Back to Journals » Infection and Drug Resistance » Volume 15
Hemorrhagic Fever with Renal Syndrome Complicated with Rhino Mucormycosis: A Case Report
Authors Guo L , Zhang J, Lei J, Wang G
Received 1 October 2022
Accepted for publication 29 November 2022
Published 6 December 2022 Volume 2022:15 Pages 7139—7145
DOI https://doi.org/10.2147/IDR.S391035
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Video abstract of "HFRS complicated with rhino MCR" [ID 391035].
Views: 106
Litao Guo,1,* Jingjing Zhang,2,* Jin’e Lei,3 Gang Wang2
1Department of Critical Care Medicine, the First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, People’s Republic of China; 2Department of Critical Care Medicine, the Second Affiliated Hospital of Xi’an Jiaotong University, Xi’an, People’s Republic of China; 3Department of Clinical Laboratory, the First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jin’e Lei, Department of Clinical Laboratory, the First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, People’s Republic of China, Email [email protected] Gang Wang, Department of Critical Care Medicine, the Second Affiliated Hospital of Xi’an Jiaotong University, Xi’an, People’s Republic of China, Email [email protected]
Abstract: Mucormycosis (MCR) is a rare but aggressive fungal disease. Rhino-orbito-cerebral mucormycosis is the most common clinical form of MCR infection, and sinonasal inoculation is the primary site of infection. The morbidity and mortality rates associated with MCR remain high. In this case report, we describe the successful use of amphotericin B in a 40-year-old male with hemorrhagic fever with renal syndrome (HFRS) complicated by rhinomucormycosis. This case report provides evidence for the successful treatment of HFRS.
Keywords: hemorrhagic fever with renal syndrome, mucormycosis, pulmonary aspergillosis, sepsis, COVID-19, acute kidney injury
Introduction
Mucormycosis (MCR) is a rare but aggressive fungal disease, and rhino-orbito-cerebral mucormycosis (ROCM), the most common clinical form of MCR infection, is typically the primary site of infection.1 ROCM typically begins in the nasal mucosa and spreads into the paranasal sinuses and spaces in the neck, orbits, and intracranial structures.2 A compromised immune system and diabetes are the most important risk factors for MCR infection.3 Since the coronavirus disease 2019 (COVID-19) outbreak, MCR coinfections have been observed in patients with COVID-19.4 Hemorrhagic fever with renal syndrome (HFRS) is an important infectious disease that endangers human health and is caused by the hemorrhagic fever virus (hantavirus), a natural epidemic disease with rodents as the main vector of infection. Fever, bleeding, congestion, hypotensive shock, and acute kidney injury (AKI) are the main clinical manifestations of HFRS, which may affect the immune system itself, resulting in vulnerability to mucormycosis. The morbidity and mortality rates of MCR remain high,5 and establishing an early diagnosis and administering first-line treatment with amphotericin B can improve the clinical outcome.6 We report the successful use of amphotericin B in a 40-year-old male patient with HFRS and rhinomucormycosis. This case study provides evidence for the successful treatment of HFRS.
Case Presentation
A 40-year-old male who was 178-cm tall and 100-kg in weight was admitted to a local hospital in November 2013 year with fever (39 °C), diarrhea, an oxygen saturation of 60%, and the gradual onset of dyspnea, confusion, and shock. Physical examination revealed bleeding spots on the chest and abdomen and florid spots on the lower limbs. Laboratory tests revealed a platelet count (PLT) of 26 × 109/L, a blood urea nitrogen (BUN) level of 13.9 mmol/L, a creatinine (CRE) level of 330 μmol/L, a urine protein level of 3+, and positivity for the HFRS anti-IgM antibody. The patient had a history of hypertension (usual blood pressure 160/100 mmHg) and fatty liver. The patient was diagnosed with HFRS and treated with ventilator-assisted ventilation, fluid replacement, and norepinephrine to increase the blood pressure. He was admitted to the Department of Intensive Care at The First Affiliated Hospital of Xi’an Jiaotong University with HFRS, septic shock, decompensated metabolic acidosis, AKI, and multiple organ failure. The laboratory test results after admission into the ICU are listed in Table 1. Because of AKI and anuria, continuous renal replacement therapy (CRRT) was administered, and ventilator-assisted ventilation, sedation and analgesia, fluid replacement, norepinephrine to increase the blood pressure, and plasma and platelet transfusions were administered.
 |
Table 1 Laboratory Test Indicators at Admission into the ICU |
On day 1 of admission, the left nasal cavity was filled with an expanded sponge because of coagulation disorders complicated by left nasal cavity bleeding. On the second day after the nasal filling, the left nasal alar became purple and swollen (Figure 1). Massive nasal necrotic lesions rapidly occurred the next day. Microbial cultures from the lesion samples revealed the growth of mucormycetes (Figure 2). The strain was identified as Rhizopus oryzae complex by mass spectrometry (Figure 3). Bronchofiberoptic bronchoalveolar lavage fluid culture confirmed Aspergillus growth, polymerase chain reaction (PCR) strains identified Aspergillus fumigatus. Bronchoalveolar lavage fluid galactomannan (GM) 1.29 (GM < 1.0 negative), and serum GM 4.99 (GM < 0.5 negative). The patient was diagnosed with rhinomucormycosis and pulmonary aspergillosis, and systemic caspofungin and voriconazole were administered. The therapeutic dose was adjusted according to organ function and drug characteristics of the patients. Caspofungin: loading dose: 70 mg/d, qd; post-35 mg/d, ivgtt; voriconazole: loading dose: 400 mg/12 h, ivgtt, bid, post-100 mg/12 h, ivgtt. Because the patient had developed multiple organ failure, such as liver and kidney failure, intravenous amphotericin B treatment was not possible. To avoid further impairment of the liver function, we developed a local treatment regimen for nasal mucormycosis. The nasal cavity was rinsed with 10 mL saline, kept dry for 1 h, then filled with a sterile dressing containing 2.5 mg/mL amphotericin B for 4 h, and finally filled with a sterile dressing containing 5% sodium bicarbonate for 1 h. These procedures were administered every 6 h. After 12 days, the left nasal scab fell off, and a large tissue defect remained (Figure 4). Computed tomography revealed no orbitocerebral involvement during the treatment.
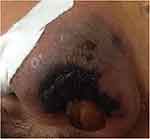 |
Figure 1 The left nasal alar revealed purple and swollen. |
 |
Figure 2 (A) Sabouraud dextrose agar: Villous colonies after 48 hours. (B) Light microscope×200: large sporangia, rhizoids, hold capsule upright vertical. |
 |
Figure 3 Mass spectrum of Rhizopus oryzae complex, 99.9% confidence. |
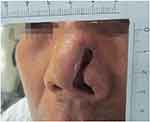 |
Figure 4 After 12 days of treatments, the left nasal scab fell off and a large tissue defect remained. |
After the treatment, the patient ‘s condition gradually stabilized, bronchoalveolar lavage fluid culture revealed no fungal growth, and lung imaging findings gradually returned to normal. Urine output and renal function recovered after weaning from the ventilator (day 11), discontinuation of caspofungin (day 13), and discontinuation of voriconazole (day 14). The patient recovered on day 28 and was discharged. During 2 years of follow-up, the patient exhibited no further signs of rhinomucormycosis.
Discussion
Mucormycosis is an opportunistic fungal infection caused by Rhizopus, Lichtheimia, and Mucor.7 Fungal spores are mainly inhaled through the respiratory tract, causing lung infection, or direct skin inoculation, causing infection.8 Mucormycosis invades the human body, causing infections that progress rapidly and spread to the systemic system, and can involve the central nervous system, orbit, paranasal sinuses, skin, lungs, gastrointestinal tract, and kidneys.1,2,9 Patient susceptibility is an important cause of mucor infection.7 Several factors are associated with susceptibility to mucormycosis. Diabetes mellitus, malignancy, organ transplantation, corticosteroids, and immunosuppressive therapy are considered risk factors for MCR.8,10,11 The incidence of MCR is lower in women than men, presumably because of the protective effect of estrogen on the development of paracoccidioidomycosis.8 Since the COVID-19 epidemic in 2020, there have been increasing numbers of reports on the incidence of MCR in patients with COVID-19 or in patients in rehabilitation.12 Opportunistic fungal infections, such as MCR, can be promoted by low oxygen levels, high iron levels, an acidic internal environment, a stress-induced hyperglycemic state, and reduced phagocyte function in patients with COVID-19.13 In recent decades, the incidence of MCR has increased worldwide, and its mortality remains high.6
Herein, we reported the case of a male patient with HFRS who presented with AKI and septic shock with stress hyperglycemia, severe hypoxia, and decompensated metabolic acidosis. These are high-risk factors for MCR infection. HFRS is characterized by AKI as the main manifestation of hantavirus infection.14 Viral infections are associated with opportunistic fungal infections, particularly Mucor and Aspergillus infections.12 Therefore, when the patient experienced nasal bleeding, nasal packing and left alar necrosis developed and rapidly progressed. We identified this strain as a Rhizopus oryzae complex using microbial culture and mass spectrometry. Bronchoalveolar lavage fluid culture confirmed Aspergillus growth, and polymerase chain reaction (PCR) identified the strains as A. fumigatus. The patient was diagnosed with nasal mucormycosis and pulmonary aspergillosis. Voriconazole is the drug-of-choice for treating pulmonary aspergillosis,15 however, studies have demonstrated that the combination of voriconazole and caspofungin has a synergistic effect and is more effective than voriconazole alone.16 The patient in this case was considered to have septic shock and was critically ill; therefore, voriconazole combined with caspofungin was administered. Dose adjustment and optimization were performed according to organ function and drug characteristics, and the pulmonary aspergillosis was controlled after treatment. Therefore, the combination of voriconazole and caspofungin can be considered for the treatment of pulmonary aspergillosis in critically ill patients.
Liposomal amphotericin B (LAmB) is the most effective antifungal agent for the treatment of mucormycosis.9 In addition, posaconazole and isavuconazole are recommended in refractory cases and in patients who are intolerant to prior antifungal agents.9 However, LAmB has more-toxic side effects, especially hepatotoxicity, and our patient had already developed liver failure; therefore, intravenous systemic use of LAmB was not possible. Considering the high mortality rate of MCR, we consulted the relevant data and developed a local treatment plan (case presentation) for MCR taking into consideration the patient ‘s condition. Topical treatment regimens are based on the following two considerations: changing the local growth environment of Mucor, and in contrast, administering local treatment with LAmB. In this patient, the left alar lesion did not spread further and was gradually controlled. Therefore, surgical debridement was not performed. Large tissue defects remained after the scab was detached. Surgical debridement is necessary if early treatment does not control disease progression or if necrotic tissue after disease control requires surgical debridement.1 Eventually, we successfully treated the MCR in this patient. The success of treatment also benefits from the improvement of the general condition, which is the result of comprehensive treatment.
Conclusion
Mucormycosis is a rare, fulminant, and aggressive disease. Its management is challenging, especially in the developing world. Early high-dose intravenous antifungal therapy is critical, and aggressive surgical debridement is necessary. HFRS increases the difficulty of treatment. This case provides a reference for the treatment of patients with severe MCR. Because this is a successful experience in a single case, whether it can be further promoted requires evidence from additional clinical case studies.
Data Sharing Statement
All the information supporting our conclusions and relevant references are included in the manuscript. There are no datasets related to this case report.
Declarations Ethics Approval and Consent to Participate
It is approved by the First Affiliated Hospital of Xi’an Jiaotong University Ethics committee. The patient was informed and written consent was documented in the patients’ medical records. All procedures performed in the present study were in accordance with the Declaration of Helsinki.
Acknowledgments
We are very thankful for the patient and all medical staff involved in the treatment of patients. This study was funded by Key Research and Development Program of Shaanxi Province of China (No. 2021SF-056), the Clinical Research Award of the First Affiliated Hospital of Xi’an Jiaotong University, China (No.XJTU1AF-CRF-2022-016).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Pai V, Sansi R, Kharche R, et al. Rhino-orbito-cerebral mucormycosis: pictorial review. Insights Imaging. 2021;12(1):167. doi:10.1186/s13244-021-01109-z
2. Chan LL, Singh S, Jones D, et al. Imaging of mucormycosis skull base osteomyelitis. AJNR Am J Neuroradiol. 2000;21(5):828–831.
3. Prakash H, Chakrabarti A. Global epidemiology of mucormycosis. J Fungus. 2019;5(1):26. doi:10.3390/jof5010026
4. Hussain S, Riad A, Singh A, et al. Global prevalence of COVID-19-associated mucormycosis (CAM): living systematic review and meta-analysis. J Fungi. 2021;7(11):985. doi:10.3390/jof7110985
5. Darwish RM, AlMasri M, Al-Masri MM. Mucormycosis: the hidden and forgotten disease. J Appl Microbiol. 2022;132(6):4042–4057. doi:10.1111/jam.15487
6. Chao CM, Lai CC, Yu WL. COVID-19 associated mucormycosis - an emerging threat. J Microbiol Immunol Infect. 2022;55(2):183–190. doi:10.1016/j.jmii.2021.12.007
7. Skiada A, Pavleas I, Drogari-Apiranthitou M. Epidemiology and diagnosis of mucormycosis: an update. J Fungi. 2020;6(4):265. doi:10.3390/jof6040265
8. Riad A, Shabaan AA, Issa J, et al. COVID-19-Associated Mucormycosis (CAM): case-series and global analysis of mortality risk factors. J Fungi. 2021;7(10):837. doi:10.3390/jof7100837
9. Ryu BU, Laylani NAR, Davila-Siliezar P, et al. Rhino-orbital mucormycosis. Curr Opin Ophthalmol. 2022;33(6):501–506. doi:10.1097/ICU.0000000000000892
10. Jeong W, Keighley C, Wolfe R, et al. The epidemiology and clinical manifestations of mucormycosis: a systematic review and meta-analysis of case reports. Clin Microbiol Infect. 2019;25(1):26–34. doi:10.1016/j.cmi.2018.07.011
11. Nicolás FE, Murcia L, Navarro E, et al. Mucorales species and macrophages. J Fungi. 2020;6(2):94. doi:10.3390/jof6020094
12. Baddley JW, Thompson GR, Chen SC, et al. Coronavirus disease 2019-associated invasive fungal infection. Open Forum Infect Dis. 2021;8(12):ofab510. doi:10.1093/ofid/ofab510
13. Singh AK, Singh R, Joshi SR, et al. Mucormycosis in COVID-19: a systematic review of cases reported worldwide and in India. Diabetes Metab Syndr Clin Res Rev. 2021;15(4):102146. doi:10.1016/j.dsx.2021.05.019
14. Tariq M, Kim DM. Hemorrhagic fever with renal syndrome: literature review, epidemiology, clinical picture and pathogenesis. Infect Chemother. 2022;54(1):1–19. doi:10.3947/ic.2021.0148
15. Zaini F, Lotfali E, Fattahi A, et al. Voriconazole resistance genes in Aspergillus flavus clinical isolates. J Mycol Med. 2020;30(2):100953. doi:10.1016/j.mycmed.2020.100953
16. Cuenca-Estrella M, Gomez-Lopez A, Garcia-Effron G, et al. Combined activity in vitro of caspofungin, amphotericin B, and azole agents against itraconazole-resistant clinical isolates of Aspergillus fumigatus. Antimicrob Agents Chemother. 2005;49(3):1232–1235. doi:10.1128/AAC.49.3.1232-1235.2005
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
