Back to Journals » Clinical and Experimental Gastroenterology » Volume 11
Hemin reduces inflammation associated with TNBS-induced colitis
Authors Mateus V , Rocha J, Mota-Filipe H , Sepodes B, Pinto R
Received 22 February 2018
Accepted for publication 26 May 2018
Published 18 September 2018 Volume 2018:11 Pages 325—334
DOI https://doi.org/10.2147/CEG.S166197
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Hoda Malaty
Vanessa Mateus,1,2 João Rocha,2 Hélder Mota-Filipe,2 Bruno Sepodes,2 Rui Pinto2,3
1H&TRC – Health and Technology Research Center, ESTeSL – Lisbon School of Health Technology, Instituto Politécnico de Lisboa, Lisbon, Portugal; 2iMed.ULisboa, Faculty of Pharmacy, University of Lisbon, Lisbon, Portugal; 3Dr. Joaquim Chaves, Laboratory of Clinical Analysis, Joaquim Chaves Saúde, Lisbon, Portugal
Purpose: Hemin is a heme-oxygenase inducer, which can confer anti-inflammatory, cytoprotective, and antiapoptotic effects. These properties are beneficial therapeutical effects to inflammatory bowel disease (IBD). IBD is a worldwide health problem characterized by chronic inflammation of intestinal epithelium, which promotes intestinal and extraintestinal symptomatology. Current treatment only induces and maintains the patient in remission and results in many side effects. The research of other pharmacologic approaches is crucial to the treatment of IBD. The aim of this study is to evaluate the effect of hemin in the 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis model.
Materials and methods: Male CD-1 mice with TNBS-induced colitis were treated with a daily dose of hemin 5 mg/kg body weight/day and 10 mg/kg body weight/day intraperitoneal, during 4 days. The evaluated parameters were fecal hemoglobin, alkaline phosphatase (ALP), myeloperoxidase, tumor necrosis factor-α, interleukin (IL)-1β, IL-10, histopathologic analysis, urea, creatinine, and alanine aminotransferase.
Results: The hemin-treated mice presented a decrease in fecal hemoglobin, ALP, and proinflammatory cytokine concentrations compared to the TNBS group. Histopathology analysis confirmed the decrease in lesion extension produced by hemin.
Conclusion: These findings suggest that hemin treatment reduces hemorrhagic focus, intestinal damage, tissue inflammation, and lesion extension associated with experimental colitis.
Keywords: inflammatory bowel disease, experimental colitis, anti-inflammatory effect, heme-oxygenase inducer
Introduction
Hemin, or ferriprotoporphyrin IX chloride, is an iron-containing metalloporphyrin, currently commercialized for the treatment of acute attacks of inducible porphyria.1,2 It is also used for the amelioration of recurrent attacks of acute intermittent porphyria that are temporally related to the menstrual cycle.2
However, hemin is also well known as a heme-oxygenase (HO) inducer.3,4 HO is the rate-limiting enzyme in heme catabolism, a process that leads to the generation of equimolar amounts of biliverdin, free iron, and carbon monoxide.5 Three mammalian HO isozymes have been identified, namely HO-1, HO-2, and HO-3.6 Under several pathologic conditions, HO-1 is expressed and is able to metabolize high amounts of free heme to produce high concentrations of its enzymatic by-products, which can have a beneficial influence in various biologic events.7 Recently, HO-1 has been the focus of considerable medical interest.7
The induction of HO-1 or its catalytic activity by either natural or synthetic compounds may represent an effective strategy to intervene in several pathologic conditions. Indeed, using gene therapy or pharmacologic modulation, the HO-1 induction has shown promising results both in vitro and in vivo.8 Many studies have reported the beneficial effect of hemin through HO-1 induction in various animal models, such as hippocampal injury, renal fibrosis, cardiac ischemia/reperfusion, lung injury, and sepsis.3,4,6 Thus, a better understanding of the heme-HO system may result in novel therapeutic strategies for some important pathologic disorders. Inclusively, HO-1 expression can confer cytoprotective, antiapoptotic, and anti-inflammatory properties, suggesting, thus, that HO-1 can be a possible therapeutic target in several kinds of gastrointestinal diseases.6
Inflammatory bowel disease (IBD), which includes Crohn’s disease and ulcerative colitis, is a chronic inflammatory disease of the gastrointestinal tract, characterized by chronic recurrent ulceration of the bowels.9 IBD affects between 7% and 10% of people worldwide, mainly of Caucasian descent,10,11 promoting significant gastrointestinal symptoms, like bloody diarrhea, abdominal pain, anemia, weight loss, and other extraintestinal manifestations.9 Currently, medical therapy of IBD consists of salicylates, corticosteroids, and immunomodulators.9 These drug treatments aim to induce or maintain the patient in remission and ameliorate the disease’s secondary effects, rather than modifying or reversing the underlying pathogenic mechanism.9,12 Even their use may result in severe side effects and complications, such as an increased rate of malignancies or infectious diseases.12 So, the research of novel pharmacologic approaches would constitute important advances in the therapy of IBD.13
Hemin treatment was tested once in an acute model of experimental colitis.14 However, it was used as a single administration, which is not in line with the need for treatment of IBD. So, biologic significance of HO-1 upregulation in gastrointestinal inflammation remains to be fully elucidated.6 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis model is one of the most widely used, because it resembles human IBD. This model is appropriated to develop and test novel therapeutic strategies for the treatment of IBD.15 As TNBS is associated with predominant activation of Th1-mediated immune response, this model promotes chronic transmural colitis that mimics some characteristics of Crohn’s disease in humans.16 The aim of this study is to evaluate the effect of hemin in the TNBS-induced colitis model.
Materials and methods
Drugs and chemicals
TNBS 5%, ferriprotoporphyrin IX chloride (hemin), and sodium hydroxide (NaOH) were purchased from Sigma Chemical Co (Sintra, Portugal). Ketamine (Imalgene® 1000) was purchased from Merial (Lisbon, Portugal). Xylazine (Rompun® 2%) was purchased from Bayer (Lisbon, Portugal). ADVIA® kit was purchased from Siemens Healthcare Diagnostics (Erlangen, Germany). Enzyme-linked immunosorbent assay (ELISA) assay kits for tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-10, and myeloperoxidase (MPO) measurements in mice were obtained from Quantikine®, R&D Systems (Minneapolis, MN, USA).
Animals
Male CD-1 mice of 6–10 weeks of age and 30–40 g in weight were obtained from Charles River (Barcelona, Spain). In the Faculty of Pharmacy (University of Lisbon) biotereum, the animals were housed at 18°C–23°C of temperature and 40%–60% of humidity, with controlled 12 hours light/dark cycle. Standard polypropylene cages were used to keep the animals with ad libitum access to food and water. Animal care was in compliance with the Directive 2010/63/EU, which represents the internationally accepted principles for laboratory animal use. The experiment was approved by the Ethics Committee for Animal Experimentation of Faculty of Pharmacy, University of Lisbon.
Chemical induction of colitis
The experimental colitis was chemically induced with TNBS as described by Mateus et al.17 Mice were left unfed during 12 hours. At day 0 (induction day), mice were anesthetized with ketamine 100 mg/kg + xylazine 10 mg/kg. An intracolonic single dose of TNBS solution (100 µL) was administered through a catheter carefully inserted until 4 cm into the colon. The Trendelenburg position was used to avoid colonic reflux. At day 4, the blood samples of mice were collected by cardiac puncture, under anesthesia. Then, mice were killed by cervical dislocation. The necropsy was initiated with a midline incision in the abdomen and the colon was removed, freed from surrounding tissues, and washed with PBS.
Experimental groups
Mice were organized in seven experimental groups: TNBS group received 100 µL of 2.5% TNBS in 50% (v/v) ethanol intrarectal (n=35); TNBS + hemin5 group and TNBS + hemin10 group were colitic mice treated daily with 5 and 10 mg/kg body weight (bw)/day intraperitoneal (IP) of hemin (dissolved in NaOH and PBS solution), since day 0, respectively (n=35 for each group); TNBS + vehicle group was colitic mice treated daily with NaOH and PBS solution (hemin vehicle) IP, since day 0 (n=20); hemin10 group received 10 mg/kg bw/day IP of hemin daily, since day 0 (n=20); ethanol group received 100 µL of 50% (v/v) ethanol intrarectal (TNBS vehicle) (n=20); and sham group received 100 µL of saline solution intrarectal (n=20). The ethanol group was used as a reference to compare the results with the other experimental groups.
Biochemical markers
Sera from the collected blood samples were separated by centrifugation at 3600 rpm for 15 minutes and were analyzed by an automated clinical chemistry analyzer (ADVIA®1200). The biochemical markers evaluated were alkaline phosphatase (ALP), alanine aminotransferase (ALT), urea, and creatinine. Fecal hemoglobin was analyzed in feces using a quantitative method by immunoturbidimetry (Kroma Systems).
MPO concentration
Neutrophilic infiltration in this experimental colitis model was indirectly quantitated through an MPO activity assay. MPO concentration was expressed in ng/mL. The colon was weighed and homogenized in phosphate buffer using an Ultra-turrax T25 (13,500 rev/min, twice for 30 seconds). The colon was centrifuged (at 15,000 rpm for 15 minutes at 4°C) and the supernatant was incubated in microtiter wells coated with biotinylated tracer antibody, which is able to recognize mouse MPO. Streptavidin-peroxidase conjugate was added to bind the biotinylated tracer antibody and was then mixed with tetramethylbenzidine substrate. The reaction was stopped by the addition of oxalic acid and assayed spectrophotometrically at 450 nm (ELISA kit HK210; Hycult Biotech, Uden, the Netherlands).
Determination of tissue cytokines
The colon samples were homogenized with an Ultra-turrax T25 (13,500 rev/min, twice for 30 seconds) in phosphate buffer and centrifuged at 15,000 rpm for 15 minutes at 4°C. The supernatant was stored at –20°C until use. The TNF-α, IL-1β, and IL-10 levels were measured spectrophotometrically at 450 nm (ELISA kit Quantikine, Hycult Biotechnology) and expressed as pg/mL.
Microscopic assessment of colitis severity
The colon samples were fixed in 10% phosphate-buffered formalin, processed routinely for paraffin embedding, sectioned at 5 µm, and stained with H&E. Adapted criteria of Corazza et al (1999) and Seamons et al (2013) were used to characterize the distal colon sections.18,19 The histopathologic score of lesions was partially determined (0–4 increasing severity) based on the 1) presence of tissue loss/necrosis, 2) severity of mucosal epithelial lesion, 3) inflammation, 4) extent 1 – the percentage of intestine affected in any manner, and 5) extent 2 – the percentage of intestine affected by the most severe lesion. The colitis severity was calculated by summing the individual lesions and the extent scores, promoting a final colitis score (max score =20). The histopathologic analyses were evaluated by two blinded independent histopathologists from Faculty of Veterinary Medicine and Institute of Molecular Medicine.
Statistical analysis
All results were analyzed using GraphPad Prism 5.0 software (GraphPad, San Diego, CA, USA). Statistical significance was determined through one-way analysis of variance followed by Tukey’s post hoc test for multiple comparisons or chi-squared test depending on the variables under study. Data are expressed as mean ± SEM. A value of P <0.05 was regarded as statistically significant.
Results
Biochemical markers
The fecal hemoglobin (Figure 1) and ALP (Figure 2) concentrations in the TNBS group were significantly higher than the ethanol group (P<0.0001). However, the concentration of these biochemical markers decreased after hemin treatment. At the end of experimental period, hemin was able to attenuate the increased fecal hemoglobin (P<0.0001) in a dose-dependent manner (P<0.0001). Furthermore, hemin treatment also decreased the ALP concentration in blood (P<0.0001) in a dose-dependent manner (P<0.0001). In this case, the administration of hemin 10 mg/kg bw/day (4 days of treatment) was able to abolish the ALP changes promoted by TNBS-induced colitis, since there is no statistical significance with ethanol group, presenting, thus, a protective effect on the enterocyte.
MPO concentration
MPO concentration was measured as a marker of neutrophil influx (Figure 3). TNBS group presented an increase of MPO concentration compared to the ethanol group (P<0.001). After hemin treatment, a dose-dependent effect was identified in the decrease of the MPO concentration; however, statistical significance difference was only registered in the highest dose (P<0.001, compared to the TNBS group).
Determination of tissue cytokines
The proinflammatory cytokines were increased in the TNBS-induced colitis (Figure 4). So, the TNBS group revealed a significant increase in TNF-α and IL-1β concentrations compared to the ethanol group (P<0.001). The TNBS + vehicle group also revealed an increased concentration of these cytokines, confirming the obtained data of TNBS group. After hemin treatment, the mice exhibited a decrease of both evaluated proinflammatory cytokines with a dose-dependent effect (P<0.001, for the highest doses compared to the TNBS group). The remaining control groups, hemin10, ethanol, and sham groups, presented quite similar TNF-α and IL-1β concentrations.
The assessment of IL-10 concentration is crucial to confirm the obtained results with TNF-α and IL-1β measurements (Figure 5). Concretely, a low IL-10 concentration was registered in the TNBS group; however, mice treated with hemin had an increase of its concentration as expected, with a dose-dependent effect (P<0.001, for the highest dose compared to the TNBS group).
Microscopic assessment of colitis severity
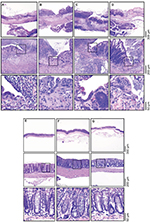
The histopathologic score of the experimental groups is translated by the representative histologic images (Figure 6). Briefly, the TNBS group displays diffuse transmural necrosis with severe hemorrhaging, involving the mucosa, submucosa, muscle layer, and serosa, and often associated with peritonitis. Colitic mice had a final score substantially higher than that of the ethanol group (P<0.0001). The histopathologic score keeps unchanged after hemin treatment. Indeed, under hemin treatment, similar lesions are seen, without any improvement in its severity. However, a slight influence on the extension of the lesions is perceptible. The increase in the hemin dose has no effect on the histopathologic images. The histopathologic images of TNBS + vehicle group show the same lesions as TNBS group. The ethanol group showed only mild epithelial erosion, and no lesions were observed in the hemin10 and sham groups.
Extraintestinal markers
The influence of hemin treatment on the renal function was evaluated by measurement of urea and creatinine concentrations (Table 1). The TNBS group exhibited a significant increase in urea and creatinine compared to the ethanol group (P<0.0001). The mice treated with hemin presented a significant decrease in urea and creatinine levels to values quite similar with the ethanol group, promoting, thus, a dose-dependent effect. There are no statistically significant differences in both renal markers between the TNBS + hemin10 and the ethanol groups.
The ALT concentration was measured to evaluate the hepatic function (Table 1). The ALT concentration in blood was significantly higher in the TNBS group compared to the ethanol group (P<0.0001). Therefore, the hemin treatment at both doses was able to abolish the ALT changes promoted by TNBS-induced colitis.
Discussion
The presence of one or more genetically known defects is one of the main factors that contribute to IBD, inducing an overreaction of the mucosal immune system to normal constituents of the mucosal microflora. Therefore, IBD is produced through a final common immunopathologic pathway consisting of a Th1 T-cell-response-mediated inflammation (Crohn’s disease) or a Th2 T-cell-response-mediated inflammation (ulcerative colitis). This implies that, regardless of the nature of the fundamental defects present, one could potentially treat IBD with therapy that addresses an essential element of the final common pathway.20
Within this context, existing conventional treatments such as salicylates, corticosteroids, and immunomodulators aim broadly to block downstream inflammatory events such as the secretion of cytokines, immunocytes, and neutrophils, regardless of the nature of the underlying T-cell response that generated these events. These agents have sustained treatment of IBD for many years despite shortcomings and toxicities.21–23 For many years there have been numerous efforts to find a new effective method that would allow controlling specifically unwanted immune responses that occur during autoimmune reaction.24 The future treatment options for IBD will not only be extended by simultaneously targeting several pathogenetic players through combinations of existing strategies, but also by the introduction of drugs with completely new targets.25 Thus, the assessment of the influence of a hemin, through a TNBS-induced colitis model in mice, can facilitate a more effective and selective treatment than the currently known.
Hemin is well known as an inducer of HO-1.3,4 HO-1 is a rate-limiting enzyme for heme metabolism and is capable of producing antioxidant and anti-inflammatory products, such as biliverdin/bilirubin and carbon monoxide.26–28 Biliverdin/bilirubin can scavenge peroxyl radicals in vitro as effectively as α-tocopherol, which is regarded as the most potent antioxidant against lipid peroxidation,29 whereas carbon monoxide can inhibit the production of proinflammatory cytokines in macrophages, such as TNF-α, IL-1β, and macrophage inflammatory protein-1, through modulation of mitogen-activated protein kinase activation.30 These findings suggest that HO-1 can be a possible therapeutic target in several kinds of gastrointestinal diseases.6
In our study, the intensity of hemorrhagic focus was evaluated by the fecal hemoglobin concentration.17,31 We observed that hemin treatment produced a considerable influence in the intensity of hemorrhagic focus, because the fecal hemoglobin concentration significantly decreased in a dose-dependent manner after a daily dose of hemin. This finding suggests that hemin-treated mice presented an amelioration of the active inflammatory disease identified in mice with TNBS-induced colitis.32,33
Regarding ALP concentration, hemin treatment was able to decrease the elevated level of ALP in blood in a dose-dependent manner, demonstrating an anti-inflammatory potential by HO-1 induction. This data is consistent with other previous findings, where anti-inflammatory drugs are able to decrease ALP level.17,31,34 Furthermore, as intestinal ALP is expressed on the enterocytes and it is responsible for the mucosal defense,35,36 our findings also suggest that hemin had a cytoprotective effect on the intestinal mucosa.
MPO activity was used as an index of quantitative inflammation and neutrophil infiltration in tissues.17,31,37 Hemin administration was able to attenuate neutrophil infiltration and inflammation with both hemin doses, in a dose-dependent manner. However, a statistical significance difference was only identified in the highest hemin dose, where MPO decreased around 60% compared with the nontreated mice. These results suggest that HO-1 activation may possibly protect colonic tissue against inflammation, consistent with other studies, where HO-1 modulators are used in models of experimental colitis.14,38,39
TNF-α and IL-1β are proinflammatory cytokines that can become dysregulated under pathologic condition of inflammation.40 Actually, mice with TNBS-induced colitis exhibited a significant increase in TNF-α and IL-1β levels;17,31 however, hemin treatment significantly decreased the level of these cytokines in a dose-dependent manner. Furthermore, hemin-treated mice also revealed a significant increase in the IL-10 concentration in a dose-dependent manner as expected, confirming the results obtained with TNF-α and IL-1β measurements. These findings suggest that HO-1 induction by hemin treatment can produce anti-inflammatory effect, suppressing the production of these proinflammatory cytokines. Moreover, HO-1 induction may still confer a protective effect, because it is able to increase anti-inflammatory cytokines, as IL-10.41
To assess whether hemin affected TNBS-induced colon damage, the colon morphology was analyzed. The histologic features of the lesions suggest that hemin was able to decrease the extension of the lesions, suggesting a beneficial effect in the inflammation of tissue because of HO-1 induction.41
As IBD can promote extraintestinal manifestations, the periodic evaluation of renal and hepatic functions should be emphasized.17,31,42–44 Single daily dose of hemin significantly recovered the renal and hepatic functions to normal levels, similar to the control group, suggesting a beneficial effect in the extraintestinal manifestations because of metabolic and physiologic changes induced by the IBD. We also can conclude that hemin does not promote renal and/or hepatic changes as adverse drug reaction, because hemin10 group had no elevated levels of these biochemical markers.
Our study suggests that hemin treatment reduces hemorrhagic focus, intestinal damage, tissue inflammation, and lesion extension associated with an acute model of experimental colitis.
Conclusion
Hemin treatment had a positive influence on the attenuation of inflammation associated with experimental colitis. This pharmaceutical approach promoted a reduction of fecal hemoglobin, ALP, MPO, and proinflammatory cytokines. Furthermore, hemin was also able to increase the anti-inflammatory cytokine, as well as abolish the renal and hepatic changes induced by rectal TNBS administration. In sum, hemin treatment decreases the severity of the disease, because it is able to improve several inflammation markers, suggesting an anti-inflammatory effect of hemin by HO-1 induction. Hemin also decreases the extension of the intestinal lesions, which is corroborated by the histologic images. These findings suggest that hemin seems to significantly inhibit the acute inflammatory response in this experimental colitis.
This study allowed exploring the effect of hemin in the development of IBD, as well as its influence on response mechanisms to intestinal injury. Moreover, it represents a truly innovative contribution to the pharmacologic treatment of IBD, identifying the pro- and anti-inflammatory responses that can modulate the establishment and development of the disease, as well as a new therapeutic target that allows attenuating the IBD and contributing to the enrichment of the therapeutic opportunities of this disease.
Disclosure
The authors report no conflicts of interest in this work.
References
Bonkowsky HL, Tschudy DP, Collins A, Doherty J, Bossenmaier I, Cardinal R, Watson CJ. Repression of the overproduction of porphyrin precursors in acute intermittent porphyria by intravenous infusions of hematin. Proc Natl Acad Sci U S A. 1971;68(11):2725–2729. | ||
Abbott Laboratories. Product Information: Panhematin®. 1997:Abbott Park. | ||
Guan L, Wen T, Zhang Y, Wang X, Zhao J. Induction of heme oxygenase-1 with hemin attenuates hippocampal injury in rats after acute carbon monoxide poisoning. Toxicology. 2009;262(2):146–152. | ||
Hualin C, Wenli X, Dapeng L, Xijing L, Xiuhua P, Qingfeng P. The anti-inflammatory mechanism of heme oxygenase-1 induced by hemin in primary rat alveolar macrophages. Inflammation. 2012;35(3):1087–1093. | ||
Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol. 1997;37:517–554. | ||
Naito Y, Takagi T, Uchiyama K, Yoshikawa T. Heme oxygenase-1: a novel therapeutic target for gastrointestinal diseases. J Clin Biochem Nutr. 2011;48(2):126–133. | ||
Abraham NG, Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol Rev. 2008;60(1):79–127. | ||
Ferrándiz ML, Devesa I. Inducers of heme oxygenase-1. Curr Pharm Des. 2008;14(5):473–486. | ||
Pithadia AB, Jain S. Treatment of inflammatory bowel disease (IBD). Pharmacol Rep. 2011;63(3):629–642. | ||
Hanauer SB. Inflammatory bowel disease: epidemiology, pathogenesis, and therapeutic opportunities. Inflamm Bowel Dis. 2006;12 Suppl 1:S3–S9. | ||
Spiegel BM. The burden of IBS: looking at metrics. Curr Gastroenterol Rep. 2009;11(4):265–269. | ||
Engel MA, Neurath MF. New pathophysiological insights and modern treatment of IBD. J Gastroenterol. 2010;45(6):571–583. | ||
Cuzzocrea S, Mazzon E, di Paola R, et al. Erythropoietin reduces the development of experimental inflammatory bowel disease. J Pharmacol Exp Ther. 2004;311(3):1272–1280. | ||
Wang WP, Guo X, Koo MW, Wong BC, Lam SK, Ye YN, Cho CH. Protective role of heme oxygenase-1 on trinitrobenzene sulfonic acid-induced colitis in rats. Am J Physiol Gastrointest Liver Physiol. 2001;281(2):G586–G594. | ||
Randhawa PK, Singh K, Singh N, Jaggi AS. A review on chemical-induced inflammatory bowel disease models in rodents. Korean J Physiol Pharmacol. 2014;18(4):279–288. | ||
Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96(3):795–803. | ||
Mateus V, Rocha J, Alves P, Mota-Filipe H, Sepodes B, Pinto RM. Anti-inflammatory effect of erythropoietin in the TNBS-induced colitis. Basic Clin Pharmacol Toxicol. 2017;120(2):138–145. | ||
Corazza N, Eichenberger S, Eugster HP, Mueller C. Nonlymphocyte-derived tumor necrosis factor is required for induction of colitis in recombination activating gene (RAG)2(-/-) mice upon transfer of CD4(+)CD45RB(hi) T cells. J Exp Med. 1999;190(10):1479–1492. | ||
Seamons A, Treuting PM, Brabb T, Maggio-Price L. Characterization of dextran sodium sulfate-induced inflammation and colonic tumorigenesis in Smad3(-/-) mice with dysregulated TGFβ. PLoS One. 2013;8(11):e79182. | ||
Strober W, Fuss I, Mannon P. The fundamental basis of inflammatory bowel disease. J Clin Invest. 2007;117(3):514–521. | ||
Hanauer S, Feagan BG, Lichtenstein GR, et al. ACCENT I Study Group. Maintenance infliximab for Crohn’s disease: the ACCENT I randomized trial. Lancet. 2002;359:1541–1549. | ||
Sandborn W, Colombel JF, Enns R, et al. International Efficacy of Natalizumab as Active Crohn’s Therapy (ENACT-1) Trial Group; Evaluation of Natalizumab as Continuous Therapy (ENACT-2) Trial Group. Natalizumab induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2005;353:1912–1925. | ||
Blonski W, Lichtenstein GR. Complications of biological therapy for inflammatory bowel diseases. Curr Opin Gastroenterol. 2006;22(1):30–43. | ||
Majewska-Szczepanik M, Góralska M, Marcińska K, Zemelka-Wiącek M, Strzępa A, Dorożyńska I, Szczepanik M. Epicutaneous immunization with protein antigen TNP-Ig alleviates TNBS-induced colitis in mice. Pharmacol Rep. 2012;64(6):1497–1504. | ||
Zundler S, Neurath MF. How will new and future therapies change our treatment of IBD? Expert Rev Clin Immunol. 2016;12(3):233–236. | ||
Willis D, Moore AR, Willoughby DA. Heme oxygenase isoform expression in cellular and antibody-mediated models of acute inflammation in the rat. J Pathol. 2000;190(5):627–634. | ||
Maines MD. The heme oxygenase system: update 2005. Antioxid Redox Signal. 2005;7(11–12):1761–1766. | ||
Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev. 2006;86(2):583–650. | ||
Stocker R, Yamamoto Y, Mcdonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235(4792):1043–1046. | ||
Otterbein LE, Bach FH, Alam J, et al. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med. 2000;6(4):422–428. | ||
Mateus V, Rocha J, Alves P, Mota-Filipe H, Sepodes B, Pinto R. Thiadiazolidinone-8 ameliorates inflammation associated with experimental colitis in mice. Pharmacology. 2018;101(1–2):35–42. | ||
Hirata I, Hoshimoto M, Saito O, et al. Usefulness of fecal lactoferrin and hemoglobin in diagnosis of colorectal diseases. World J Gastroenterol. 2007;13(10):1569–1574. | ||
Mooiweer E, Fidder HH, Siersema PD, Laheij RJ, Oldenburg B. Fecal hemoglobin and calprotectin are equally effective in identifying patients with inflammatory bowel disease with active endoscopic inflammation. Inflamm Bowel Dis. 2014;20(2):307–314. | ||
Kumar VS, Rajmane AR, Adil M, Kandhare AD, Ghosh P, Bodhankar SL. Naringin ameliorates acetic acid induced colitis through modulation of endogenous oxido-nitrosative balance and DNA damage in rats. J Biomed Res. 2014;28(2):132–145. | ||
Malo MS, Alam SN, Mostafa G, et al. Intestinal alkaline phosphatase preserves the normal homeostasis of gut microbiota. Gut. 2010;59(11):1476–1484. | ||
Nagalingam NA, Kao JY, Young VB. Microbial ecology of the murine gut associated with the development of dextran sodium sulfate-induced colitis. Inflamm Bowel Dis. 2011;17(4):917–926. | ||
Rachmilewitz D, Simon PL, Schwartz LW, Griswold DE, Fondacaro JD, Wasserman MA. Inflammatory mediators of experimental colitis in rats. Gastroenterology. 1989;97(2):326–337. | ||
Jun CD, Kim Y, Choi EY, et al. Gliotoxin reduces the severity of trinitrobenzene sulfonic acid-induced colitis in mice: evidence of the connection between heme oxygenase-1 and the nuclear factor-kappaB pathway in vitro and in vivo. Inflamm Bowel Dis. 2006;12(7):619–629. | ||
Varga C, Laszlo F, Fritz P, et al. Modulation by heme and zinc protoporphyrin of colonic heme oxygenase-1 and experimental inflammatory bowel disease in the rat. Eur J Pharmacol. 2007;561(1–3):164–171. | ||
Műzes G, Molnár B, Tulassay Z, Sipos F. Changes of the cytokine profile in inflammatory bowel diseases. World J Gastroenterol. 2012;18(41):5848–5861. | ||
Zhang L, Zhang Y, Zhong W, Di C, Lin X, Xia Z. Heme oxygenase-1 ameliorates dextran sulfate sodium-induced acute murine colitis by regulating Th17/Treg cell balance. J Biol Chem. 2014;289(39):26847–26858. | ||
Larsen S, Bendtzen K, Nielsen OH. Extraintestinal manifestations of inflammatory bowel disease: epidemiology, diagnosis, and management. Ann Med. 2010;42(2):97–114. | ||
Oikonomou K, Kapsoritakis A, Eleftheriadis T, Stefanidis I, Potamianos S. Renal manifestations and complications of inflammatory bowel disease. Inflamm Bowel Dis. 2011;17(4):1034–1045. | ||
Rojas-Feria M, Castro M, Suárez E, Ampuero J, Romero-Gómez M. Hepatobiliary manifestations in inflammatory bowel disease: the gut, the drugs and the liver. World J Gastroenterol. 2013;19(42):7327–7340. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.