Back to Journals » Research Reports in Clinical Cardiology » Volume 6
Hemodynamic device-based optimization in cardiac resynchronization therapy: concordance with systematic echocardiographic assessment of AV and VV intervals
Authors Oliveira M, Branco L, Galrinho A, da Silva N, Cunha PS, Valente B, Feliciano J, Pimenta R, Delgado AS, Ferreira RC
Received 10 February 2015
Accepted for publication 5 May 2015
Published 6 August 2015 Volume 2015:6 Pages 97—103
DOI https://doi.org/10.2147/RRCC.S82540
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Kones
Mário M Oliveira, Luisa M Branco, Ana Galrinho, Nogueira da Silva, Pedro S Cunha, Bruno Valente, Joana Feliciano, Ricardo Pimenta, Ana Sofia Delgado, Rui Cruz Ferreira
Santa Marta Hospital, Lisbon, Portugal
Background: Inappropriate settings of atrioventricular (AV) and ventriculo-ventricular (VV) intervals can be one of the factors impacting response to cardiac resynchronization therapy (CRT). Optimal concordance of AV and VV intervals between echocardiographic-based assessment and a device-based automatic programming with a hemodynamic sensor was investigated, together with left ventricle (LV) reverse remodeling after 6 months of regular automatic device-based optimization.
Methods: We evaluated blindly 30 systematic echocardiographic examinations during 6 months in 17 patients (12 men, 64±10 years, in sinus rhythm and New York Heart Association class III; 76% with non-ischemic dilated cardiomyopathy, LV ejection fraction [LVEF] <35%, QRS 130 milliseconds and LV dyssynchrony) implanted with the SonRtip lead and a cardioverter-defibrillator device. Dyssynchrony (AV, VV, or intraventricular) was evaluated by an experienced operator blinded to the device programming, using conventional echocardiography, tissue synchronization imaging, tissue Doppler imaging, radial strain, and 3D echocardiography.
Results: Either no AV or VV dyssynchrony (n=11; 36.7%) or a slight septal or lateral delay (n=13; 43.3%) was found in most echocardiography examinations (80%). AV or VV dyssynchrony requiring further optimization was identified in one-fifth of the examinations (20%). At 6 months, 76.5% patients were responders with LV reverse remodeling, of which 69% were super-responders (LVEF >40%). A statistically significant increase in LVEF was observed between baseline and 6 months post implant (P<0.01). One patient died from non-cardiac causes.
Conclusion: Concordance between echocardiographic methods and device-based hemodynamic sensor optimization was found in most examinations (80%) post CRT. After 6 months of systematic optimization with SonR, patients showed a statistically significant increase in LVEF, with a high rate of reverse remodeling.
Keywords: cardiac resynchronization therapy, hemodynamic sensor, atrioventricular delay, interventricular delay, reverse remodeling
Introduction
Cardiac resynchronization therapy (CRT) has demonstrated significant clinical benefits and left ventricular (LV) reverse remodeling in selected patients with heart failure (HF), severe LV dysfunction, and a wide QRS complex.1 An increase in cardiac output and hemodynamic parameters has been associated with improvement in functional capacity and quality of life and decrease in symptoms and hospitalization due to HF and mortality. However, up to one-third of patients with advanced HF do not exhibit a positive response to CRT despite appropriate selection.2 Suboptimal atrioventricular (AV) timings have been reported as important contributors to this high proportion of non-responders to CRT.2 AV delay optimization, although recommended, is often poorly performed in clinical practice. Hence, the medical community appears to wait for further evidence on the benefit of AV and ventriculo-ventricular (VV) optimization of CRT programming in clinical outcomes. Despite the use of non-invasive techniques, including echocardiography and electrogram device-based algorithms, to optimize AV and VV delay parameters, there is lack of consensus on this issue, with discrepancy between the findings from echocardiographic methods and automatic intracardiac AV and VV interval optimization.3 Echocardiography has been considered the gold standard tool in patients undergoing optimization of their AV and VV timings. Nevertheless, it is relatively costly, time consuming, requires skill and expertise to be accurate, and, because optimal intervals often change over time, may require systematic validation to improve CRT effectiveness. The availability of an effective automatic optimization algorithm capable of a systematic adaptive optimization of AV and VV intervals in concordance with echocardiographic-based methods could change clinical practice in this important field. In order to investigate further this question, the concordance of optimal AV and VV intervals between echocardiographic-based assessment and an automatic device programming using the available hemodynamic sensor algorithm SonR was evaluated in the first 6 months after CRT implant, and CRT response was recorded up to 6 months of automatic optimization.
Methods
Population
The study was carried out in 17 consecutive patients following successful CRT implantation. Before CRT, patients were in New York Heart Association (NYHA) class III with a clinical history of non-ischemic dilated cardiomyopathy or ischemic cardiomyopathy, sinus rhythm with left bundle branch block (LBBB), QRS width ≥130 milliseconds, and intraventricular dyssynchrony, based on echocardiography evaluation, demonstrated in all cases. Patients were excluded according to the following criteria: any sustained ventricular tachyarrhythmia, acute coronary syndrome, coronary artery bypass graft or percutaneous transluminal coronary angioplasty within the previous 4 weeks, correctable valvular disease as a cause of HF, a cerebrovascular accident within the previous 3 months, atrial tachyarrhythmias, post heart transplant, renal failure requiring dialysis, previous CRT implant, unavailability to travel to hospital for echocardiographic examination, and life expectancy <1 year. All subjects gave written informed consent to participate in the study. The study was conducted in compliance with Good Clinical Practice guidelines, consistent with the Declaration of Helsinki.
Implanted devices
All patients received a CRT system combined with a cardioverter-defibrillator (CRT-D; Paradym RF SonR, Sorin), with the atrial SonRtip™ bipolar lead (Sorin CRM SAS, Clamart, France) containing the SonR micro-accelerometer into the tip of the active fixation lead, previously described.4 The SonR hemodynamic sensor detects cardiac muscle vibrations using the accelerometer that reflects the first heart sound (SonR signal) and computes AV and VV delay based on hemodynamic sensed data. The choice of right and left ventricular (RV and LV) leads was left at implanting investigators’ discretion. Figure 1 shows the schematic anatomy of the coronary sinus branches and the distribution of the LV lead tip location in the population studied.
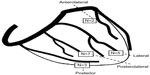  | Figure 1 Anatomical location of the left ventricular lead tip for all 17 patients as defined by fluoroscopy (RAO view). |
Patients were discharged with the SonR sensor function on. The algorithm automatically optimizes AV and VV intervals each week, based on measurement changes in the SonR signal, which were shown previously to be correlated with hemodynamic improvement (LV dP/dtmax).5
Methods of evaluation
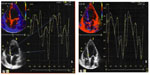
In all, 1 month and 6 months after CRT implantation, patients underwent standard device interrogation followed by echocardiographic evaluation performed by an experienced operator, 12–16 hours after automatic AV and VV optimization by the SonR technology. Concordance analysis was obtained from 30 systematic echocardiographic examinations scheduled at rest, undertaken using Vivid E9 (General Electrics, Vingmed, Milwaukee, WI, USA) with software for resynchronization analysis and a probe for 3D and multiplane echocardiography. The operator was blinded to the CRT device programming. AV dyssynchrony was studied by analyzing pulsed Doppler transmitral flow recordings at the tip of the mitral valve leaflets, with optimal AV delay identified according to the longest LV filling time without any truncation of the A wave; interventricular dyssynchrony was identified by a difference of >40 milliseconds between LV and RV pre-ejection time using conventional echocardiography; and intraventricular dyssynchrony was evaluated by conventional echocardiography searching septal flash or dyssynchronous apical movement and by tissue synchronization imaging, with a difference >65 milliseconds between the time-to-peak systolic velocities of septal and lateral walls and anterior septum and posterior wall, in a conventional way and using 3D probe with multiplane tissue synchronization imaging, and/or with a standard deviation of all 12 segments or four basal segments greater than 33 milliseconds and/or radial strain with a difference >130 milliseconds between different segments. VV dyssynchrony was present when there was a difference of the time to peak >65 milliseconds between the septal and lateral walls of the LV and/or when interventricular dyssynchrony was detected, as described above.
Additionally, responders’ rates were evaluated at 6 months of automatic CRT optimization with the hemodynamic sensor. Responders were defined as patients having a sustained improvement in at least one NYHA functional class after 6 months, with a decrease of >15% of their LV end-systolic volume (LVESV) compared to the baseline.6,7 The patients having a final LV ejection fraction (LVEF) >40% and an improvement in LVEF ≥20% above baseline values were considered as super-responders.6,7
Statistical analysis
Continuous data are expressed as mean ± SD. Comparison of the continuous parametric variables of the echocardiographic findings at the baseline and at 6 months was performed using paired tests: if the variables followed a normal distribution, a paired t-test was used; otherwise, a sign paired test was applied. A value of P<0.05 was considered statistically significant. Analyses were performed with the statistical software program GraphPad Prism 6.
Results
Population
We followed 17 consecutive patients (12 men; 64.4±10.5 years; in sinus rhythm and NYHA class III), with severe LV dysfunction (LVEF <35%), LBBB, QRS width ≥130 milliseconds, and intraventricular dyssynchrony before CRT. Most patients (76%) presented with non-ischemic dilated cardiomyopathy, and 24% had ischemic cardiomyopathy. Baseline conditions of the patients enrolled in the study are summarized in Table 1.
Reverse remodeling
Echocardiographic data obtained pre-CRT-D and 1 month and 6 months after CRT-D implantation are shown in Table 2. There was a sustained significant improvement in LVEF since the 1st month, and both LV end-systolic and end-diastolic volumes decreased during the 6 months compared to the baseline, while LV and left atrium diameters showed a slight non-significant decrease. At the end of the 6-month follow-up period, 13 patients (76.5%) were responders with LV reverse remodeling and four patients (23.5%) were non-responders. Among the group of responders, nine (69.2%) were classified as super-responders.
Concordance between automatic hemodynamic sensor and echocardiography methods
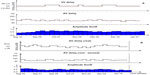
All patients had the SonR sensor on with AV and/or VV intervals automatically changed over time. Observation data showed frequent variations in all patients, confirmed via device interrogation in the outpatient clinic. Figure 2 shows examples of the sensor amplitude evolution and of the automatic programmed AV and VV intervals during the 6-month follow-up period in a super-responder patient (Figure 2A) and in a non-responder (Figure 2B).
In 24 out of 30 systematic post CRT echocardiographic examinations (80%), there was no AV or VV dyssynchrony (11/24) or a slight septal or lateral delay was detected (13/24) (Figure 3). In these cases, the automatically programmed AV and VV intervals were considered sufficient to allow synchronization. In six examinations (20%), AV or VV dyssynchrony was identified maintaining the SonR sensor switched on during the first 6 months follow-up period and stalling the decision regarding further optimization according to the clinical and echocardiographic response. Four cases were excluded from the 6 months analysis of concordance between both methods due to development of persistent atrial fibrillation (n=1), loss of LV capture (n=1), decision to undergo optimization based on echocardiography in a non-responder to CRT (n=1), and death caused by pneumonia (n=1).
Discussion
In this initial experience using systematic device programming based on a hemodynamic sensor, we demonstrated that the large majority of post CRT echocardiographic evaluations were in concordance with the AV and VV intervals automatically programmed. Using advanced state-of-the-art echocardiography techniques for mechanical dyssynchrony mapping, we showed, for the first time, a strong agreement between optimization with the hemodynamic sensor and echocardiographic analysis by an experienced echocardiography operator blinded to programming of AV and VV intervals. Concordance regarding synchronization was found in 80% of the evaluations, while AV or VV dyssynchrony was identified in only 20%. These data also confirm the frequent variations over time in individually optimized AV and VV delays, as previously suggested by other authors.8–14 Finally, these single-center findings, using a device with an algorithm that allows weekly automatic reprogramming of optimal AV and VV intervals, were corroborated by a high rate of reverse remodeling and super-responders at 6 months, with a sustained improvement in LVEF and a significant reduction in LV volumes. Reverse remodeling, as a response to CRT, has been defined as a decrease in LVESV of at least 10% or 15% with an absolute improvement of LVEF >5%.6–8 In previous studies based on these criteria, a marked percentage of patients seemed not to benefit from CRT. In fact, pooled data have demonstrated that one-third of patients undergoing CRT fail to experience symptomatic benefit, with only 60% exhibiting a favorable echocardiographic response12,13 and approximately 20% being considered super-responders.12 Comparable echocardiographic responder rates (decrease in LVESV ≥15%) of 63% and 60% were reported at 6-month follow-up by Poller et al15 and Shetty et al,16 respectively. In the present study, the rates of clinical responders with reverse remodeling were almost 80%, and the majority of responders were classified as super-responders. These results may have a potential benefit in outcomes since reduction in LVESV and increase in LVEF appear to be good predictors of long-term prognosis after CRT.14
Recently, the SonR signal was found to be associated with better clinical outcomes in CRT patients in a pilot study (CLEAR).10 Also, a CLEAR post hoc analysis studied retrospectively the association between the frequency of AV and VV intervals’ optimization and clinical outcomes in CRT-P patients.17 The authors concluded that systematic optimization (at implant, at 3 months and 6 months), irrespective of the optimization method used, either echocardiography or hemodynamic sensor-based algorithm, was associated with improved long-term clinical response. To our knowledge, no other studies have evaluated the course of LV reverse remodeling and the concordance between automatic programming of AV and VV intervals and echocardiographic evaluation of dyssynchrony in a population submitted to CRT. Our study suggests a potential role for individual and periodic automatic device optimization in patients undergoing CRT. Nevertheless, such improvements in reverse remodeling and LV function need to be confirmed in large prospective studies, with longer follow-up periods. The RESPOND CRT,18 a double-blinded, randomized, multicenter trial to assess the clinical effectiveness and reverse remodeling at 1 year after CRT-D using the SonR-based automatic optimization algorithm, which completed its enrollment recently, will certainly bring some answers to the impact of systematic automatic optimization of CRT programming in clinical practice.
Study limitations
This was a single-center observational study with consecutive patients undergoing implantation of a CRT device with a nouvelle hemodynamic algorithm for automatic programming optimization. The study sample was small, with a short follow-up period and with non-ischemic cardiomyopathy as the etiology of HF in the majority of the patients. However, considering the consistent improvements in LV remodeling reported in consecutive echocardiographic assessments and the mechanical synchrony benefits, confirmed by an experienced operator blinded to CRT programming, our data support the potential benefits of the SonR-based algorithm to identify the optimal AV and VV delays at weekly intervals in patients with severe HF, LBBB, and low LVEF who are candidates for CRT.
Conclusion
Concordance between echocardiographic methods and device-based hemodynamic sensor optimization of AV and VV delays was found in most examinations (80%) post CRT. After 6 months of systematic optimization with SonR, patients showed a statistically significant increase in LVEF with a high rate of reverse remodeling. These observations warrant further larger scale studies to validate the power of an automated hemodynamic-based method in CRT-D patients.
Acknowledgments
The authors would like to thank Daniela Oliveira, MSc, for data collection and Anne Rousseauplasse, PhD, and Frédérique Maneval, MSc, for editorial assistance. The abstract of this paper was presented at the Cardiostim 2014 as a poster with interim findings. However, the study has never been published.
Disclosure
The authors report no conflicts of interest in this work.
References
Brignole M, Auricchio A, Baron-Esquivias G, et al. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Eur Heart J. 2013;34(29):2281–2329. | |
Mullens W, Grimm RA, Verga T, et al. Insights from a cardiac resynchronization optimization clinic as part of a heart failure disease management program. J Am Coll Cardiol. 2009;53(9):765–773. | |
Kamdar R, Frain E, Warburton F, et al. A prospective comparison of echocardiography and device algorithms for atrioventricular and interventricular interval optimization in cardiac resynchronization therapy. Europace. 2010;12(1):84–91. | |
Sacchi S, Contardi D, Pieragnoli P, Ricciardi G, Giomi A, Padeletti L. Hemodynamic sensor in cardiac implantable electric devices: the endocardial acceleration technology. J Healthc Eng. 2013;4(4):453–464. | |
Delnoy PP, Marcelli E, Oudeluttikhuis H, et al. Validation of a peak endocardial acceleration-based algorithm to optimize cardiac resynchronization: early clinical results. Europace. 2008;10(7):801–808. | |
Galrinho A, Branco LM, Oliveira MM, et al. Cardiac resynchronization therapy-clinical and echocardiographic characteristics of responders and exceptional responders. Port J Cardiol. 2009;28(9):959–969. | |
Rickard J, Kumbhani DJ, Popovic Z, et al. Characterization of super-response to cardiac resynchronization therapy. Heart Rhythm. 2010;7(7):885–889. | |
Houthuizen P, Bracke FA, van Gelder BM. Atrioventricular and interventricular delay optimization in cardiac resynchronization therapy: physiological principles and overview of available methods. Heart Fail Rev. 2011;16(3):263–276. | |
O’Donnell D, Nadurata V, Hamer A, Kertes P, Mohamed U. Long-term variations in optimal programming of cardiac resynchronization therapy devices. Pacing Clin Electrophysiol. 2005;28(Suppl 1):S24–S26. | |
Ritter P, Delnoy PP, Padeletti L, et al. A randomized pilot study of optimization of cardiac resynchronization therapy in sinus rhythm patients using a peak endocardial acceleration sensor vs standard methods. Europace. 2012;14(9):1324–1333. | |
Dupuis JM, Kobeissi A, Vitali L, et al. Programming optimal atrioventricular delay in dual chamber pacing using peak endocardial acceleration: comparison with a standard echocardiographic procedure. Pacing Clin Electrophysiol. 2003;26(1 pt 2):210–213. | |
Leung SK, Lau CP, Lam CT, et al. Automatic optimization of resting and exercise atrioventricular interval using a peak endocardial acceleration sensor: validation with Doppler echocardiography and direct cardiac output measurements. Pacing Clin Electrophysiol. 2000; 23(11 pt 2):1762–1766. | |
Padeletti L, Porciani MC, Ritter P, et al. Atrioventricular interval optimization in the right atrial appendage and interatrial septum pacing: a comparison between echo and peak endocardial acceleration measurements. Pacing Clin Electrophysiol. 2000;23(11 pt 1):1618–1622. | |
Ritter P, Padeletti L, Gillio-Meina L, Gaggini G. Determination of the optimal atrioventricular delay in DDD pacing. Comparison between echo and peak endocardial acceleration measurements. Europace. 1999;1(2):126–130. | |
Poller WC, Dreger H, Schwerg M, Bondke H, Melzer C. Not left ventricular lead position, but the extent of immediate asynchrony reduction predicts long-term response to cardiac resynchronization therapy. Clin Res Cardiol. 2014;103(6):457–466. | |
Shetty AK, Duckett SG, Ginks MR, et al. Cardiac magnetic resonance-derived anatomy, scar, and dyssynchrony fused with fluoroscopy to guide LV lead placement in cardiac resynchronization therapy: a comparison with acute haemodynamic measures and echocardiographic reverse remodelling. Eur Heart J Cardiovasc Imaging. 2013;14(7):692–699. | |
Delnoy PP, Ritter P, Naegele H, et al. Association between frequent cardiac resynchronization therapy optimization and long-term clinical response: a post hoc analysis of the clinical evaluation on advanced resynchronization (CLEAR) pilot study. Europace. 2013;15(8):1174–1181. | |
Brugada J, Brachmann J, Delnoy PP, et al. Automatic optimization of cardiac resynchronization therapy using SonR-rationale and design of the clinical trial of the SonRtip lead and automatic AV-VV optimization algorithm in the paradym RF SonR CRT-D (RESPOND CRT) trial. Am Heart J. 2014;167(4):429–436. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.