Back to Journals » Journal of Hepatocellular Carcinoma » Volume 9
Hepatic Arterial Infusion Chemotherapy as a Timing Strategy for Conversion Surgery to Treat Hepatocellular Carcinoma: A Single-Center Real-World Study
Authors Wang J, Zheng Z , Wu T, Li W, Wang J, Pan Y , Peng W, Hu D, Hou J, Xu L , Zhang Y , Chen M, Zhang R, Zhou Z
Received 28 June 2022
Accepted for publication 9 September 2022
Published 14 September 2022 Volume 2022:9 Pages 999—1010
DOI https://doi.org/10.2147/JHC.S379326
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Jörg Trojan
Jiongliang Wang,1,2,* Zhikai Zheng,1,2,* Tianqing Wu,1,2,* Wenxuan Li,1,2,* Juncheng Wang,1,2 Yangxun Pan,1,2 Wei Peng,1,2 Dandan Hu,1,2 Jiajie Hou,1,2 Li Xu,1,2 Yaojun Zhang,1,2 Minshan Chen,1,2 Rongxin Zhang,2,3 Zhongguo Zhou1,2
1Department of Liver Surgery, Sun Yat-sen University Cancer Center, Guangzhou, People’s Republic of China; 2Sun Yat-sen University Cancer Center; State Key Laboratory of Oncology in South China; Collaborative Innovation Center for Cancer Medicine, Guangzhou, People’s Republic of China; 3Department of Colorectal Surgery, Sun Yat-sen University Cancer Center, Guangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhongguo Zhou, Department of Liver Surgery, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Dongfeng Road East 651, Guangzhou, Guangdong, 510060, People’s Republic of China, Tel +86-20-87343117, Email [email protected] Rongxin Zhang, Department of Colorectal Surgery, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Dongfeng Road East 651, Guangzhou, Guangdong, 510060, People’s Republic of China, Tel +86-411-84672130, Email [email protected]
Objective: To evaluate whether surgery-related complications are increased after hepatic arterial infusion chemotherapy (HAIC) using oxaliplatin plus fluorouracil/leucovorin for conversion compared with primary hepatocellular carcinoma (HCC) resection and the optimal timing of conversion surgery (CS).
Background: HAIC has been widely used for advanced HCC, especially initially unresectable HCC, to facilitate conversion to curative-intent resection in approximately 23.8% of cases. However, the optimal timing of surgery to reduce surgical complications must be clarified.
Methods: Data from 320 HCC patients, including 107 initially unresectable patients in the HAIC-Surgery group and 213 patients in the Surgery group, were retrospectively collected and analyzed. Survival outcomes and the incidence of surgery-related complications were compared.
Results: There was no significant difference in recurrence-free survival (RFS) between the HAIC-Surgery group and the Surgery group (HR: 1.140, 95% CI: 0.8027– 1.618, p=0.444). The HAIC-Surgery group had a higher incidence of surgery-related complications than the Surgery group [biliary leakage (10.3% vs 4.2%, p=0.035), abdominal bleeding (10.3% vs 3.8%, p=0.020), pleural effusion (56.1% vs 23.0%, p< 0.0001) and ascites effusion (17.8% vs 5.2%, p< 0.0001)]. In the HAIC-Surgery group, postoperative liver function decreased and abdominal bleeding increased with more preoperative HAIC cycles (Spearman=0.229, p=0.042, Spearman=0.198, p=0.041, respectively). The pathological complete remission (pCR) rate after 3– 5 HAIC cycles was significantly higher than that after 1– 2 cycles (29.4% vs 13.2%, p=0.043).
Conclusion: The prognosis of advanced HCC after conversion surgery is comparable to that after direct surgery. Rather than increasing pCR, more HAIC cycles can exacerbate liver dysfunction and surgery-related complications.
Keywords: hepatocellular carcinoma, conversion therapy, hepatic artery chemotherapy infusion
Introduction
HCC is the sixth leading cause of cancer and the third most lethal malignancy worldwide.1,2 Although hepatic resection has been considered a standard radical treatment for resectable HCCs,3,4 approximately 60% of patients with HCC lose the chance of surgery.4,5 Targeted therapy combined with immunotherapy as the standard pharmacological treatment effectively prolong the survival of patients with advanced liver cancer.6–9 Therefore, The scheme of targeted therapy combined with immunotherapy was recommended to be the preferred option in first-line setting for advanced primary liver cancer in both the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines) for hepatocellular carcinoma and the Guidelines for diagnosis and treatment of primary liver cancer in China. Recently, several studies have reported favorable results either in response rate or survival when HAIC is based on oxaliplatin plus fluorouracil/leucovorin (FOLFOX) alone or accompanied with sorafenib used for advanced HCC.10–12 Additionally, FOLFOX-based HAIC, either as a single agent or in combination with other treatment modalities, is an attractive conversion therapy approach for unresectable patients.13–16
It is widely believed that successful conversion treatment can provide patients an opportunity for prolonged survival in cases of initially unresectable disease.17,18 This approach directly delivers chemotherapeutic agents into tumor-associated hepatic arterial branches locally at high concentrations,19 achieving strong antitumor efficacy and lower systemic toxicity through a greater first-pass effect in the liver.20 However, a series of complications can occur due to the liver damage caused by chemotherapy drugs. A pattern of hepatic vascular injury has been reported in patients receiving neoadjuvant therapy with 5-FU and leucovorin in combination with oxaliplatin or irinotecan before the resection of liver metastases from colorectal cancer.21 Histologically, oxaliplatin-induced liver injury is associated with damage related to sinusoidal obstruction syndrome (SOS).22 Accordingly, HAIC with the FOLFOX regimen has resulted in some chemotherapy-related complications during treatment, such as leucopenia, vomiting, hyperbilirubinemia, AST elevation, and ascites.23,24 Furthermore, among colorectal cancer patients with liver metastases, those who have been successfully converted with chemotherapy regimens based on oxaliplatin and fluorouracil are more likely to suffer from perioperative complications.25–28
In fact, in addition to the possible liver damage caused by HAIC, the duration of HAIC treatment and the use of concomitant medication remain controversial. In addition, reports on the post-transformation-related complications of HAIC with the FOLFOX regimen have mainly focused on colorectal cancer with liver metastases, and there is still a lack of research reports on primary liver tumors. Thus, in this retrospective study, we aimed to evaluate whether the incidence of complications will increase after HAIC conversion therapy compared with HCC resection and the optimal timing of surgery.
Methods
Patients
Data from patients who were diagnosed with HCC according to the American Association for the Study of Liver Diseases practice guidelines and treated by hepatic resection or HAIC-Surgery between January 2015 and June 2021 were retrieved. The inclusion criteria were as follows: Surgery group: (a) pathological diagnosis of hepatocellular carcinoma; (b) Barcelona clinic liver cancer (BCLC) stage A or B; (c) Child–Pugh Grade A and (d) residual liver volume >40% after resection. HAIC-Surgery group: (a) pathological diagnosis of hepatocellular carcinoma; (b) Child–Pugh Grade A; (c) before HAIC, tumors not amenable to radical surgical resection due to insufficient surgical margins after assessment by multi-disciplinary treatment (MDT) group, or an estimated <40% residual liver volume (FLV) remaining after resection and (d) after HAIC, residual liver volume >40% after resection. Cases were excluded if they met any of the following criteria: (a) a previous history of HCC treatment; (b) signs of vascular invasion or distant metastasis on imaging; (c) severe underlying cardiac, pulmonary, or renal diseases; or (d) a second primary malignancy. This study was approved by the Institutional Review Board of Sun Yat-sen University Cancer Center (SYSUCC, Guangzhou, China) and was performed following the Declaration of Helsinki of 1975 as revised in 1983.
Propensity Score Analysis
Propensity score matching (PSM) was applied to reduce selection bias by equating the 2 groups. All possible clinicopathological covariates, including age, sex, degree of tumor differentiation, China liver cancer staging (CNLC), tumor size, tumor number, alpha-fetoprotein (AFP), alanine transaminase (ALT), albumin (ALB), total bilirubin (TBIL), and hepatitis B surface antigen (HBsAg), which might have increased the incidence of complications, were included when performing PSM. Using NCSS 10 Statistical Software (LLC, Kaysville, UT, USA), the greedy method was used for matching the study groups at a 2:1 ratio with a caliper width of 0.2-fold the standard deviation of the propensity score between the two groups. The standardized mean difference (SMD) was used to evaluate the covariate balance after PSM.
HAIC Treatment and Hepatic Resection After Conversion
A catheter was placed and fixed in the tumor feeding artery for the FOLFOX-based chemotherapy infusion at the following dosage: 130 mg/m2 oxaliplatin infusion for 3 hours; 200 mg/m2 leucovorin infusion for 2 hours; and 1200 mg/ m2 5-FU continuous infusion for 23 hours. HAIC treatment was repeated every 3 weeks. Hepatectomy was performed after careful evaluation by 2 experienced surgeons when the estimated residual liver volume was >30–40% after resection.29 Hepatectomy was performed using cutting ultrasonic aspiration (CUSA) and an ultrasonic scalpel via open or laparoscopic surgery.
Follow-Up and Major Complication Evaluation
Blood cell counts, liver function tests, and serum AFP levels were determined before each cycle. Adverse events/complications were graded according to the Clavien–Dindo version before each cycle. Biliary leakage: According to the definition of biliary fistula by the International Liver Surgery Study Group (ISGLS), biliary leakage was defined as leakage occurring ≥3 days after surgery and a bilirubin concentration at least 3 times the normal plasma bilirubin concentration in the drainage fluid or bile accumulation or bilirubin in the drainage fluid. Cases of biliary peritonitis required interventional or surgical treatment.30 Abdominal bleeding: Abdominal bleeding was defined as a decrease in hemoglobin of more than 3 g/dl compared to preoperative values and at least two doses of postoperative hemostatic drugs.31 Pleural effusion: Pleural effusion was defined as perioperative or first postoperative follow-up imaging depicting pleural effusion compared with preoperative chest imaging. Ascites effusion: Ascites effusion was defined as perioperative or first postoperative follow-up imaging revealing ascites compared with preoperative abdominal imaging. Evaluation of changes in liver function: Deterioration in liver function was defined as a difference between the postoperative albumin-bilirubin scoring model (ALBI) grade and preoperative ALBI grade greater than 1; otherwise, a difference between the postoperative ALBI grade and preoperative ALBI grade less than or equal to 1 was defined as no deterioration in liver function.
Statistical Analyses
RFS was measured from the date of liver resection until disease progression or recurrence or the last follow-up visit. Survival curves were analyzed using the Kaplan–Meier method and the Log rank test. Categorical variables were analyzed using the chi-square test, and continuous variables were analyzed using Student’s t test. The correlation coefficient and p value were calculated using Spearman’s rank correlation analysis. A p<0.05 was considered statistically significant. All data were processed with the Statistics for Social Sciences package version 24.0 (IBM Corp).
Result
Characteristics of the Total Study Cohort
Among a total of 320 patients included in our original study, 213 (66.6%) and 107 (33.4%) patients were included in the HAIC-Surgery and Surgery groups, respectively (Figure 1). The baseline data of these patients are shown in Table 1. Before the PSM analysis, most characteristics were not significantly different between HAIC-Surgery and Surgery groups, except a higher proportion of patients in the HAIC-surgery group had a significantly more advanced CNLC stage after HAIC (Ia 26.0% vs 47.9%; Ib 38.9% vs 36.4%; IIa 9.2% vs 6.0%; IIb 15.3% vs 3.7%; III 10.7% vs 6.0%, p<0.0001) and a higher tumor number (18.3% vs 2.3%, p<0.0001) (Table 1). After 2:1 PSM of the 320 patients (213 in the Surgery group and 107 in the HAIC-Surgery group), most of the characteristics analyzed in this work were balanced between the two groups (Figure 2). Finally, no significant differences were observed between the patients in the two groups in terms of confounding factors in subsequent analyses.
 |
Table 1 Baseline Characteristics of Patients with Hepatocellular Carcinoma |
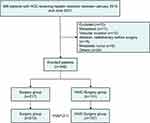 |
Figure 1 Flow diagram of patients with hepatocellular carcinoma who underwent either hepatectomy or HAIC-hepatectomy. |
 |
Figure 2 A visualization of the Propensity Score Matching. |
Survival Outcomes
Downstaging occurred in 58 of 107 patients, accounting for 54.2% of the population who underwent HAIC before liver resection. In addition, Similar rates of partial hepatectomy (75.6% vs 77.6%) and hepatolobectomy(24.4% vs 22.4%) were performed in the two groups. The median follow-up period was 39.8 months. The median RFS was 38.7 months for the Surgery group and 22.9 months for the HAIC-Surgery group. The Surgery group did not show superiority over the HAIC-Surgery group in terms of RFS (HR: 1.140, 95% CI: 0.8027–1.618, p=0.444, Figure 3).
 |
Figure 3 Kaplan-Meier curves for RFS in the Surgery and HAIC-Surgery groups. Vertical bars indicate censoring of patients alive at their last follow-up. |
Complications
The complications observed in this study are shown in Table 2. We found higher cumulative incidences of biliary leakage (10.3% vs 4.2%, p=0.035), abdominal bleeding (10.3% vs 3.8%, p=0.020), pleural effusion (56.1% vs 23.0%, p<0.0001) and ascites effusion (17.8% vs 5.2%, p<0.0001) in the HAIC-Surgery group than in the surgery group. However, there were no significant differences in the rate of complications between the two groups, including pulmonary infection (5.6% vs 5.2%, p=0.868), bowel obstruction (0 vs 2.3%, p=0.263), fever (≥38.1°C) (7.5% vs 12.7%, p=0.160), and nausea and vomiting (15.9% vs 17.4%, p=0.738). Although the HAIC-Surgery group had a higher rate of complications, it was comparable to the Surgery group in terms of the number of patients experiencing grade ≥ 3 adverse effects. (Table 2)
 |
Table 2 Study Surgery-Related Complications for Patients Who Underwent Either Hepatectomy or Hepatectomy After HAIC |
It should be noted that both HAIC and liver resection have an impact on liver function to some extent. Here, we used the ALBI grade to comprehensively evaluate the liver function of the two groups before and after surgery. Moreover, to reflect the changes in liver function and to better describe the possible impact of HAIC on postoperative liver function, we compared the ALBI grade between the two groups of patients before and after surgery. Supplementary Table 1 and Figure 4 show that most patients in both the HAIC-Surgery group and the Surgery group had decreased postoperative liver function, especially the latter group (70.1% vs 55.4%, p=0.011).
Analysis of Complications Related to the HAIC Cycle
Among the 107 patients in the HAIC-Surgery group in our study, the patients underwent 1 to more than 5 HAIC cycles before reaching the standard of conversion surgery for resection. Here, we performed a correlation analysis of the HAIC cycle with pre-HAIC tumor size and clinical stage (CNLC). As shown in Table 3, the number of HAIC cycles had a significant positive correlation with tumor size (Spearman=0.311, p=0.001) and clinical stage (Spearman=0.301, p=0.002), indicating that when the tumor burden is greater, more HAIC cycles are needed to achieve conversion.
 |
Table 3 Correlation Analysis of Tumor Stage and Tumor Size with HAIC Cycles of Treatment |
Biliary leakage (10.3%), abdominal bleeding (10.3%), pleural effusion (56.1%), and ascites effusion (17.8%) were the 4 most common complications after HAIC conversion surgery. As shown in Table 4, among these 4 complications, only abdominal bleeding (Spearman=0.198, p=0.041) had a positive correlation with the number of HAIC cycles, which means that as the number of HAIC cycles increases, the incidence of abdominal bleeding gradually increases. Furthermore, Table 4 shows that receiving 3–8 cycles of HAIC treatment was more likely to cause abdominal bleeding (16.7% vs 3.8%, p=0.028) than receiving 1–2 cycles of HAIC. However, there was no significant correlation between biliary leakage (p=0.323), pleural effusion (p=0.201), or ascites effusion (p=0.575) and the number of HAIC cycles (Table 4).
 |
Table 4 Correlation Analysis of 4 Major Postoperative Complications with HAIC Cycles of Treatment |
In addition to the above complications, postoperative liver function changes also showed a positive correlation with the number of HAIC cycles. We used the difference between the postoperative ALBI score (that is, the day after the operation, Spearman=0.229, p=0.042) and the preoperative ALBI score as the standard for liver function changes. Based on the results shown in Supplementary Table 2 (the table summarizes only the changes in ALBI grade before and after surgery) and Figure 4, it can be concluded that with an increase in the number of HAIC cycles, the postoperative liver function decreases significantly.
Analysis of pCR Related to the HAIC Cycle
Pathological complete response (pCR) was defined as no histological evidence of a malignant tumor in the primary tumor site via multipoint sampling. Among the 107 patients who underwent surgery after HAIC treatment included in this study, 22 patients met the criteria for pCR (Supplementary Table 3). Moreover, the rate of pCR in patients who received 3–5 cycles of HAIC was significantly higher than that in patients who received 1–2 cycles (29.4% vs 13.2%, p=0.043).
Discussion
In this study, we tried to demonstrate that the optimal timing of CS after HAIC. Compared with the patients in the surgery group, the number of advanced patients in the HAIC-Surgery group was significantly higher. However, some patients were downstaged and even reached the criteria for surgical resection after HAIC treatment. Nonetheless, patients in the HAIC-Surgery group were staged later than those in the Surgery group. Therefore, we performed a PSM for the tumor stage to enable two groups of patients comparable in preoperative stage, which reduces or avoids the influence of tumor stage on the results of subsequent analysis. According to our observations, postoperative complications such as biliary leakage, abdominal bleeding, pleural effusion, and ascites effusion were significantly higher in the HAIC-Surgery group than in the Surgery group. At the meantime, we found the short-term survival benefit (RFS) of patients who underwent HAIC-Surgery is comparable to those direct surgery cohort on early stage.
Recently, there has been growing evidence that HAIC with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX) can provide a significant survival benefit and is safe for patients with advanced HCC.32–35 However, the use of FOLFOX chemotherapy regimens, including oxaliplatin and fluorouracil, has been associated with damage to the liver parenchyma, described as SOS,22 and vascular hepatic lesions (hemorrhagic centrilobular necrosis, HCN; nodular regenerative hyperplasia, NRH), which may progress to fibrosis after long-term chemotherapy.21 Furthermore, previous studies have shown higher AST and ALT levels in patients with high-grade SOS, suggesting postoperative liver failure.36,37 Our study showed that patients in the HAIC- Surgery group had a more pronounced decrease in postoperative liver function than those in the Surgery group, which appeared to be associated with the liver damage (SOS, HCN, RNH, etc.) caused by oxaliplatin and fluorouracil. Furthermore, hepatic sinusoidal injury and portal damage or increases in spleen size related to hypertension caused by oxaliplatin-based chemotherapy could cause some sequelae, such as ascites, hemorrhoidal and variceal bleeding, or persistent thrombocytopenia.25–28 Considering the above study results, we also investigated whether patients who underwent HAIC conversion therapy were more likely to develop biliary leakage, abdominal bleeding, pleural effusion, and ascites effusion after surgery. In essence, we believe that these complications are still related to the decrease in liver function caused by oxaliplatin-induced liver parenchymal injury. Coincidentally, the review by Zhao et al clarified that postoperative major morbidity and liver surgery-specific complications increased in patients with severe SOS resulting from oxaliplatin-based chemotherapy and steatohepatitis after partial hepatectomy.38
Although the liver injury caused by preoperative chemotherapy may cause postoperative complications, HAIC is still an important approach to achieve tumor downstaging. What is more important is to explore the relationship between the number of HAIC cycles and the incidence of complications so that a relative balance can be achieved between the conversion effect and the occurrence of complications. In a study by Karoui et al, complication occurrence after liver resection was associated with the number of preoperative chemotherapy cycles in colorectal cancer liver metastases. Patients who received 6 cycles of chemotherapy showed increased morbidity compared to patients with less than 6 cycles (54% vs 19%; n = 45; p=0.047).39 Similarly, another study also showed that treatment with more than 12 cycles of preoperative chemotherapy resulted in a higher reoperation rate and a longer hospital length of stay.21 At the same time, our study also revealed a significant correlation (Spearmen=0.198, p=0.041) between postoperative abdominal bleeding and preoperative HAIC treatment. The administration of 3 to 8 cycles of HAIC preoperatively are significantly more likely to lead to the development of abdominal bleeding than 1–2 cycles preoperatively. The frequency of chemotherapy and obvious complications in this study is quite different from the results of previous studies, which may be related to the method of preoperative chemotherapy administration. Relatedly, liver metastases from colorectal cancer are typically treated with systemic chemotherapy, while HAIC increases the concentration of chemotherapy drugs in the liver, which may cause more liver toxicity. On the other hand, in this study, the number of patients receiving more than 6 cycles HAIC was small, which may also yield biased results. Although complications other than abdominal bleeding were not significantly correlated with the duration of HAIC, our results showed that the degree of postoperative liver function deterioration gradually increased with an increase in the duration of HAIC (Spearmen=0.229, p=0.042). The only chemotherapy-associated liver injury that negatively affected postoperative outcomes was NRH, which was associated with a higher liver failure rate (7.8% vs 2.8%, p = 0.037).40 Since HAIC with the FOLFOX regimen is generally reviewed after every 2 cycles, we suggest that surgical resection be performed after 4 cycles to reach the standard for conversion surgery and reduce the occurrence of postoperative complications. Of course, because postoperative complications increase with an increasing number of HAIC cycles, it is not necessary to continue treatment through the 4th cycle of HAIC if patients can reach the standard for surgery after 2 cycles.
We also investigated whether the need for more preoperative HAIC cycles was associated with a advanced stage. Obviously, we found that the advanced the preoperative tumor stage was (Spearmen=0.301, p=0.002), the larger the tumor volume (Spearmen=0.311, p=0.001), and the more HAIC cycles were required for downstaging and conversion. In addition, we confirmed that 3–5 cycles of HAIC achieved the highest pathological complete response (pCR) rate (29.4%). In our study, pCR was more likely to be achieved after 3–5 cycles of HAIC than after fewer than 3 cycles of HAIC (29.4% vs 13.2%, p=0.043). However, we did not find that pCR increased with the number of HAIC cycles in this study. This may be related to the small number of included patients who received more than 5 cycles of HAIC. For pCR, some studies have shown that the tumor-free survival of patients after liver cancer conversion resection is related to the degree of pathological remission, with the postoperative tumor-free survival of patients with pathological remission being longer.41,42 However, Kishi et al showed that after more than 8 cycles of chemotherapy, the probability of major pathological remission in colorectal cancer patients with liver metastases did not increase significantly, but the proportion of liver injury caused by chemotherapy did.43 Importantly, SOS resulting from oxaliplatin has also been associated with early tumor recurrence and decreased long-term survival.44 Therefore, a higher number of HAIC cycles does not the pCR rate and may negatively affect prognosis due to the liver damage caused by multiple cycles of HAIC.
Due to the high ORR rate of HAIC,24,32 combined targeted therapy or immunotherapy is used to improve the survival of patients with advanced HCC. Clinical trials in recent years have shown that the combination of HAIC with targeted therapy, immunotherapy and a combination of treatment methods has further improved the transformation rate of liver cancer.13,45–47 However, as a single center retrospective study, we still need more prospective clinical studies to verify these clinical findings. In addition, in order to avoid confounding factors, patients with immune checkpoint inhibitors and molecular targeted therapy were not involved in this study.
Conclusion
In conclusion, we found that the prognosis of advanced HCC after conversion surgery is comparable to that after direct surgery. The optimal number of cycles for HAIC conversion therapy should not exceed 4; a higher number of cycles does not increase pCR and can cause more severe liver dysfunction postoperatively and increase the incidence of complications, such as abdominal bleeding and pleural effusion.
Data Sharing Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author (Zhongguo Zhou).
Ethics Approval and Consent to Participate
This study was conducted according to the ethical guidelines of the 1975 Declaration of Helsinki. This research was approved by the institutional review board of Sun Yat-sen University Cancer Center (Ethical review no. B2022-238-01). The study used retrospective anonymous clinical data that were obtained after each patient agreed to treatment.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work is funded by Sun Yat-sen University Cancer Center physician scientist funding (No. 16zxqk04), Wu Jieping Medical Foundation - special fund for tumor immunity (320.6705.2021-02-76).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi:10.3322/caac.21660
2. IARC. Latest global cancer data Cancer burden rises to 19.3 million new cases and 10.0 million cancer deaths in 2020; 2020.
3. Qiu J, Peng B, Tang Y, et al. CpG methylation signature predicts recurrence in early-stage hepatocellular carcinoma: results from a multicenter study. J Clin Oncol. 2017;35:734–742. doi:10.1200/JCO.2016.68.2153
4. Cucchetti A, Zhong J, Berhane S, et al. The chances of hepatic resection curing hepatocellular carcinoma. J Hepatol. 2020;72:711–717. doi:10.1016/j.jhep.2019.11.016
5. Shaya FT, Breunig IM, Seal B, et al. Comparative and cost effectiveness of treatment modalities for hepatocellular carcinoma in SEER-Medicare. Pharmacoeconomics. 2014;32(1):63–74. doi:10.1007/s40273-013-0109-7
6. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–1905. doi:10.1056/NEJMoa1915745
7. Finn RS, Ikeda M, Zhu AX, et al. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol. 2020;38:2960–2970. doi:10.1200/JCO.20.00808
8. Ren Z, Xu J, Bai Y, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, Phase 2–3 study. Lancet Oncol. 2021;22:977–990. doi:10.1016/S1470-2045(21)00252-7
9. Abou-Alfa GK, Lau G, Kudo M, et al. Tremelimumab plus Durvalumab in Unresectable Hepatocellular Carcinoma. NEJM Evid. 2022;1. doi:10.1056/EVIDoa2100070
10. Kudo M. Treatment of advanced hepatocellular carcinoma with emphasis on hepatic arterial infusion chemotherapy and molecular targeted therapy. Liver Cancer. 2012;1:62–70. doi:10.1159/000342402
11. Ikeda M, Shimizu S, Sato T, et al. Sorafenib plus hepatic arterial infusion chemotherapy with cisplatin versus sorafenib for advanced hepatocellular carcinoma: randomized Phase II trial. Ann Oncol. 2016;27:2090–2096. doi:10.1093/annonc/mdw323
12. Ueshima K, Kudo M, Tanaka M, et al. Phase I/II study of sorafenib in combination with hepatic arterial infusion chemotherapy using low-dose cisplatin and 5-fluorouracil. Liver Cancer. 2015;4:263–273. doi:10.1159/000367751
13. Xu L, Zhang YJ, Wang XH, et al. Transarterial infusion chemotherapy (TAI) combined with Sintilimab in locally advanced, potentially resectable hepatocellular carcinoma (HCC). J Clin Oncol. 2020;38:e16593–e16593. doi:10.1200/JCO.2020.38.15_suppl.e16593
14. He M, Li Q, Zou R, et al. Sorafenib plus hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin vs sorafenib alone for hepatocellular carcinoma with portal vein invasion: a randomized clinical trial. JAMA Oncol. 2019;5:953–960. doi:10.1001/jamaoncol.2019.0250
15. Gourd K, Lai C, Reeves C. ESMO Virtual Congress 2020. Lancet Oncol. 2020;21:1403–1404. doi:10.1016/S1470-2045(20)30585-4
16. Li B, Qiu J, Zheng Y, et al. Conversion to resectability using transarterial chemoembolization combined with hepatic arterial infusion chemotherapy for initially unresectable hepatocellular carcinoma. Ann Surg Open. 2021;2(2):e057. doi:10.1097/AS9.0000000000000057
17. Zhang ZF, Luo YJ, Lu Q, et al. Conversion therapy and suitable timing for subsequent salvage surgery for initially unresectable hepatocellular carcinoma: what is new? World J Clin Cases. 2018;6:259–273. doi:10.12998/wjcc.v6.i9.259
18. Lau WY, Lai EC. Salvage surgery following downstaging of unresectable hepatocellular carcinoma--A strategy to increase resectability. Ann Surg Oncol. 2007;14:3301–3309. doi:10.1245/s10434-007-9549-7
19. Bartkowski R, Berger MR, Aguiar JL, et al. Experiments on the efficacy and toxicity of locoregional chemotherapy of liver tumors with 5-fluoro-2’-deoxyuridine (FUDR) and 5-fluorouracil (5-FU) in an animal model. J Cancer Res Clin Oncol. 1986;111:42–46. doi:10.1007/BF00402774
20. Kuan HY, Smith DE, Ensmiger WD, et al. Regional pharmacokinetics of 5-bromo-2’-deoxyuridine and 5-fluorouracil in dogs: hepatic arterial versus portal venous infusions. Cancer Res. 1996;56:4724–4727.
21. Aloia T, Sebagh M, Plasse M, et al. Liver histology and surgical outcomes after preoperative chemotherapy with fluorouracil plus oxaliplatin in colorectal cancer liver metastases. J Clin Oncol. 2006;24:4983–4990. doi:10.1200/JCO.2006.05.8156
22. Rubbia-Brandt L, Audard V, Sartoretti P, et al. Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol. 2004;15:460–466. doi:10.1093/annonc/mdh095
23. Choi JH, Chung WJ, Bae SH, et al. Randomized, prospective, comparative study on the effects and safety of sorafenib vs. hepatic arterial infusion chemotherapy in patients with advanced hepatocellular carcinoma with portal vein tumor thrombosis. Cancer Chemother Pharmacol. 2018;82(3):469–478. doi:10.1007/s00280-018-3638-0
24. Lyu N, Lin Y, Kong Y, et al. FOXAI: a phase II trial evaluating the efficacy and safety of hepatic arterial infusion of oxaliplatin plus fluorouracil/leucovorin for advanced hepatocellular carcinoma. Gut. 2018;67:395–396. doi:10.1136/gutjnl-2017-314138
25. Slade JH, Alattar ML, Fogelman DR, et al. Portal hypertension associated with oxaliplatin administration: clinical manifestations of hepatic sinusoidal injury. Clin Colorectal Cancer. 2009;8:225–230. doi:10.3816/CCC.2009.n.038
26. Overman MJ, Maru DM, Charnsangavej C, et al. Oxaliplatin-mediated increase in spleen size as a biomarker for the development of hepatic sinusoidal injury. J Clin Oncol. 2010;28:2549–2555. doi:10.1200/JCO.2009.27.5701
27. Arotçarena R, Calès V, Berthelémy P, et al. Severe sinusoidal lesions: a serious and overlooked complication of oxaliplatin-containing chemotherapy? Gastroentérologie Clinique et Biologique. 2006;30:1313–1316. doi:10.1016/S0399-8320(06)73542-4
28. Agarwal V, Sgouros J, Smithson J, et al. Sinusoidal obstruction syndrome (veno-occlusive disease) in a patient receiving bevacizumab for metastatic colorectal cancer: a case report. J Med Case Rep. 2008;2:227. doi:10.1186/1752-1947-2-227
29. Yang P, Qiu J, Li J, et al. Nomograms for pre- and postoperative prediction of long-term survival for patients who underwent hepatectomy for multiple hepatocellular carcinomas. Ann Surg. 2016;263:778–786. doi:10.1097/SLA.0000000000001339
30. Schaible A, Schemmer P, Hackert T, et al. Location of a biliary leak after liver resection determines success of endoscopic treatment. Surg Endosc. 2017;31:1814–1820. doi:10.1007/s00464-016-5178-1
31. Rahbari NN, Garden OJ, Padbury R, et al. Post-hepatectomy haemorrhage: a definition and grading by the International Study Group of Liver Surgery (ISGLS). HPB. 2011;13:528–535. doi:10.1111/j.1477-2574.2011.00319.x
32. Lyu N, Kong Y, Mu L, et al. Hepatic arterial infusion of oxaliplatin plus fluorouracil/leucovorin vs. sorafenib for advanced hepatocellular carcinoma. J Hepatol. 2018;69:60–69. doi:10.1016/j.jhep.2018.02.008
33. Li QJ, He MK, Chen HW, et al. Hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin versus transarterial chemoembolization for large hepatocellular carcinoma: a randomized Phase III trial. J Clin Oncol. 2022;40:150–160. doi:10.1200/JCO.21.00608
34. He MK, Le Y, Li QJ, et al. Hepatic artery infusion chemotherapy using mFOLFOX versus transarterial chemoembolization for massive unresectable hepatocellular carcinoma: a prospective non-randomized study. Chin J Cancer. 2017;36:83. doi:10.1186/s40880-017-0251-2
35. Qin S, Bai Y, Lim HY, et al. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J Clin Oncol. 2013;31:3501–3508. doi:10.1200/JCO.2012.44.5643
36. Soubrane O, Brouquet A, Zalinski S, et al. Predicting high grade lesions of sinusoidal obstruction syndrome related to oxaliplatin-based chemotherapy for colorectal liver metastases: correlation with post-hepatectomy outcome. Ann Surg. 2010;251:454–460. doi:10.1097/SLA.0b013e3181c79403
37. Nakano H, Oussoultzoglou E, Rosso E, et al. Sinusoidal injury increases morbidity after major hepatectomy in patients with colorectal liver metastases receiving preoperative chemotherapy. Ann Surg. 2008;247:118–124. doi:10.1097/SLA.0b013e31815774de
38. Zhao J, van Mierlo KMC, Gomez-Ramirez J, et al. Systematic review of the influence of chemotherapy-associated liver injury on outcome after partial hepatectomy for colorectal liver metastases. Br J Surg. 2017;104:990–1002. doi:10.1002/bjs.10572
39. Karoui M, Penna C, Amin-Hashem M, et al. Influence of preoperative chemotherapy on the risk of major hepatectomy for colorectal liver metastases. Ann Surg. 2006;243:1–7. doi:10.1097/01.sla.0000193603.26265.c3
40. Vigano L, De Rosa G, Toso C, et al. Reversibility of chemotherapy-related liver injury. J Hepatol. 2017;67:84–91. doi:10.1016/j.jhep.2017.02.031
41. Zhang W, Hu B, Han J, et al. 174P A real-world study of PD-1 inhibitors combined with TKIs for HCC with major vascular invasion as the conversion therapy: a prospective, non-randomized, open-label cohort study. Ann Oncol. 2020;31:S1307. doi:10.1016/j.annonc.2020.10.195
42. Yarchoan M, Zhu QF, Durham JN, et al. Feasibility and efficacy of neoadjuvant cabozantinib and nivolumab in patients with borderline resectable or locally advanced hepatocellular carcinoma (HCC). J Clin Oncol. 2021;39:335. doi:10.1200/JCO.2021.39.3_suppl.335
43. Kishi Y, Zorzi D, Contreras CM, et al. Extended preoperative chemotherapy does not improve pathologic response and increases postoperative liver insufficiency after hepatic resection for colorectal liver metastases. Ann Surg Oncol. 2010;17:2870–2876. doi:10.1245/s10434-010-1166-1
44. Tamandl D, Klinger M, Eipeldauer S, et al. Sinusoidal obstruction syndrome impairs long-term outcome of colorectal liver metastases treated with resection after neoadjuvant chemotherapy. Ann Surg Oncol. 2011;18:421–430. doi:10.1245/s10434-010-1317-4
45. He MK, Liang RB, Zhao Y, et al. Lenvatinib, toripalimab, plus hepatic arterial infusion chemotherapy versus lenvatinib alone for advanced hepatocellular carcinoma. Ther Adv Med Oncol. 2021;13:17588359211002720. doi:10.1177/17588359211002720
46. Yang X, Lin J, Zhao H. Potential areas of interest in a trial of sorafenib plus hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin for Hepatocellular Carcinoma. JAMA Oncol. 2019. doi:10.1001/jamaoncol.2019.4049
47. Kudo M, Ueshima K, Yokosuka O, et al. Sorafenib plus low-dose cisplatin and fluorouracil hepatic arterial infusion chemotherapy versus sorafenib alone in patients with advanced hepatocellular carcinoma (SILIUS): a randomised, open label, Phase 3 trial. Lancet Gastroenterol Hepatol. 2018;3:424–432. doi:10.1016/S2468-1253(18)30078-5
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

