD due to liver tissue hypoxia in hepatic steatosis.31 Thirdly, the process of aging has been considered as a risk factor for the development and progression of COPD and liver fibrosis. Aging is generally related with increased oxidative stress and reduced tolerance to oxidative damage.32 Moreover, immunosenescence is unavoidable with increasing age, resulting in various clinical consequences including increased susceptibility to infection, malignancy, and autoimmunity.33,34 Increased inflammatory reaction mainly composed of CD4+ lymphocytes and macrophages expressing TH2 cytokines is the main factor involved in the higher susceptibility to liver fibrosis with increasing age.35 Increased CD4+ and CD8+ T cells in the airway and lung parenchyma are also major COPD-related changes.36
This study has several limitations. Firstly, because of the absence of a control group, we were not able to assess the impact of liver fibrosis on the clinical outcomes in COPD compared to that in a control group. In addition, the degree of liver fibrosis was noninvasively assessed using FIB-4 without detailed histological information. However, the simple-to-use FIB-4 has been validated and widely used to assess the degree of liver fibrosis and cirrhosis in chronic liver disease.8–10 Thirdly, NAFLD is a major liver disease that progresses to liver fibrosis, and metabolic syndrome is an essential comorbidity that contributes to the progression of liver fibrosis. Unfortunately, NAFLD and metabolic syndrome presence-related data were not available for this study. Lastly, because of the unavailability of data on cause of death, only all-cause mortality was assessed, and thus, the effect of liver fibrosis on mortality from COPD was not evaluated.
Conclusion
A higher fibrotic burden assessed using FIB-4 at the diagnosis of COPD was independently predictive of the increased risk of all-cause mortality and acute exacerbation in patients with COPD without chronic liver diseases. FIB-4, a non-invasive, simple-to-use, and readily available tool in daily clinical practice, could play a role as a prognostic tool representing liver fibrosis in patients with COPD.
Abbreviations
AFP, Alpha-fetoprotein; AST, aspartate aminotransferase; ALT, alanine aminotransferase; CAOD, Coronary artery occlusive disease; COPD, Chronic obstructive pulmonary disease; COPD AE, Chronic obstructive pulmonary disease acute exacerbation; DCP, des-gamma-carboxy-prothrombin; FEV1, Forced expiratory volume in 1 second; FVC, Forced Vital capacity; FIB-4, Fibrosis-4 index; GOLD Stage, Global initiative for chronic Obstructive Lung Disease stage, HBeAg; hepatitis B e antigen; HDL-C, High-density lipoprotein cholesterol; ICS, inhaled corticosteroid; INR, international normalized ratio; IRB; International Review Board, LDL-C, Low-density lipoprotein cholesterol; LAMA, long-acting muscarinic antagonist; LABA, long-acting beta-2 agonist; NAFLD, Non-alcoholic fatty liver disease; SABA, short-acting beta-2 agonist; SAMA, short-acting muscarinic antagonist; PFT, Pulmonary function test.
Disclosure
The authors report no conflicts of interest in this work. This research was supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education (2016R1D1A1B03933125).
References
1. Smith MC, Wrobel JP. Epidemiology and clinical impact of major comorbidities in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2014;9:871–888. doi:10.2147/COPD.S49621
2. Viglino D, Jullian-Desayes I, Minoves M, et al. Nonalcoholic fatty liver disease in chronic obstructive pulmonary disease. Eur Respir J. 2017;49(6):1601923. doi:10.1183/13993003.01923-2016
3. Kwak MS, Kim E, Jang EJ, Lee CH. The association of non-alcoholic fatty liver disease with lung function: a survey design analysis using propensity score. Respirology. 2018;23(1):82–88. doi:10.1111/resp.13127
4. Lee CH, Choi SH, Chung GE, Park B, Kwak MS. Nonalcoholic fatty liver disease is associated with decreased lung function. Liver Int. 2018;38(11):2091–2100. doi:10.1111/liv.13860
5. Moon SW, Kim SY, Jung JY, et al. Relationship between obstructive lung disease and non-alcoholic fatty liver disease in the Korean population, Korea National Health and Nutrition Examination Survey, 2007–2010. Int J Chron Obstruct Pulmon Dis. 2018;13:2603–2611. doi:10.2147/COPD.S166902
6. Mantovani A, Lonardo A, Vinco G, et al. Association between non-alcoholic fatty liver disease and decreased lung function in adults: a systematic review and meta-analysis. Diabetes Metab. 2019;45(6):536–544. doi:10.1016/j.diabet.2019.04.008
7. Song JU, Jang Y, Lim SY, et al. Decreased lung function is associated with risk of developing non-alcoholic fatty liver disease: a longitudinal cohort study. PLoS One. 2019;14(1):e0208736. doi:10.1371/journal.pone.0208736
8. Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43(6):1317–1325. doi:10.1002/hep.21178
9. Kim BK, Kim DY, Park JY, et al. Validation of FIB-4 and comparison with other simple noninvasive indices for predicting liver fibrosis and cirrhosis in hepatitis B virus-infected patients. Liver Int. 2010;30(4):546–553. doi:10.1111/j.1478-3231.2009.02192.x
10. Suh B, Park S, Shin DW, et al. High liver fibrosis index FIB-4 is highly predictive of hepatocellular carcinoma in chronic hepatitis B carriers. Hepatology. 2015;61(4):1261–1268. doi:10.1002/hep.27654
11. Chon YE, Jung ES, Park JY, et al. The accuracy of noninvasive methods in predicting the development of hepatocellular carcinoma and hepatic decompensation in patients with chronic hepatitis B. J Clin Gastroenterol. 2012;46(6):518–525. doi:10.1097/MCG.0b013e31825079f1
12. Chun HS, Kim BK, Park JY, et al. Design and validation of risk prediction model for hepatocellular carcinoma development after sustained virological response in patients with chronic hepatitis C. Eur J Gastroenterol Hepatol. 2019. doi:10.1097/meg.0000000000001512
13. Iwasaki Y, Tomiyama H, Shiina K, et al. Liver stiffness and arterial stiffness/abnormal central hemodynamics in the early stage of heart failure. Int J Cardiol Heart Vasc. 2018;20:32–37. doi:10.1016/j.ijcha.2018.07.001
14. Jeong SM, Kwon H, Park S, et al. Favorable impact of non-alcoholic fatty liver disease on the cerebral white matter hyperintensity in a neurologically healthy population. Eur J Neurol. 2019;26(12):1471–1478. doi:10.1111/ene.14029
15. Miyata M, Kuroda M, Unakami M, Tasaki K, Migita K, Ohira H. Validation of the fibrosis-4 (FIB-4) index in the diagnosis of liver disease of rheumatoid arthritis patients treated with methotrexate. Mod Rheumatol. 2018. doi:10.1080/14397595.2018.1542962.1-7
16. Up Kim S, Kim BK, Park JY, et al. Fibrosis-4 index at diagnosis can predict all-cause mortality in patients with rheumatoid arthritis: a retrospective monocentric study. Mod Rheumatol. 2018. doi:10.1080/14397595.2018.1558760.1-16
17. Park HJ, Park JY, Jung SM, Song JJ, Park YB, Lee SW. Fibrosis-4 index at diagnosis is associated with all-cause mortality in patients with microscopic polyangiitis and granulomatosis with polyangiitis. BMC Gastroenterol. 2019;19(1):90. doi:10.1186/s12876-019-1007-z
18. Divo M, Cote C, de Torres JP, et al. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186(2):155–161. doi:10.1164/rccm.201201-0034OC
19. Fabbri LM, Luppi F, Beghe B, Rabe KF. Complex chronic comorbidities of COPD. Eur Respir J. 2008;31(1):204–212. doi:10.1183/09031936.00114307
20. Lonardo A, Nascimbeni F, Ponz de Leon M. Nonalcoholic fatty liver disease and COPD, is it time to cross the diaphragm? Eur Respir J. 2017;49(6):1700546. doi:10.1183/13993003.00546-2017
21. Viglino D, Plazanet A, Bailly S, et al. Impact of non-alcoholic fatty liver disease on long-term cardiovascular events and death in chronic obstructive pulmonary disease. Sci Rep. 2018;8(1):16559. doi:10.1038/s41598-018-34988-2
22. Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149(2):389–397.e310. doi:10.1053/j.gastro.2015.04.043
23. Vallet-Pichard A, Mallet V, Nalpas B, et al. FIB-4, an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007;46(1):32–36. doi:10.1002/hep.21669
24. Tanni SE, Pelegrino NR, Angeleli AY, Correa C, Godoy I. Smoking status and tumor necrosis factor-alpha mediated systemic inflammation in COPD patients. J Inflamm (Lond). 2010;7:29. doi:10.1186/1476-9255-7-29
25. Saito A, Horie M, Nagase T. TGF-beta signaling in lung health and disease. Int J Mol Sci. 2018;19(8):2460. doi:10.3390/ijms19082460
26. Osawa Y, Hoshi M, Yasuda I, Saibara T, Moriwaki H, Kozawa O. Tumor necrosis factor-alpha promotes cholestasis-induced liver fibrosis in the mouse through tissue inhibitor of metalloproteinase-1 production in hepatic stellate cells. PLoS One. 2013;8(6):e65251. doi:10.1371/journal.pone.0065251
27. Krommidas G, Kostikas K, Papatheodorou G, et al. Plasma leptin and adiponectin in COPD exacerbations, associations with inflammatory biomarkers. Respir Med. 2010;104(1):40–46. doi:10.1016/j.rmed.2009.08.012
28. Fabregat I, Moreno-Caceres J, Sanchez A, et al. TGF-beta signalling and liver disease. FEBS J. 2016;283(12):2219–2232. doi:10.1111/febs.13665
29. Chiang CH, Chuang CH, Liu SL. Transforming growth factor-beta1 and tumor necrosis factor-alpha are associated with clinical severity and airflow limitation of COPD in an additive manner. Lung. 2014;192(1):95–102. doi:10.1007/s00408-013-9520-2
30. Kang HH, Kim IK, Lee HI, et al. Chronic intermittent hypoxia induces liver fibrosis in mice with diet-induced obesity via TLR4/MyD88/MAPK/NF-kB signaling pathways. Biochem Biophys Res Commun. 2017;490(2):349–355. doi:10.1016/j.bbrc.2017.06.047
31. Mesarwi OA, Shin MK, Bevans-Fonti S, Schlesinger C, Shaw J, Polotsky VY. Hepatocyte hypoxia inducible factor-1 mediates the development of liver fibrosis in a mouse model of nonalcoholic fatty liver disease. PLoS One. 2016;11(12):e0168572. doi:10.1371/journal.pone.0168572
32. Kim IH, Kisseleva T, Brenner DA. Aging and liver disease. Curr Opin Gastroenterol. 2015;31(3):184–191. doi:10.1097/MOG.0000000000000176
33. Busse PJ, Mathur SK. Age-related changes in immune function, effect on airway inflammation. J Allergy Clin Immunol. 2010;126(4):
34. Ito K, Barnes PJ. COPD as a disease of accelerated lung aging. Chest. 2009;135(1):173–180. doi:10.1378/chest.08-1419
35. Mahrouf-Yorgov M, Collin de l’Hortet A, Cosson C, et al. Increased susceptibility to liver fibrosis with age is correlated with an altered inflammatory response. Rejuvenation Res. 2011;14(4):353–363. doi:10.1089/rej.2010.1146
36. Sharma G, Hanania NA, Shim YM. The aging immune system and its relationship to the development of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2009;6(7):573–580. doi:10.1513/pats.200904-022RM
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


