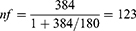Back to Journals » Journal of Blood Medicine » Volume 13
Hematological Profiles and Clinical Outcome of COVID-19 Among Patients Admitted at Debre Markos Isolation and Treatment Center, 2020: A Prospective Cohort Study
Authors Atnaf A , Shiferaw AA , Tamir W, Akelew Y, Toru M , Tarekegn D , Bewket B , Reta A
Received 9 July 2022
Accepted for publication 21 October 2022
Published 31 October 2022 Volume 2022:13 Pages 631—641
DOI https://doi.org/10.2147/JBM.S380539
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Martin H Bluth
Aytenew Atnaf,1 Abtie Abebaw Shiferaw,1 Workineh Tamir,1 Yibeltal Akelew,1 Milkiyas Toru,1 Daniel Tarekegn,2 Bekalu Bewket,3 Alemayehu Reta1
1Department of Medical Laboratory Science, College of Health Sciences, Debre Markos University, Debre Markos, Ethiopia; 2Department of Public health, College of Health Sciences, Debre Markos University, Debre Markos, Ethiopia; 3Department of Nursing, College of Health Sciences, Debre Markos University, Debre Markos, Ethiopia
Correspondence: Aytenew Atnaf, Department of Medical Laboratory Sciences, College of Health Sciences, Debre Markos University, P.O.Box 269, Debre Markos, Ethiopia, Tel +251 924474452, Email [email protected]
Background: Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is coronavirus isolated from SARS patients. As far as the researchers’ knowledge, there was paucity of studies conducted in Ethiopia, particularly in the study area. As immune protection is arisen from our blood cells, assessing their level will provide a clue for controlling the disease and monitoring the prognosis. This study will also provide additional information for clinical intervention and patient management.
Purpose: This study aimed to investigate the hematological profile and clinical outcome of coronavirus disease-19 (COVID-19) among patients admitted to the Debre Markos Isolation and Treatment Center (DMITC).
Material and Methods: A prospective cohort study was conducted among 136 COVID-19 adult patients at DMITC from January 1, 2020 to March 30, 2021. Data related to clinical, hematological profiles and socio-demographic factors were collected, entered into Epi data, and analyzed using STATA 14.2 software. Multivariable logistic regression was applied to determine the predictor variable and a p-value < 0.05 was considered significant.
Results: Of 136 COVID-19 patients, 28.68% had died. The mean age of patients was 47.21± 1.29 years. The hematological profile of the patients revealed that 28% had abnormal leukocyte, 23% abnormal lymphocyte, 44.85% abnormal granulocyte, 22.06% abnormal monocyte, 30.15% abnormal RBC and 87% abnormal platelet counts. The prevalence of anemia was 13.24%.
Conclusion: Leukocytosis (mainly granulocytosis and monocytosis) and lymphopenia, were the predominant abnormal findings of complete blood cell count (CBC) analysis of the patient’s blood. Most of the patients had abnormally low platelet counts. RBC count and hematocrit determination were the only significant predictors of death. The clinician could manage cases according to the hematological findings of the patients. Further experimental studies should be conducted to determine hematological parameter changes and the clinical outcome of the disease.
Keywords: leukocytosis, hematological profile, COVID-19, clinical outcome, Debre Markos
Introduction
Background
The novel coronavirus was isolated from pneumonia cases in Wuhan city, China, 2019.1 The disease caused by the novel coronavirus is called Coronavirus disease of 2019 (COVID-19), given by WHO2 and the virus causing the disease called Severe acute respiratory syndrome coronaviruses-2 (SARS-CoV-2), given by the Coronavirus Study Group of the International Committee on Taxonomy of Viruses. SARS-CoV-2 is classified under the family Coronaviridae, genus Betacoronavirus, subgenus Sarbecovirus.3 A study4 suggested that the human receptor for SARS-COV-2 could be angiotensin-converting enzyme 2 (ACE2), similar to other SARS-CoV viruses’portal of entry into human cells are through ACE2.5 The 5’-terminal two-thirds of the genome encodes a polyprotein (non-structural proteins) that are involved in genome transcription and replication whereas the 3’ end encodes structural proteins, including envelope glycoproteins spike (S), envelope (E), membrane (M) and nucleocapsid (N).6 The rate of its transmission is higher than SRAS-CoV and the reason could be the occurrence of genetic recombination at S protein in the RBD region of SARS-CoV-2.7 The human-to-human spreading of the virus occurs due to close contact with an infected person, exposure to coughing, sneezing, respiratory droplets, or aerosols. These aerosols can penetrate the human body (lungs) via inhalation through the nose or mouth.8–10
The exact pathogenesis of SARS-CoV-2 infection in humans is poorly understood. However, some studies stated that the pathogenesis is related to dysregulation of inflammatory mediators and presence of different strain of the virus.11 Most patients have mild symptoms and good prognosis after infection, but some patients developed severe and die from multiple organ complications.12 The virus can affect the respiratory system, gastrointestinal system, renal system, circulatory system. The pathological changes in these organs may be caused directly by the cytopathic effect mediated by local replication of the SARS‐CoV-2; or indirectly as a result of systemic responses to respiratory failure or the harmful immune response induced by viral infection.13
There is no specific antiviral drug, but there are vaccines against the virus which are approved to alter the immune function that helps the patient to recover from illness.12
The complications of COVID-19 are pulmonary embolism, venous thromboembolism, and arterial thrombotic events.14 Patients with COVID-19 infection can also present with acute encephalopathy and changes in their level of consciousness.15 Complications were more common in patients with cardiac injury, acute respiratory distress syndrome (SARS), acute kidney injury, electrolyte disturbances, hypoproteinemia and coagulation disorders.16
Studies had found increased white blood cell and neutrophil counts17 but decreased lymphocyte counts. In addition, there was decreased T cells (both regulatory and helper cells) and NK cells.11
However, as far as the researchers’ knowledge, there were few studies done in Ethiopia. As a result, we investigated the hematological profile and clinical outcome of COVID-19 among patients admitted at in Debre Markos Isolation and treatment center (DMITC). This study will provide the hematological profile and clinical outcome of COVID-19 patients which will be important to clinicians for management of patients.
Materials and Methods
Study Design, Area and Period
A prospective cohort study was conducted among COVID-19 patients at DMITC from January 1, 2020 to March 30, 2021. DMITC was established at the end of April 2020 in the new Campus of Health Science in Debre Markos town. Debre Markos town, the capital city of East Gojjam zone, is found 299km from Addis Ababa and 264 km from Bahir Dar.
Population
COVID-19 confirmed adult patients admitted to the treatment center during the study period at DMITC site were the study population.
Sample Size Determination and Sampling Technique
In Ethiopia, there were few studies conducted on hematological profile and clinical outcome of COVID-19 patients. As a result, the sample size was determined using single population proportion formula but not calculated for associated factors. It was calculated using the assumption of 50% clinical outcomes, 95% confidence level, and a 5% margin of error. The sample size was determined using 50% proportion clinical outcome (death/recovery=0.5) 95% confidence level (Za/2) =1.96 and margin of error 0.05. The total sample size calculated was 384, but the number of COVID-19 confirmed cases in the last 3 months in the COVID 19 treatment center were 180, which is less than 10,000 and needs population correction formula.
As a result, the corrected sample size is
About 10% of non- response rate was added to the calculated sample size (123) and giving 136, that is 123+ (123/10) =136.
Systematic random sampling technique was used to obtain 136 study participants. The k value was determined by 135/180=0.75 ͠, 1. Therefore, we recruited study participants in every other order of registration sequence.
Data Collection Tools and Procedures
Data were collected from COVID-19 positive patients using a predesigned structured questionnaire. The researchers trained five data collectors; two general practitioners, two nurses, and two laboratory technologists on the aim and objectives of the research, blood sample collection and processing procedures, and infection prevention mechanism to prevent COVID-19 infection at DMITC. The trained data collectors explained the aim of the study to patients or their clinician in order to obtain consents and samples. Socio-demographic variables and COVID-19 related clinical data were collected by trained health professionals working in DMITC. Data were filled on questionnaire and kept confident, and the consistency of data filled was rechecked every day.
Measurement of Variables
Hematological profile and clinical outcome of COVID-19 patients were determined using independent factors; socio-demographic variables (age, sex, residence, etc) and factors related to change in hematological profile of COVID-19.
Hematological Profile
About 3mL of blood samples were collected from COVID-19 patients during admission and discharge. These samples were analyzed by Mindray BC-3000 Plus CBC hematology analyzer (MINDRAY, China) for complete blood cell count (CBC) determinations and altitude values have been adjusted using WHO guidelines for red cell count and hemoglobin measurements. Mean value of each measurement of the parameter for a patient was used. Mean absolute counts of white blood cells, granulocytes, lymphocytes, and Monocytes were used to categorize abnormal cell counts as “low” and “high”. We used CBC reference range of Debre Markos Comprehensive and Specialized Hospital to categorize the cell count as normal and abnormal.
Data Management, Analysis and Interpretation
Data were entered into the Epi-Data version 4.2 software and analyzed with STATA 14.2 software. Data cleaning was carried out to check the frequency, accuracy, consistency and missed values; any identified errors were corrected. Frequencies and proportion were used to describe the socio-demographic characteristics of the patients. Bivariate logistic regression was done using independent variables in order to identify candidate variables (p-value <0.25) for the multivariable logistic regression. Multivariate logistic regression model was made and predictors of abnormal hematological profiles were determined among the selected candidate variables fitted to multivariable logistic regression model. The statistical significance was determined in the multivariate analysis at a confidence interval of 95% at a p-value <0.05.
Results
Socio-Demographic Characteristics
A total of 136 study participants were involved in the study. From these participants, 85 (62.5%) were males and 51 (31.5%) females. The mean age of the patients was about 47 ± 1.29 (SD) years.
Majority (30.88%) of the patients were in the age range 40–49 years followed by 60 years and above. The least number of patients were found in the age range ≤19 years. Most of them (85.29%) had no history of smoking but 97.04% drank alcohol. From the total patients, nearly one-fourth of them (27.94%) used traditional medicine for the treatment of COVID-19 disease (Table 1).
 |
Table 1 Socio-Demographic Characteristics of COVID-19 Patients, 2020 |
Clinical Profile and Outcome of COVID-19 Patients
Of 136 COVID-19 patients, most (97.79%) of them had symptomatic clinical manifestation. From the clinical manifestations, joint pain (95.59%), cough (94.12%), headache and vomiting (93.38%), loss of appetite (93.38%), respiratory distress syndrome (73.53%) and fever (68.38%) were the predominant symptoms, respectively. The mean respiratory rate, temperature, pulse rate, systolic blood pressure, diastolic blood pressure, and oxygen saturation of the patients was 28.87±0.56, 37.33±0.1104.8±1.86, 109.98±1.73, 68.04±0.74, 89.75±0.35 respectively (Table 2).
 |
Table 2 Clinical Profile of COVID-19 Patients Admitted at DMU COVID-19 Treatment Center, 2020 |
There were different comorbidities identified with COVID-19. Of the COVID-19 patients, 20.59% had hypertension, 12.50% diabetes mellitus, 2.94% HIV, 1.47% malignancy, 8.09% asthma, 11.03% chronic obstructive pulmonary disease (COPD), 2.94% chronic kidney disease CKD, 5.88% chronic liver disease (CLD), 30.88% congestive heart failure (CHF) and 55.88% other comorbidities (Table 3).
 |
Table 3 Comorbidities of COVID-19 Patients Admitted at DMU COVID-19 Treatment Center, 2020 |
The outcome status of COVID-19 positive patients during discharge revealed that 39 patients (28.68%) had died and 97 (71.32%) recovered from the disease. From the total deaths, 69.23% were males. More males (59.79%) had recovered from the disease than females (40.21%) (Table 4).
 |
Table 4 Clinical Outcome Status of COVID-19 Positive Patients During Discharge |
There were 58 mild, 47 severe and 31 critical cases of COVID-19 during their admission. From the mild case only one patient died, but 12 severe cases and 26 critical cases passed away. The case fatality rate (83.87%) was higher in critical patients (Figure 1).
 |
Figure 1 Severity of COVID-19 among patients admitted at DMU COVID-19 treatment center, 2020. |
Hematological Profile of COVID-19 Patients
The total white blood cells and differential counts of 136 COVID-19 patients were performed, and majority of them (72%) had normal white blood cell counts and 28% had abnormal leukocyte counts (5.88% low WBC count versus 22.06% high WBC counts). Among COVID-19 patients of low WBC counts, two died and six (75%) recovered. Similarly, 27.55% patients who had normal WBC count died and 72.45% recovered. However, one-third (33.33%) of the patients who had high WBC count died and 66.67% recovered.
The lymphocyte counts of the study participants indicated that 16.91% low, 77.21% normal and 5.88% high counts, respectively. The prevalence of abnormal lymphocyte counts (low and high cell counts) was about 23%. Patients with 30.43% of low and 12.5% of high lymphocyte counts died. However, patients with 69.57% of low, 70.48% of normal and 87.5% high lymphocyte counts had recovered from the disease.
The granulocyte counts of COVID-19 patients were 17.65% low, 55.15% normal and 27.21% high. From patients who had abnormal granulocyte counts (low and high counts), 16.67% of low and 35.14% of high counts died. Most of the patients who had low (83.33%), normal (70.67%) and high (64.86%) granulocyte counts were recovered.
The monocyte count of the patients indicated that only three patients had low cell counts. One of the three died and two of them recovered. About 55% of the patients had normal monocyte count; 28.30% of them had died and 71.7% had recovered. Of the total monocyte counts performed, 27.21% of the patients had high cell counts. From patients who had high counts, 29.63% died and 70.37% recovered.
The result of red blood cell (RBC) count of the study participants revealed that 11.76% had low and 18.38% high cell counts. And, 43.75% of the patients of low RBC counts and 16% of the high RBC counts died, whereas 56.25% of low and 84% of the high RBC counts recovered.
The platelet count abnormality was seen in nearly 87% (95% CI; 81–92.53) of the patients. From this abnormality, 64.71% had low and 22.06% high platelet counts. Among patients who had low platelet count, 31.82% died and 68.18% recovered. In contrast, patients with high platelet count, 20% died and most of them (80%) had recovered (Table 5).
 |
Table 5 Cell Count Abnormality of COVID-19 Patients Admitted at DMU COVID Treatment Center 2020 |
The overall prevalence of anemia was 13.24%. The prevalence was the same in both sexes that is 6.62% (Figure 2).
 |
Figure 2 Prevalence of anemia among COVID-19 patients admitted at DMU COVID treatment center 2020. |
The multivariable logistic regression result of hematological profile between death and recovery indicated that only red cell count (RBC) and hematocrit (Hct) were statistically significant predictors of death due to SARS-COV-2 (P<0.05) (Table 6).
 |
Table 6 Multivariable Logistic Regression of Hematological Profile of COVID-19 Patients, 2020 |
Discussion
The pandemic coronavirus has tremendous social, economic, environmental and health related negative impacts on different countries.18–20 Studies have shown that the disease severity is associated with hematological abnormalities like anemia, leukocytosis, neutrophilia, and combined neutrophilia-lymphopenia.21–24 However, few studies show the exact hematological abnormality predictor related to disease severity or clinical outcome.
The mean age (47.21+2.9) of the patients was in line with the study done in India (49±17.4).25
The current study had shown that most of the patients (97.79%) had symptomatic clinical manifestation. This finding is higher than a study done in Wuhan, China, which was 60%.26 The higher finding might be related to increased severity of SARS-COV-2 or reduced immunity of the population.
Of the manifestations: joint pain, cough, headache, vomiting, loss of appetite, respiratory distress syndrome, and fever were the common symptoms. The mean oxygen saturation level (89.75%) of the patients was lower than the National Institute of Health recommended reference range (92–96%) for both inpatient and outpatient COVID-19-positive patients.27 The clinical outcome of the patients revealed that 28.68% of the patients died and majority of the deaths were males. On the contrary, the number of male patients recovered from the disease were higher than female patients. There were different comorbidities identified with COVID-19. The common comorbidities were hypertension, diabetes mellitus, HIV, malignancy, asthma, chronic obstructive pulmonary disease (COPD), chronic kidney disease CKD, chronic liver disease (CLD), and congestive heart failure (CHF). These comorbidities increased the death rate of patients in the study area.
The hematological profile of patients indicated that 28% of COVID-19 patients had abnormal WBC count; 5.88% leukopenia and 22.06% leukocytosis which is in line with a study done in Wuhan, China, indicated that 9% leukopenia and 24% leukocytosis.26 However, leukocytosis in the current study is higher than 17.32%, but leukopenia is lower than 14.71% in Wuhan.25 The lymphocyte count of the patients revealed that 16.9% of the patients had lymphopenia which is lower than 25.2% in China,28 but lymphocytosis was seen in only 5.88% of the patients. The current finding of lymphopenia was lower than 35% in Wuhan, China.26 Lymphopenia is a common feature in viral disease, which has been related to the cytopathic effect due to the affinity of the virus for angiotensin-converting enzyme inhibitors of lymphocytes.29 The other possible explanation associated with lymphopenia is increased inflammatory response which leads to increased granulocyte production but lymphocyte apoptosis.30 Nearly, one-third of lymphopenia patients died but only 12.5% of the patients with lymphocytosis died. This finding is related with the review done by Huang et al 2020 which stated that patients who had lymphopenia had poor outcome.31
Abnormal platelet count was detected nearly among 87% of the study participants in the present study which is in line with a systematic review study done by Bhattacharjee et al (84.5%).32 Nearly two-third of the platelet count abnormalities (64.71%) were thrombocytopenia which is higher than studies done in Wuhan, China (20.7%) and by Lippi et al (55%).33 The possible mechanism of thrombocytopenia in COVID-19 infected patients might be related to direct effect of the virus on the bone marrow cells or platelets by reducing platelet synthesis, creating dysfunctional marrow microenvironment; liver damage by the virus leading to decreased thrombopoietin production; pulmonary endothelial damage followed by platelet aggregation in the lungs, subsequent formation of microthrombi, and platelet consumption; and finally, the destruction of platelets by cross reacting antibodies.32 A study had shown that thrombocytopenia increased the risk of COVID-19 severity over five folds.33,34 The prevalence of anemia in the current study (13.24%+2.92) was lower than 24.7% in Austria and 25.6% and 61% in Italy, respectively.35–37 According to the WHO classification, the type of anemia detected among patients was mild anemia.38 The difference might be related to the difference in the study population, the immunity of patients and severity of the disease or other underlying clinical conditions. The increased prevalence of anemia among COVID-19 patients are associated with SARS-CoV-2 infection which may lead to hemolytic anemia directly through cytopathic injury of red cells or indirectly through formation of auto-antibodies.39
Conclusion
A concrete study has shown that COVID-19 has three critical clinical phases; phase I (initial phase), phase II (propagating phase) and phase III (complicating phase).40 The study indicated that the hematological changes are categorized under propagating phase (Phase II).s.
The majority of the cases were recovered from COVID-19. The hematological finding of COVID-19 patients indicated that nearly one-third of the patients had abnormal WBCs, and RBC counts. Moreover, most of the patients had abnormally low platelet cell counts. Of the white cell count abnormalities, leukocytosis, lymphocytopenia, granulocytosis, and monocytosis were the common findings. Mild anemia was observed among patients. RBC count and hematocrit level were the only significant predictors of death (P<0.05). The case fatality rate was higher in critical cases than in any other stages of severity of the disease.
The clinician could manage cases according to the hematological findings of the patients. Further large-scale experimental studies should be conducted to determine hematological parameter changes and associated factors. The main limitation of this study is that we could not perform coagulation tests like PT, aPTT, INR and D-dimer as well as inflammatory markers due to lack of reagents and equipment during the study period in the study setting.
Abbreviations
aPTT, activated partial thromboplastin time; CBC, complete blood count; COVID-19, coronavirus disease 19; INR, international normalized ratio; MCHC, mean cell hemoglobin concentration; MCV, mean cell volume; MCH, mean cell hemoglobin; PT, prothrombin time, RBC, red blood cell; RDW, red cell distribution width; SARS-COV-2, severe acute respiratory syndrome; WBC, white blood cell.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Informed Consent
The study complies with the Declaration of Helsinki. Ethical approval was obtained from Debre Markos University Health Science College Institutional Research Ethics Review Committee (IRERC) with reference number HSC/R/C/Ser/Co/202/11/13. Then, an informed written consent was obtained from each participant after explaining aim of the study. The study participants were briefed on the study’s purpose, procedures, potential risks, and benefits. In addition, they were told that they had the right to withdraw from the study which would not endanger their access to treatment.
Acknowledgments
The authors thank data collectors and study participants involved in this research, and all individuals for their cooperation in this study. In addition, we thank Debre Markos University for financial support of the research project.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The funding was obtained from Debre Markos University.
Disclosure
The authors declared that there are no competing interests.
References
1. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi:10.1016/S0140-6736(20)30183-5
2. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi:10.1056/NEJMoa2001017
3. Ciotti M, Angeletti S, Minieri M, et al. COVID-19 outbreak: an overview. Chemotherapy. 2019;64(5–6):215–223. doi:10.1159/000507423
4. Wu F, Zhao S, Yu B, et al. Complete genome characterisation of a novel coronavirus associated with severe human respiratory disease in Wuhan, China. bioRxiv. 2020;2020:1.
5. Dong N, Yang X, Ye L, Chen K, Chan EWC, Chen S. Genomic and protein structure modelling analysis depicts the origin and pathogenicity of 2019-nCoV, a new coronavirus which caused a pneumonia outbreak in Wuhan, China. F1000Research. 2020;9(121):121. doi:10.12688/f1000research.22357.2
6. Cui J, Li F, Shi Z-L. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019;17(3):181–192. doi:10.1038/s41579-018-0118-9
7. Shereen MA, Khan S, Kazmi A, Bashir N, Siddique R. COVID-19 infection: origin, transmission, and characteristics of human coronaviruses. J Adv Res. 2020;24:91–98. doi:10.1016/j.jare.2020.03.005
8. Phan LT, Nguyen TV, Luong QC, et al. Importation and human-to-human transmission of a novel coronavirus in Vietnam. N Engl J Med. 2020;382(9):872–874. doi:10.1056/NEJMc2001272
9. Riou J, Althaus CL. Pattern of early human-to-human transmission of Wuhan 2019 novel coronavirus (2019-nCoV), December 2019 to January 2020. Eurosurveillance. 2020;25(4):2000058. doi:10.2807/1560-7917.ES.2020.25.4.2000058
10. Parry J. China coronavirus: cases surge as official admits human to human transmission. Br Med J. 2020;m236. doi:10.1136/bmj.m236
11. Upadhyay J, Tiwari N, Ansari MN. Role of inflammatory markers in Corona virus disease (COVID-19) patients: a review. Exp Biol Med. 2020;245(15):1368–1375. doi:10.1177/1535370220939477
12. Lin L, Lu L, Cao W, Li T. Hypothesis for potential pathogenesis of SARS-CoV-2 infection–a review of immune changes in patients with viral pneumonia. Emerg Microbes Infec. 2020;9(1):727–732. doi:10.1080/22221751.2020.1746199
13. Ding Y, He L, Zhang Q, et al. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS‐CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J Pathol. 2004;203(2):622–630. doi:10.1002/path.1560
14. Klok F, Kruip M, Van der Meer N, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147.
15. Filatov A, Sharma P, Hindi F, Espinosa PS. Neurological complications of coronavirus disease (COVID-19): encephalopathy. Cureus. 2020;12:1.
16. Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802. doi:10.1001/jamacardio.2020.0950
17. Bairwa M, Kumar R, Beniwal K, Kalita D, Bahurupi Y. Hematological profile and biochemical markers of COVID-19 non-survivors: a retrospective analysis. Clin Epidemiol Glob Health. 2021;11:100770. doi:10.1016/j.cegh.2021.100770
18. Roy D, Ghosh R, Dubey S, Dubey MJ, Benito-León J, Ray BK. Neurological and neuropsychiatric impacts of COVID-19 pandemic. Can J Neurol Sci. 2021;48(1):9–24. doi:10.1017/cjn.2020.173
19. Kitamura Y, Karkour S, Ichisugi Y, Itsubo N. Evaluation of the economic, environmental, and social impacts of the COVID-19 pandemic on the Japanese tourism industry. Sustainability. 2020;12(24):10302. doi:10.3390/su122410302
20. Cheval S, Mihai Adamescu C, Georgiadis T, Herrnegger M, Piticar A, Legates DR. Observed and potential impacts of the COVID-19 pandemic on the environment. Int J Environ Res Public Health. 2020;17(11):4140. doi:10.3390/ijerph17114140
21. Araya S, Wordofa M, Mamo MA, et al. The magnitude of hematological abnormalities among COVID-19 patients in Addis Ababa, Ethiopia. J Multidiscip Healthc. 2021;14:545. doi:10.2147/JMDH.S295432
22. Rahman A, Niloofa R, Jayarajah U, De Mel S, Abeysuriya V, Seneviratne SL. Hematological abnormalities in COVID-19: a narrative review. Am J Trop Med Hyg. 2021;104(4):1188. doi:10.4269/ajtmh.20-1536
23. Yuan X, Huang W, Ye B, et al. Changes of hematological and immunological parameters in COVID-19 patients. Int J Hematol. 2020;112(4):553–559. doi:10.1007/s12185-020-02930-w
24. Lippi G, Mattiuzzi C. Hemoglobin value may be decreased in patients with severe coronavirus disease 2019. Hematol Transfus Cell Ther. 2020;42:116–117. doi:10.1016/j.htct.2020.03.001
25. Gujar RK, Meena A, Chouhan SS, Likhar K. Hematological profiles of COVID-19 patients at the Ratlam district, Madhya Pradesh State, India. Bioinformation. 2021;17(7):686. doi:10.6026/97320630017686
26. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi:10.1016/S0140-6736(20)30211-7
27. Shenoy N, Luchtel R, Gulani P. Considerations for target oxygen saturation in COVID-19 patients: are we under-shooting? BMC Med. 2020;18(1):1–6. doi:10.1186/s12916-020-01735-2
28. Mao J, Dai R, Du R-C, Zhu Y, Shui L-P, Luo X-H. Hematologic changes predict clinical outcome in recovered patients with COVID-19. Ann Hematol. 2021;100(3):675–689. doi:10.1007/s00277-021-04426-x
29. Xu R, Cui B, Duan X, Zhang P, Zhou X, Yuan Q. Saliva: potential diagnostic value and transmission of 2019-nCoV. Int J Oral Sci. 2020;12(1):1–6. doi:10.1038/s41368-020-0080-z
30. Tan L, Wang Q, Zhang D, et al. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct Target Ther. 2020;5(1):1–3. doi:10.1038/s41392-019-0089-y
31. Huang I, Pranata R. Lymphopenia in severe coronavirus disease-2019 (COVID-19): systematic review and meta-analysis. J Intensive Care. 2020;8(1):36. doi:10.1186/s40560-020-00453-4
32. Bhattacharjee S, Banerjee M. Immune thrombocytopenia secondary to COVID-19: a systematic review. SN Compr Clin Med. 2020;2(11):2048–2058. doi:10.1007/s42399-020-00521-8
33. Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Anal Chim Acta. 2020;506:145–148. doi:10.1016/j.cca.2020.03.022
34. Yang X, Yang Q, Wang Y, et al. Thrombocytopenia and its association with mortality in patients with COVID‐19. J Thromb Haemost. 2020;18(6):1469–1472. doi:10.1111/jth.14848
35. Bellmann-Weiler R, Lanser L, Barket R, et al. Prevalence and predictive value of anemia and dysregulated iron homeostasis in patients with COVID-19 infection. J Clin Med. 2020;9(8):2429. doi:10.3390/jcm9082429
36. Bergamaschi G, Borrelli de Andreis F, Aronico N, et al. Anemia in patients with Covid-19: pathogenesis and clinical significance. Clin Exp Med. 2021;21(2):239–246. doi:10.1007/s10238-020-00679-4
37. Zuin M, Rigatelli G, Quadretti L, Fogato L, Zuliani G, Roncon L. Prognostic role of anemia in COVID-19 patients: a meta-analysis; infect. Dis Rep. 2021;13:930–937. doi:10.3390/idr13040085
38. World Health Organization. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Geneva: World Health Organization; 2011.
39. Al-kuraishy HM, Al-Gareeb AI, Kaushik A, Kujawska M, Batiha GES. Hemolytic anemia in COVID-19. Ann Hematol. 2022;101(9):1887–1895. doi:10.1007/s00277-022-04907-7
40. Turk C, Turk S, Malkan U, Haznedaroglu IC. Three critical clinicobiological phases of the human SARS-associated coronavirus infections; 2020.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.