Back to Journals » Journal of Asthma and Allergy » Volume 13
Helminth Induced Immunoregulation and Novel Therapeutic Avenue of Allergy
Authors Ayelign B , Akalu Y , Teferi B, Molla MD , Shibabaw T
Received 23 July 2020
Accepted for publication 17 September 2020
Published 7 October 2020 Volume 2020:13 Pages 439—451
DOI https://doi.org/10.2147/JAA.S273556
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Luis Garcia-Marcos
Birhanu Ayelign,1 Yonas Akalu,2 Banchamlak Teferi,3 Meseret Derbew Molla,4 Tewodros Shibabaw4
1Department of Immunology and Molecular Biology, School of Biomedical and Laboratory Sciences, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia; 2Department of Physiology, School of Medicine, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia; 3Department of Clinical Pharmacy, School of Pharmacy, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia; 4Department of Biochemistry, School of Medicine, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
Correspondence: Birhanu Ayelign
Department of Immunology and Molecular Biology, School of Biomedical and Laboratory Sciences, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
Email [email protected]
Abstract: Allergic diseases are increasing at an alarming rate worldwide, particularly in developed countries. In contrast, there is a decrease in the prevalence of helminthic infections and other neglected diseases. The hygiene hypothesis elaborates parasitic infection, and allergy-associated diseases have an inverse relationship. Acute helminthic infection and allergic reaction stimulate Type 2 helper cells (Th2) immune response with up-regulation of cytokines IL-4-, IL-5-, and IL-13-mediated IgE and mast cell production, as well as eosinophilia. However, people who chronically suffer from helminthic infections are demarcated through polarized Th2 resulting in alternative macrophage activation and T regulatory response. This regulatory system reduces allergy incidence in individuals that are chronically diseased through helminth. As a result, the excretory-secretory (ES) substance derived from parasites and extracellular vesicular components can be used as a novel therapeutic modality of allergy. Therefore, the aim of this review meticulously explored the link between helminth infection and allergy, and utilization of the helminth secretome for therapeutic immunomodulation.
Keywords: allergy, helminth, IgE, Treg, hygiene hypothesis, immunotherapy
Introduction
Helminths are multicellular worms that colonize around 1/3 of the population of the globe, approximately two billion people are infected.1 It is more pronounced in low-income countries like sub-Saharan Africa, including Ethiopia.1,2 In the developing world, inadequate water supply, crowded living conditions, lack of health care access, and a low level of education makes people more vulnerable to helminthic infection. Many scholars have shown that in developing countries helminthic infection is still endemic while the allergic disease is rare.3,4 It is widely spread in tropical rural children due to poor sanitation.5 Although helminthic infections are a global concern, some species may be found only in particular regions. The problem may even vary in different regions within a certain country. Typical examples are Schistosoma spp. (S. haematobium, S. mansoni, and S. japonicum) is more commonly found in sub-Saharan Africa/South America and East Asia, whereas filarial (Wuchereria bancrofti, loa loa, onchocerciasis, and Brugia malayi) are highly prevalent in Southeast Asia.6 Together, these parasite's infections cause anaemia, stunted growth, cognitive impairment, fatigue, infertility, liver fibrosis, and bladder cancer.7
In contrast, the developed world now faces different health problems, mainly cardiovascular disease, metabolic disease, hyperinflammatory disease, and associated autoimmunity.8 In the last twenty years, hypersensitive illnesses, such as asthma/wheeze, allergic rhinitis (AR), or eczema, the so-called “allergic diseases” have become highly prevalent in the developed world.9
The increased prevalence of allergy is associated with a sanitized living environment of the industrialized world rather than genetic variables, the phenomenon is called hygiene hypothesis.10 At the same time, allergic disease increase as lifestyle become more urbanized in low-income countries. Many research hypotheses elaborate that allergic hyper-reactivity is due to dysregulated mucosal Th2 response reaction to decreased Th1 response.11,12 In fact, some of the latest clinical and epidemiological reports show that there is a reverse correlation of helminthic infection with allergy. The common feature between helminthic infection and allergy is the elevation of immunoglobin E (IgE) due to the sensitization of the Th2 immune response along with interleukins, such as IL-4, IL-5 and IL-13 cytokine synthesis.13 In helminthic infection, this is usually rare, toughly regulated antibody Isotype is significantly elevated. It is commonly recognized that IgE receptors and inimitable cellular response did not involve the targeting of offensive particles in pollen, dust mites, and animal dander.
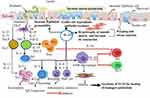
Indeed, the IgE alliance has developed to combat helminths, which are to be phagocytized and the allergy in a hypersensitive individual is a misdirected anti-parasite reaction.14 An allergy happens in individuals with atopy; which is a response of hypersensitivity caused by either antibody or cell-mediated immunological mechanisms.14 Inflammatory reactions that resulted due to a multifaceted collaboration of genetic and environmental factors lead to hypersensitive/allergic responses.1 In high-income or industrialized countries, hypersensitivity diseases like asthma are becoming a very common public health burden in recent decades.15 The leading cause of hypersensitivity or allergic diseases is associated with the maturation of T cell to Th2 immune responses. In return, it encourages the stimulation of IgE, mast cell, eosinophils, goblet cell, and secretary mucin.12 Allergic disorders associated with inflammation begin with mast cell degranulation, which then results in the activation of mediators that further activate inflammatory cells like eosinophil, monocyte, and neutrophil resulting in the release of cytokines and long-lasting mediators that cause tissue and specific organ damage.1,6 A cell located within germinal centres of lymph nodes, T follicular helper (Tfh) cells are also essential in the generation of antibody-secreting plasma cells. As such, they are of potential importance in allergen-specific IgE production in allergic disease.16,17 Furthermore, Type II innate lymphoid cells (ILC2) are a novel population of lineage-negative cells, which play an important role in orchestrating the type 2 response to helminths and allergens to high levels of Th2 cytokines IL-5 and IL-13.18 ILC2 is present both in human respiratory and gastrointestinal tissue as well as in skin. Type 2 responses are initiated by allergens or helminths that disrupt the epithelial barriers and induce secretion of IL-25, IL-33 and TSLP. Those epithelium-derived cytokines activate ILC2 cells, which directly secrete type 2 cytokines, and DCs, which induce TH2 responses. The secretion of type 2 cytokines by ILC2 cells feeds back on the epithelium to induce mucus secretion by goblet cells (IL-13) and tissue repair (amphiregulin (Areg)).19,20 Secretion of IL-9 and IL-5 by ILC2 cells leads to the recruitment and activation of mast cells and eosinophils. The activation of T cells in lymphoid organs further amplifies the secretion of type 2 cytokines, and the production of IL-4 by T cells in lymphoid organs leads to the production of IgE by B cells. Together, the responses triggered by secretion of type 2 cytokines from both ILC2 and TH2 cells orchestrate allergic inflammation, helminth expulsion and tissue repair.20
In most patients, IgE Isotype mediates the hypersensitivity reaction of other immunoglobulins.21 IgE-mediated allergy is a pyogenic syndrome associated with polymorphism in cytokine genetics, receptors, and transcriptional factor-related with skewness of Th0 to Th2 immunity and expression of IgE receptors. High Th2 secreted protein, IgE, and eosinophilia are all usual biological responses to helminthic pathogens (Figure 1).14 Moreover, helminth's vigorously restrained host cell response against it enhance alternatively activated macrophages (AAM), transforming growth factor ß (TGF-ß), and IgG4 antibodies that work against IgE mediated allergic reaction.14,22 However, some diseases (particularly Ascaris species) are connected with enhanced allergy, and it can be associated with cross-reactivity between worm antigen (Tropomyosin’s), which is similar to allergy-causing particles in insects, and dust-mites.23 It has been commonly observed in elevated densities of helminth infections, that asthma, and other allergic illnesses, have not increased as significantly as in more developed countries. Moreover, a negative correlation of allergy with helminthiasis had been founded in the endemic areas of helminthic infections. The latest studies on students infected with hookworm and Ascaris in Ethiopia and Ecuador, respectively, showed that individuals infected with helminths are less likely to be allergic.4,24 Similarly, a study done in Israel on Ethiopian immigrants showed that helminthic infection is significantly associated with low allergy and low skin prick test (SPT) reactivity, however, one year after immigration to Israel, allergy and SPT reactivity increased significantly in all immigrants.25 The inverse correlation of these studies may be due to helminthic infection-associated immune suppression or genomic aspects that predispose a hypersensitivity reaction and/or resistance to infection.26
Helminths and Hygiene Hypothesis
The “hygiene hypothesis” was first formulated in 1989 by an epidemiologist, Dr. Strachan, who reported an inverse relationship between family size and development of atopic disorders. He proposed that a lower incidence of infection in early childhood, transmitted by unhygienic contact with older siblings or acquired prenatally could be a cause for the rise in allergic diseases.27 Then, Greenwood illustrates the reverse association with immune dysregulation and the pathogenesis of parasitic diseases.28 He hypothesized that infection in early childhood protects from allergic associated illnesses, including hay fever, in larger families.28 In the principle of the hygiene hypothesis, the absence of parasitic infection or living in a sterilized environment with reduced exposure to bacteria or protozoa or helminth infection in early life results in increased incidence of allergic disease through an exaggerated Th2 allergic response to a harmless antigen.29 The association between reduced exposure to infectious agents and a higher prevalence of allergy seems now to be confirmed by many research studies. It is very clear that the hygiene hypothesis has generally been associated with increasing urbanization and living in a sterilized environment. Interestingly, increasing urbanization is also associated with a reduced exposure to a diversified infectious agent, and an exponential rising of allergic diseases, now confirmed by a different epidemiological study.30 Distinct inquiries about these discoveries proposed an expanded predominance of rhinitis indications within the higher socioeconomic groups. An epidemiological study on socio-economic variation and allergic diseases showed that children and adults from very rich families are present with a high prevalence of hay fever and eczema.31 Moreover, socioeconomic variations in the prevalence of allergic diseases are evident not only in industrialized countries like Britain and Italy, but also in urban African countries like Ghana.31 It has been postulated that people living with a limited exposure to bacterial and viral pathogens during early childhood result in an insufficient stimulation of Th1 cells, which in turn cannot counterbalance the expansion of Th2 cells and results in a predisposition to allergy.32
Hygiene hypotheses were introduced through the investigation of children in Africa, particularly in Gabon.29 In Gabon, an increased prevalence of schistosomiasis results in an increased expression of the Th2 phenotype among exposed individuals. Surprisingly, allergic reactivity was decreased among exposed schoolchildren as compared to uninfected ones.3 In Gabon, increased prevalence of schistosomiasis results in an increased expression of the Th2 phenotype among exposed individuals. Surprisingly, allergic reactivity was decreased among exposed schoolchildren as compare to uninfected ones.33 Accordingly, they conclude that helminthic infections in many, but not all, are associated with the suppression of allergic reactions.28 Similarly, another study also showed that allergic sensitivity was elevated among anti-helminthic treated children in countries having the higher endemic status of the parasite, which shows the presence of an association in allergic protection and parasitic infection34,35 (Figure 1).
The immediate response to allergens or other toxic products might cause the release of damage-associated molecular patterns (DAMPs), including high-mobility group box 1 (HMGB1), adenosine triphosphate (ATP), double-stranded deoxyribonucleic acid (dsDNA), and f-actin resulting epithelial cell stress and death. In response to DAMP generation, alarmin cytokines and thymic stromal lymphopoietin (TSLP) are secreted.36 Innate lymphoid cell-generated IL-13 reaches maturation as well as chemotaxis’s of dendritic cells (DCs) to drain the lymph node, by which the presentation of antigen for naive T cells takes place.37
Besides this, when a patient is previously exposed to helminths, T regulatory cell (Treg) generates immunoregulatory cytokines (TGF-β and IL-10) to decrease T and dendritic cell responses. Modified Th2 activitates the generation of TGF-ß, IL-10, and antigen-specific IgG4 that has a major effect by inhibiting IgE-associated allergic reactivity for helminth antigens. Hence, permanent antigenic infection with the parasite is related to a Th2 modified activation, which may decrease allergies.38
Parasite as well as associated allergen secretions are a major factor to diminish immunity and reduce allergy through the induction of Treg by activating the TGF-β pathway; enzymes generated from the pathway catalyze the breakdown of eotaxin, a chemokine essential for eosinophils migration; release of apyrases, enzymes that catalyze the oxidation of inflammatory DAMP ATP into non-inflammatory adenosine monophosphate (AMP); generated substrate that hinders the secretion of IL-33; one of the substrates that decrease DC maturation into Toll-like receptor (TLR) signals; as well as the saturation of eosinophil by polyclonal IgE antibody.28
Host Immune Response to Helminthic Infection
Helminthic infections are the leading cause of chronic infections in humans, which are characterized by the ability to survive for many years within the host cell.1 Some of those may not be pathological unless exposed byaggravating factors. The question is how those helminths can survive for a prolonged time within the immunocompetent host cells harmoniously? What substances of the helminthic products are involved in immunomodulation? This is because of the development of sophisticated survival strategies. Modulation and manipulation of our immune system are supposed to be the main mechanism/strategy for survival.39 Alteration of immune response by helminthic infection may have a negative impact on host cells, especially, when they negatively affect the immune cell response to infections. But in some conditions, the helminthic infection can ensure a positive impact on the host cells, due to the immune tolerance activity of them with the host cells. Researchers have suggested that helminthic infections could be involved in the control of autoimmune disorders, thereby preventing excessive inflammatory responses. Therefore, helminthic infection may be a novel anti-inflammatory therapeutic tool and may have a beneficial role in the control of autoimmune allergies.39
Most helminthic infections stimulate maturation of Th2 cytokines (IL-4, IL-5, IL-9, and IL-13) along with the deterring of Th1 cytokine response (IL-12 and interferon-gamma (IFN-γ).40 In response to this, the activity of IgE, mast cell, and eosinophil will be initiated. The stimulated IgE diminishes the worm's fitness as well as its fecundity via antibody-dependent cellular cytotoxicity (ADCC). Mast cell degranulation and release of protease helps the degradation of the junction of the epithelial cell layer and participates in the weep and sweep process. Most of the intestinal helminthic infections are responsible to induce the maturation of Th2 cytokines (producing IL-4 and IL-13), which promotes worm expulsion from the gastrointestinal tract (GIT). The signaling cascade of IL-13 and IL-4 is through the activation of the IL‑4Rα-STAT6 in the intestinal mucosal epithelium that enhances goblet cell differentiation and mucus production.41,42 Similar to Th2 cells, alarmins and parasite-derived ES products can also stimulate innate lymphoid cells (ILC2s) and secrete Th2-related cytokines, including IL-5, IL-9, and IL-13. Consequently, it plays a key role in the expulsion of the parasite from the intestinal lumen by promoting goblet cell mucus secretion and smooth muscle contraction that increases its permeability for flushing of the parasite in the lumen of the intestine. These modes of host cell immune response against the helminthic infection in the intestinal epithelium are called the “weep and sweep” process. Moreover, Th2 related cytokines produced by ILC2 contribute to airway hyper-reactivity and acute allergic immune response.43 In addition, the tuft cell is a brush cell of epithelial layers of many hollow organ systems like the intestine and respiratory system. The study showed that these cells are a potent source of alarmin cytokines (IL-25) and eicosanoids associated with allergic immunity, and the neurotransmitter acetylcholine required to coordinate type 2 immunity during helminthic infection.44,45
Moreover, IL-33 and IL-18 as members of the IL-1 superfamily act as alerting immune molecules to the injured epithelial cell and generate a strong type 2 immune response. In addition, after it binds with the IL-1 receptor-like 1 (IL1RL1), also known as the ST2 receptor, found on the membrane of Th2 cells and ILC2s, IL-33 plays a role in inducing proliferation and repairing of the epithelium results in healing of the mucosa of the gut.46–48 Interleukin-25, also known as IL-17E, which is a member of the IL-17 cytokine family, likewise, acts as a sensor of epithelial insult or damage and induces production of type 2 cytokines by activation of the IL-4, IL-5, and IL-13 gene expression in ILCs.25 Interleukin-25 also promotes allergic responses that themselves can lead to tissue damage and remodelling.45,48 Even though its mechanism is not yet clear, epithelial cells produce TSLP. As with IL-33 and IL-25, TSLP induces Th2-type responses, resistance to helminths49 and limits the development of a type 1 response. It can also act on other immune cells such as monocytes, granulocytes, and B cells (Figure 2).
An experimental study in mice suggests, to coordinate the immune-protective activity of the host cell for digestive system, nematodes need the release of CD4+T cells.34 Helminthic infection promotes the secretion of the intestinal mucosa and stimulates the ooze of fluid into the intestinal lumen. It also enhances the contractility of intestinal smooth muscle. This process is mainly mediated by IL-13 and IL-4 secreted by Th2 cells as well as an innate helper cell.46
Both IL-13 and IL-4 use similar receptors for their action in type2 immunity. Interleukin-4 receptor (IL-4Rα) acts as a common bottleneck component of both IL-13 and IL-4. From many biological roles, in helminthic infection, and for the activation of both eosinophils and AAM when the well-known cytokine that activates eosinophils is IL-5. As a Th2 cell, the innate helper cell called nuocyte, also known as non-B, non-T cell, is significantly secreting IL-13 and IL-4 during helminth infection.46,50–53 Other cytokines like IL-10 and numerous cellular communications are also vital to control gastrointestinal worms. However, the maturation of the Th1 pathway stimulates gastrointestinal larva colonization. As a result, IL-12, along with IL-18, induces T cell differentiation to the Th1 phenotype, thereby generating IL-12, IL-18, and IFN-γ. This inhibits larva eviction seemingly via Th2 cell maturation interference and will improve the survival of larvae. In some helminthic infections, a durable Th2 comeback does not defend the host from all parts of the problem.54 In some cases, hepatic schistosomiasis associated hepatic fibrosis, portal hypertension and gastrointestinal bleeding may rarely appear due to over-reactivity induced inflammation. The Th2 cytokines, particularly IL-13, are the greatest stimulator of hepatic fibrosis in Murine models of the disease.55 The adult schistosomiasis worm found in mesenteric veins also greatly affects the immune hyperactivity.41,56 Then the adult worms produce ova in the intestine and/or liver and cause inflammatory reactions due to helminths associated maturation of Th2 cytokines. During intestinal infection, the above process allows the eggs to pass through the intestinal wall and mix with feces for excretion. The balance between Th1 and Th2 reaction to the ova limits hepatocellular damage or fibrosis.57
The most central feature of helminth infection is the promotion of Treg activity, Treg expansion can also account for the inhibition of allergic responses in mouse models. Parasites have evolved multiple strategies to exploit the Treg pathway, within their host immune-modulatory molecules like IL-10 and transforming growth factor-beta (TGF-β) can affect both Th1 and Th2 function.58,59 Interleukin-10 inhibits macrophage and dendritic cell function and suppresses the secretion of important pro-inflammatory cytokines like TNF-α, IL-12, IL-1, nitric oxide, and numerous chemokine (Figure 2). In parallel, IL-10 also inhibits the CD28 co-stimulative pathway by activating cytotoxic T-lymphocyte-associated protein 4 (CTLA4) to promote T cell tolerance.59 The finding showed that in murine schistosomiasis, IL-10 down-regulated the intensity of the granulomatous response as well as the development of IFN-γ and IL-4 during the chronic stage of the disease, and it is essential for IFN-γ modulation in an individual. Transforming growth factor-beta is also a significant regulatory cytokine, like IL-10, which acts in schistosome granulomas to restrict the activity of the Th1 cell.60
Immunological Mechanisms of Helminth Mediated Modulation of Allergy
Helminth's means of ensuring their survival in the host body system are through limiting inflammatory responses and creating an immunoregulator microenvironment.13 Here are mechanisms by which helminthic infection can control the host immune response. These regulatory mechanisms are the expansion of regulatory cells (example: Treg cell, Breg cell, AAM), manipulation of TLR and signaling, suppression of Th1/Th2 cell, and associated cytokines. However, their cellular mechanism is still unknown and under research.61 The beneficial effect of helminthic infection on allergic diseases, particularly airway inflammation or asthma, anaphylaxis, and other autoimmune diseases via stimulation of Treg cells (its anti-inflammatory cytokine secretions) and activation of TLR.62
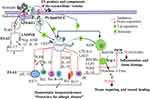
Inducing the development of Treg and/or AAM, helminth or its derived products could directly slow down allergen-specific Th2 responses. T regulatory cells and AAM can directly inhibit Th2 cell proliferation through a cell contact-dependent mechanism or by the synthesis of common immunomodulatory mediators such as IL-10 and TGF-ß. The Treg cell is the most potent inhibitor of inflammation via up-regulating the above-mentioned immunosuppressive molecules and IL-35 and repressing genes involved in pro-inflammatory function.63–66 As a result of the production of IL-10, helminthic infection diminishes the risk of allergic disease by inhibiting IFN-γ secreting cell (Th1), IL-4, IL-13, IL-5 and IL-9 producing cell (Th2), and IL-17 secreting cell (Th17) (Figure 3).10,66 Together with IL-10 and TGF-ß, IL-35 is the other novel anti-inflammatory molecule produced by CD4+CD25+Treg cell and suppresses both differentiation as well as the proliferation of Th17 cells.65 In addition, Treg can also release granzymes (Gzm) and perforin that directly suppress effector B-cell or reprogramme IgE secreting B-cell to IgG4 and IgA.66–68 Supporting this idea, a scientific study elaborates that a patient with a chronic helminthic infection has immune homeostatic tolerance mediated by the production of regulatory cytokines (e.g. IL-10) and raised IgG4 than a patient without a history of helminthic infection.68 This is characterized by higher IgG4/IgE ratios and increased IL-10 and TGF-β as a biomarker of chronic infection. The large amounts of non-specific IgE induced by the helminth infection might also contribute to reduced allergic responses by saturating the IgE receptors on mast cells, basophils and eosinophils, thereby inhibiting the binding of allergen-specific IgE and the degranulation of these cells. Helminth-derived products might also interact directly with allergen-specific IgE-induced cross-linking of granulocyte FceR or the signaling pathways.69 The mechanism behind helminth induced production of large amounts of polyclonal IgE is not yet clear. However, the negative association between helminth-specific polyclonal IgE and allergic response is called the IgE blocking hypothesis.69
The way those cells understand and sense the foreign microbes are through the presence of pattern recognition receptors (PRRs) namely, TLR, nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs), retinoic acid-inducible gene-like receptors (RIG-like receptors), and C-type lectin receptors (CLRs).61 Toll-like receptors, a family of sensor proteins, assist the innate immune system to discriminate between “self” and “non-self” antigen.70 This TLR detects specific and conserved microbial domains called pathogen-associated molecular pattern (PAMP) which acts as ligands such as lipids, lipoproteins, proteins, RNAs, and DNA of the microbe’s structure.71,72 Expansion of the Treg cell and its allergic suppression is mediated by some helminthic product mediated controls of TLRs and its down-signaling cascade. Extracellular vesicles (EV) is one of the mechanisms for the delivery of helminthic products into the host cell via receptor or clathrin-mediated endocytosis, like the receptor-mediated endocytosis of cholesterol.73 For example, SEA and SJMHE1 of S. mansoni and S. japonicum, respectively induce Treg cell proliferation as well as its anti-allergic activities via the TLR2 dependent manner.74–76 In support of this theses, the same study showed that SJMHE1 products of S. japonicum can also increase Treg cell and anti-inflammatory cytokines such as IL-10 and TGF-β mediated with TLR-2 and considered as a protective role on delayed type hypersensitivity (DTH).75 Likewise, a study done in the UK elaborates that Acanthocheilonema viteae derived excretions-secretions (ES-62), can prevent pathology associated with chronic asthma via blocking of the Th17 response, ILC2, and reversal of Th2 cell polarization and decreased secretion of their perspective inflammatory cytokines.77 ES-62 has a range of immunomodulatory effects, many of which involve destabilization of TLR4 and its signaling to induce an anti-inflammatory immunological phenotype76,78,79 and prophylactic modulation of collagen-induced arthritis (CIA) (Figure 3).80 Collectively, even though there are controversies over the influence of TLR on Treg cell, it is generally accepted that TLR2, 4, or 5 engagements can enhance Treg cell function, survival, and its proliferation.
An experimental study found that helminth mediated AAM, that directly inhibited T-cell effector functions, results in allergic disorders suppression.81 The hypothesis that non-specific IgE induced by the helminth infection protects against the degranulation of basophil or mast cells through increasing IgE receptor on those cells, and thereby inhibits the binding of allergen-specific IgE on these cells, is now out of favour and there is little evidence to support it.82 As Treg, AAM can also suppress the release of IL-5 and Il-13 cytokines from ILC2 through cell contact-dependent inhibition mechanism mediated by IL-10 and TGF-β. Equivalent with Treg, an alternatively activated macrophage secretes immune-modulating biomarkers such as arginase-1 (arg-1), resistin-like molecule alpha (RELMα), and chitinase 3‑like protein 3 (CLP3).83 Arginine is a semi-essential amino acid. The alternatively activated macrophage metabolized by two main enzymatic systems such as arginase 1 (stimulated by IL-4, IL-6, IL-10, IL-13, TGF-β) and iNOS (stimulated by IL-1, TNF-α, IFN-α, IFN-β, IFN-γ), is actively involved in immune response.13,84,85 Arginase 1 helps to provide proline amino acid as a substrate for collagen synthesis during the repair of extracellular matrices, wound healing, and fibrosis in response to mucosal epithelium damage by helminthic infection.83,84 Moreover, competing with the substrate required by iNOS for NO synthesis, arginase-1 consumes arginine to ornithine and urea and can actually play a role in regulating arginine availability.85 Therefore, by depriving arginine, which is needed for T cell activation, arg-1 is a potent suppressor of inflammation (Figure 3).13,86 On the other hand, as Treg, regulatory B cells (Breg) produce IL-10, which may potentially regulate T-cell mediated inflammatory response and allergic reaction in host cells,87 of which the Treg cells are obviously the greatest group of cells studied. Another study reported that IL-10 producing Breg cells down-regulate experimental autoimmune encephalomyelitis, collagen-induced arthritis, IBD and protects against Schistosoma induced anaphylaxis.33,82,88 It is also possible that the presentation of allergens by DCs required for the activation and production of Th2 cells is affected by the infection, leading to a reduction in allergic responses. Even though animal laboratory data point out the inhibitory function of IL-10 and/or Treg cells in allergic reactions, very limited evidence indicates that this is also true in humans.69,89
After the patient diagnosis with X-linked polyendocrinopathy syndrome (IPEX) characterized by a high incidence of autoimmune and allergic diseases present with mutations in FOXP3 with low levels of circulating Treg cell.90 Evidence confirmed that the pathogenesis of allergic diseases is inhibited by the effect of Treg cells; in contrast, individuals with the FOXP3 mutated gene, result in the inactivation of natural Treg subtypes, which in turn leads to immune-associated pathogenesis such as allergy. Simultaneously, the compartment of the B-cell becomes altered, which resulted from diminished IgE, and an increased IgA and IgG response.15 For instance, TGF-ß and IL-10 have been observed in patients diagnosed with Onchocerciasis,91 while patients infected with Brugia malayi show overexpression of the transcription factor, FOXP3 and elevated effect of TGF-ß and CTLA4.87 In addition, studies conducted in Kenya and Gabon showed that Schistosome-infected individuals had higher CD4+CD25hi and CD4+CD25hiFoxP3 T-cell levels in comparison with healthy control groups.92 Prominently, CD4+CD25hiFoxP3 Treg cells have a great immunologic effect on host immunity; however, future research must analyze the effect of Treg and B-cell sub-types in allergic associated immune-modulatory activities.87
Conclusion and Future Perspectives of Helminthic Therapy to Allergy
Nowadays, the prevalence of allergic diseases such as allergic rhinitis, atopic dermatitis, asthma, and exacerbated COPD has increased significantly, with allergies affecting up to 25% of the population in industrialized societies and found as a major public health burden with a high socioeconomic impact.93 Due to improved living standards, decline in family size, sanitation, and hygiene, burdens also shared by urban areas of developing countries.
Subsequently, strategies for disease intervention must aim not only at controlling signs and symptoms but also at preventing long-lasting implications.94 Despite this, in future microbiota or helminthic products or even live helminth infections will be involved in the human body as a novel therapeutic avenue for the treatment of a wide range of allergic and autoimmune diseases.95–97 The immune disorder can be treated through the deliberate infestation of helminth ova or larvae.3 It is done by choosing species with low pathogenicity and dose (inadequate threshold of pathogenesis), the immune response could be efficiently modulated, and allergic reactions can be altered in patients. More than ten clinical studies of worm therapy are currently at various steps of assessment and a few are complete and subject to analysis.98
Moreover, experimental human infection with some parasitic worms confers protection against inflammatory diseases in Phase 2 clinical trials. Parasitic worms manipulate the immune system by secreting immunoregulatory molecules that offer promise as a novel therapeutic modality for inflammatory diseases. We identify a protein secreted by hookworms, anti-inflammatory protein-2 (AIP-2), that suppressed airway inflammation in a mouse model of asthma, reduced expression of costimulatory markers on human dendritic cells (DCs), and suppressed proliferation, ex vivo, of T cells from human subjects with house dust mite allergy.99 Experimental and epidemiological evidence shows that parasites, particularly helminths, play a central role in balancing the host's immunity. It was demonstrated that parasites can modulate immune responses via their excretory/secretory (ES) and some specific proteins. Extracellular vesicles (EVs) in parasitological studies have been mostly employed for immunotherapy of autoimmune diseases, vaccination, and diagnosis. Moreover, EVs derived from helminths modulate the immune system via provoking anti-inflammatory cytokines.32,100 The most commonly used method has been with the pig whipworm T. suis, which was closely associated with the human-infective T. trichiura, and is accomplished through the delivery of T. suis ova (TSO) collected from pigs. The study found that TSO worked as a protective role for patients with inflammatory bowel disease (IBD), ulcerative colitis, and Crohn disease.3,12 From a study in the Netherlands, Schistosoma egg antigens (SEA) showed Treg expansion, Th2 modification, and IL-10 production through the TLR2-dependent pathway.101 In addition, S. mansoni eggs secrete a glycoprotein known as omega-1 inhibiting TLR-induced DC activation. Research done on the effect of ES-62 from A. viteae on mast cell degranulation, verified that ES-62 inhibits mast cell degranulation and the following inflammation by interfering with IgE binding (FcεR).102 Another study proves that the administration of a HSP60 derived protein fragment from S. japonicum (SJMHE1) to mutant TLR2 mice abolished Treg cell proliferation.103 As shown in Figure 3 stimulation of TLR2 enhances both the Treg population and its suppressive activity by releasing IL-10 and TGF-β.75 Similarly, ES products from F. hepatica (FhepES) act as a down regulator of nitric oxide (NO) and IFN-γ production by down-modulating TLR-induced DC activation, whereas production of Arg-1 was up-regulated.104
Another study, conducted in the United Kingdom, showed hookworm parasite Nectar americanus (N. americanus) therapies; N. americanus infections showed an improvement in the respiratory activities for patients with bronchial asthma.3 Similarly, the same study in Australia, illustrated that N. americanus infection can potentially reduce the immunopathology of Crohn disease.105 Taken together, the observed data showed that helminthic therapy is now emerging and some of which indicate promising immunotherapies in vivo. However, there was also a counter report which implicated tolerance induction following immunotherapy for allergic diseases. There has been growing evidence of the induction of B regulatory cells, which are capable of secreting large amounts of IL-10 following AIT treatment.106 Furthermore, a double blind, placebo controlled clinical trial of T. suis ova by Bager et al., illustrated that repeated treatment with helminth had no therapeutic effect on allergic rhinitis.98
Moreover, it needs a parasite molecular component evaluation for their efficacy and effective immunomodulators. In conclusion, chronic helminth disease can protect against allergic disorders by intensively inhibiting the host immune system, resulting in a particular T-cell hyporesponsiveness mediated through the activation of a regulatory network. Therefore, detailed characterization of ES products “secretome” as well as elements of extracellular vesicle, and understanding the mechanism of how helminthic parasites regulate host allergic reaction helps us to obtain the potential therapeutic components. In the future, considering the current research output at hand, it suggests that it is possible and promising that helminth antigens called helminthic derived products and/or live helminth will be a new therapeutic avenue and druggable targets for protection against allergic diseases.
Abbreviations
AAM, alternative activated macrophages; AD, atopic dermatitis; AMP, adenosine mono phosphate; ATP, adenosine tri phosphate; DAMP, damage-associated molecular pattern; DC, dendritic cell; HMGB1, high mobility group box 1; IgE, immunoglobulin E; IL, interleukin; ILC, innate lymphocyte cell; TGF-ß, transforming growth factor B; Th, T helper; Treg, T regulatory cell; TSO, tricuris sis ova.
Data Sharing Statement
All data used are included in the manuscript report.
Ethics Approval and Consent to Participate
Not applicable as the study was done from published articles.
Consent for Publication
Not applicable as the study was done from published articles.
Acknowledgment
We would like to thank the authors of the original article that allowed us to generate this report.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was not supported by any funding agency.
Disclosure
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
1. van Riet E, Hartgers FC, Yazdanbakhsh M. Chronic helminth infections induce immunomodulation: consequences and mechanisms. Immunobiology. 2007;212(6):475–490. doi:10.1016/j.imbio.2007.03.009
2. De Silva NR, Brooker S, Hotez PJ, et al. Soil-transmitted helminth infections: updating the global picture. Trends Parasitol. 2003;19(12):547–551. doi:10.1016/j.pt.2003.10.002
3. Maizels RM. Parasitic helminth infections and the control of human allergic and autoimmune disorders. Clin Microbiol Infect. 2016;22(6):481–486. doi:10.1016/j.cmi.2016.04.024
4. Chico ME, Vaca MG, Rodriguez A, et al. Soil‐transmitted helminth parasites and allergy: observations from Ecuador. Parasite Immunol. 2019;41(6):e12590. doi:10.1111/pim.12590
5. Ramsey N, DiNardo A, Lupinek C, et al. Helminth infection and allergic sensitization in eswati children. Ann Allergy Asthma Immunol. 2018;121(5):S57. doi:10.1016/j.anai.2018.09.185
6. Maizels RM. Infections and allergy—helminths, hygiene and host immune regulation. Curr Opin Immunol. 2005;17(6):656–661. doi:10.1016/j.coi.2005.09.001
7. Buck JC, De Leo GA, Sokolow SH. Concomitant immunity and worm senescence may drive schistosomiasis epidemiological patterns: an eco-evolutionary perspective. Front Immunol. 2020;11:160. doi:10.3389/fimmu.2020.00160
8. Patel SP, Järvelin M-R, Little MP. Systematic review of worldwide variations of the prevalence of wheezing symptoms in children. Environ Health. 2008;7(1):57. doi:10.1186/1476-069X-7-57
9. Bashir MEH, Andersen P, Fuss IJ, et al. An enteric helminth infection protects against an allergic response to dietary antigen. J Immunol. 2002;169(6):3284–3292. doi:10.4049/jimmunol.169.6.3284
10. Flohr C, Quinnell R, Britton J. Do helminth parasites protect against atopy and allergic disease? Clin Exp Allergy. 2009;39(1):20–32. doi:10.1111/j.1365-2222.2008.03134.x
11. Stewart D, Nichol A. Inflammation, immunity and allergy. Anaesth Intensive Care Med. 2018;19(10):534–539. doi:10.1016/j.mpaic.2018.08.011
12. Cruz AA, Cooper PJ, Figueiredo CA, et al. Global issues in allergy and immunology: parasitic infections and allergy. J Allergy Clin Immunol. 2017;140(5):1217–1228. doi:10.1016/j.jaci.2017.09.005
13. Allen JE, Maizels RM. Diversity and dialogue in immunity to helminths. Nat Rev Immunol. 2011;11(6):375. doi:10.1038/nri2992
14. Fitzsimmons CM, Falcone FH, Dunne DW. Helminth allergens, parasite-specific IgE, and its protective role in human immunity. Front Immunol. 2014;5:61. doi:10.3389/fimmu.2014.00061
15. Cooper PJ. Interactions between helminth parasites and allergy. Curr Opin Allergy Clin Immunol. 2009;9(1):29. doi:10.1097/ACI.0b013e32831f44a6
16. Varricchi G, Harker J, Borriello F, et al. T follicular helper (Tfh) cells in normal immune responses and in allergic disorders. Allergy. 2016;71(8):1086–1094. doi:10.1111/all.12878
17. King C, Tangye SG, Mackay CR. T follicular helper (TFH) cells in normal and dysregulated immune responses. Annu Rev Immunol. 2008;26(1):741–766. doi:10.1146/annurev.immunol.26.021607.090344
18. Lund S, Walford H, Doherty T. Type 2 innate lymphoid cells in allergic disease. Curr Immunol Rev. 2013;9(4):214–221. doi:10.2174/1573395510666140304235916
19. Scanlon ST, McKenzie AN. Type 2 innate lymphoid cells: new players in asthma and allergy. Curr Opin Immunol. 2012;24(6):707–712. doi:10.1016/j.coi.2012.08.009
20. Licona-Limón P, Kim LK, Palm NW, et al. Th 2, allergy and group 2 innate lymphoid cells. Nat Immunol. 2013;14(6):536–542. doi:10.1038/ni.2617
21. Johansson S, Hourihane JB, Bousquet J, et al. A revised nomenclature for allergy: an EAACI position statement from the EAACI nomenclature task force. Allergy. 2001;56(9):813–824. doi:10.1034/j.1398-9995.2001.t01-1-00001.x
22. Zhu N, Gong Y, Chen X, et al. Association between the polymorphisms of interleukin-4, the interleukin-4 receptor gene and asthma. Chin Med J. 2013.
23. Hagel I, Cabrera M, Hurtado M, et al. Infection by ascaris lumbricoides and bronchial hyper reactivity: an outstanding association in Venezuelan school children from endemic areas. Acta Trop. 2007;103(3):231–241. doi:10.1016/j.actatropica.2007.06.010
24. Stein M, Greenberg Z, Boaz M, et al. The role of helminth infection and environment in the development of allergy: a prospective study of newly-arrived Ethiopian immigrants in Israel. PLoS Negl Trop Dis. 2016;10(1):e0004208. doi:10.1371/journal.pntd.0004208
25. Stein M, Greenberg Z, Boaz M, Handzel ZT, Meshesha MK, Bentwich Z. The role of helminth infection and environment in the development of allergy: a prospective study of newly-arrived Ethiopian immigrants in Israel. PLoS neglected tropical diseases. 2016 Jan 11;10(1):e0004208.
26. Dagoye D, Bekele Z, Woldemichael K, et al. Wheezing, allergy, and parasite infection in children in urban and rural Ethiopia. Am J Respir Crit Care Med. 2003;167(10):1369–1373. doi:10.1164/rccm.200210-1204OC
27. Bloomfield S, Stanwell‐Smith R, Crevel R, et al. Too clean, or not too clean: the hygiene hypothesis and home hygiene. Clin Exp Allergy. 2006;36(4):402–425. doi:10.1111/j.1365-2222.2006.02463.x
28. Parker W. Reconstituting the depleted biome to prevent immune disorders. Evol Med Rev. 2010.
29. van den Biggelaar AH, Rodrigues LC, van Ree R, et al. Long-term treatment of intestinal helminths increases mite skin-test reactivity in Gabonese school children. J Infect Dis. 2004;189(5):892–900. doi:10.1086/381767
30. Nicolaou N, Siddique N, Custovic A. Allergic disease in urban and rural populations: increasing prevalence with increasing urbanization. Allergy. 2005;60(11):1357–1360. doi:10.1111/j.1398-9995.2005.00961.x
31. Strachan DP. Family size, infection and atopy: the first decade of the’hygiene hypothesis’. Thorax. 2000;55(Suppl 1):S2. doi:10.1136/thorax.55.suppl_1.S2
32. Yazdanbakhsh M, Kremsner PG, Van Ree R. Allergy, parasites, and the hygiene hypothesis. Science. 2002;296(5567):490–494. doi:10.1126/science.296.5567.490
33. Smits HH, Everts B, Hartgers FC, et al. Chronic helminth infections protect against allergic diseases by active regulatory processes. Curr Allergy Asthma Rep. 2010;10(1):3–12. doi:10.1007/s11882-009-0085-3
34. Halim TY, Steer CA, Mathä L, et al. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity. 2014;40(3):425–435. doi:10.1016/j.immuni.2014.01.011
35. Wilson MS, Cheever AW, White SD, et al. IL-10 blocks the development of resistance to re-infection with Schistosoma mansoni. PLoS Pathog. 2011;7(8):e1002171. doi:10.1371/journal.ppat.1002171
36. Buonocore S, Ahern PP, Uhlig HH, et al. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature. 2010;464(7293):1371. doi:10.1038/nature08949
37. Fallon PG, Mangan NE. Suppression of T H 2-type allergic reactions by helminth infection. Nat Rev Immunol. 2007;7(3):220. doi:10.1038/nri2039
38. Medeiros M
39. Helmby H. Helminths and our immune system: friend or foe? Parasitol Int. 2009;58(2):121–127. doi:10.1016/j.parint.2009.02.001
40. Else K, Finkelman F, Maliszewski C, et al. Cytokine-mediated regulation of chronic intestinal helminth infection. J Exp Med. 1994;179(1):347–351. doi:10.1084/jem.179.1.347
41. Babu S, Nutman TB. Immune responses to helminth infection. Clin Immunol. 2019;437–447e431.
42. Artis D, Grencis R. The intestinal epithelium: sensors to effectors in nematode infection. Mucosal Immunol. 2008;1(4):252–264. doi:10.1038/mi.2008.21
43. Lambrecht BN, Hammad H. The airway epithelium in asthma. Nat Med. 2012;18(5):684. doi:10.1038/nm.2737
44. von Moltke J, Ji M, Liang H-E, et al. Tuft-cell-derived IL-25 regulates an intestinal ILC2–epithelial response circuit. Nature. 2016;529(7585):221–225. doi:10.1038/nature16161
45. Fallon PG, Ballantyne SJ, Mangan NE, et al. Identification of an interleukin (IL)-25–dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J Exp Med. 2006;203(4):1105–1116. doi:10.1084/jem.20051615
46. Neill DR, Wong SH, Bellosi A, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464(7293):1367–1370. doi:10.1038/nature08900
47. Lopetuso LR, Scaldaferri F, Pizarro TT. Emerging role of the interleukin (IL)-33/ST2 axis in gut mucosal wound healing and fibrosis. Fibrogenesis Tissue Repair. 2012;5(1):18. doi:10.1186/1755-1536-5-18
48. Saenz SA, Taylor BC, Artis D. Welcome to the neighborhood: epithelial cell‐derived cytokines license innate and adaptive immune responses at mucosal sites. Immunol Rev. 2008;226(1):172–190. doi:10.1111/j.1600-065X.2008.00713.x
49. Ramalingam TR, Pesce JT, Mentink-Kane MM, et al. Regulation of helminth-induced Th2 responses by thymic stromal lymphopoietin. J Immunol. 2009;182(10):6452–6459. doi:10.4049/jimmunol.0900181
50. Saenz SA, Siracusa MC, Perrigoue JG, et al. IL25 elicits a multipotent progenitor cell population that promotes TH 2 cytokine responses. Nature. 2010;464(7293):1362–1366.
51. Price AE, Liang H-E, Sullivan BM, et al. Systemically dispersed innate IL-13–expressing cells in type 2 immunity. Proc Natl Acad Sci. 2010;107(25):11489–11494. doi:10.1073/pnas.1003988107
52. Barlow JL, McKenzie AN. Nuocytes: expanding the innate cell repertoire in type‐2 immunity. J Leukoc Biol. 2011;90(5):867–874. doi:10.1189/jlb.0311160
53. Gronke K, Diefenbach A. Tuft cell-derived IL-25 activates and maintains ILC2. Immunol Cell Biol. 2016;94(3):221. doi:10.1038/icb.2016.10
54. Finkelman FD, Shea-Donohue T, Goldhill J, et al. Cytokine regulation of host defense against parasitic gastrointestinal nematodes: lessons from studies with rodent models. Annu Rev Immunol. 1997;15(1):505–533. doi:10.1146/annurev.immunol.15.1.505
55. Mentink-Kane MM, Cheever AW, Thompson RW, et al. IL-13 receptor α 2 down-modulates granulomatous inflammation and prolongs host survival in schistosomiasis. Proc Natl Acad Sci. 2004;101(2):586–590. doi:10.1073/pnas.0305064101
56. Butterworth A, Hagan P. Immunity in human schistosomiasis. Parasitol Today. 1987;3(1):11–16. doi:10.1016/0169-4758(87)90091-3
57. Hoffmann KF, Wynn TA, Dunne DW. Cytokine-mediated host responses during schistosome infections; walking the fine line between immunological control and immunopathology. Adv Parasitol. 2002;52:265–307.
58. Maizels RM, McSorley HJ. Regulation of the host immune system by helminth parasites. J Allergy Clin Immunol. 2016;138(3):666–675. doi:10.1016/j.jaci.2016.07.007
59. Akdis CA, Blaser K. Mechanisms of interleukin‐10‐mediated immune suppression. Immunology. 2001;103(2):131–136. doi:10.1046/j.1365-2567.2001.01235.x
60. Maizels RM, McSorley HJ, Smyth DJ. Helminths in the hygiene hypothesis: sooner or later? Clin Exp Immunol. 2014;177(1):38–46. doi:10.1111/cei.12353
61. Zakeri A, Hansen EP, Andersen SD, et al. Immunomodulation by helminths: intracellular pathways and extracellular vesicles. Front Immunol. 2018;9:2349.
62. Weinstock JV, Elliott DE. Helminth infections decrease host susceptibility to immune-mediated diseases. J Immunol. 2014;193(7):3239–3247. doi:10.4049/jimmunol.1400927
63. van der Veeken J, Gonzalez AJ, Cho H, et al. Memory of inflammation in regulatory T cells. Cell. 2016;166(4):977–990. doi:10.1016/j.cell.2016.07.006
64. Kitani A, Fuss I, Nakamura K, et al. Transforming growth factor (TGF)-β1–producing regulatory T cells induce Smad-mediated interleukin 10 secretion that facilitates coordinated immunoregulatory activity and amelioration of TGF-β1–mediated fibrosis. J Exp Med. 2003;198(8):1179–1188. doi:10.1084/jem.20030917
65. Castellani M, Anogeianaki A, Felaco P, et al. IL-35, an anti-inflammatory cytokine which expands CD4+ CD25+ treg cells. J Biol Regul Homeost Agents. 2010.
66. Schmidt A, Oberle N, Krammer PH. Molecular mechanisms of treg-mediated T cell suppression. Front Immunol. 2012;3:51. doi:10.3389/fimmu.2012.00051
67. Satoguina JS, Weyand E, Larbi J, et al. T regulatory-1 cells induce IgG4 production by B cells: role of IL-10. J Immunol. 2005;174(8):4718–4726. doi:10.4049/jimmunol.174.8.4718
68. Meiler F, Klunker S, Zimmermann M, et al. Distinct regulation of IgE, IgG4 and IgA by T regulatory cells and toll‐like receptors. Allergy. 2008;63(11):1455–1463. doi:10.1111/j.1398-9995.2008.01774.x
69. Erb KJ. Helminths, allergic disorders and IgE‐mediated immune responses: where do we stand? Eur J Immunol. 2007;37(5):1170–1173. doi:10.1002/eji.200737314
70. Totura AL, Whitmore A, Agnihothram S, et al. Toll-like receptor 3 signaling via TRIF contributes to a protective innate immune response to severe acute respiratory syndrome coronavirus infection. MBio. 2015;6(3):e00638–00615. doi:10.1128/mBio.00638-15
71. Le Goffic R, Balloy V, Lagranderie M, et al. Detrimental contribution of the toll-like receptor (TLR) 3 to influenza A virus–induced acute pneumonia. PLoS Pathog. 2006;2(6):e53. doi:10.1371/journal.ppat.0020053
72. Takeda K, Akira S. TLR signaling pathways. In: Seminars in Immunology. Elsevier; 2004:3–9.
73. Loebrich S. The role of F-actin in modulating Clathrin-mediated endocytosis: lessons from neurons in health and neuropsychiatric disorder. Commun Integr Biol. 2014;7(3):e28740. doi:10.4161/cib.28740
74. van der Kleij D, Latz E, Brouwers JF, et al. A novel host-parasite lipid cross-talk. J Biol Chem. 2002;277(50):48122–9
75. Wang X, Zhou S, Chi Y, et al. CD4+ CD25+ Treg induction by an HSP60‐derived peptide SJMHE1 from Schistosoma japonicum is TLR2 dependent. Eur J Immunol. 2009;39(11):3052–3065. doi:10.1002/eji.200939335
76. Zakeri A, Borji H, Haghparast A. Interaction between helminths and toll-like receptors: possibilities and potentials for asthma therapy. Int Rev Immunol. 2016;35(3):219–248. doi:10.3109/08830185.2015.1096936
77. Coltherd J, Rodgers D, Lawrie R, et al. The parasitic worm-derived immunomodulator, ES-62 and its drug-like small molecule analogues exhibit therapeutic potential in a model of chronic asthma. Sci Rep. 2016;6(1):19224. doi:10.1038/srep19224
78. Melendez AJ, Harnett MM, Pushparaj PN, et al. Inhibition of FcεRI-mediated mast cell responses by ES-62, a product of parasitic filarial nematodes. Nat Med. 2007;13(11):1375–1381. doi:10.1038/nm1654
79. Al-Riyami L, Pineda MA, Rzepecka J, et al. Designing anti-inflammatory drugs from parasitic worms: a synthetic small molecule analogue of the Acanthocheilonema viteae product ES-62 prevents development of collagen-induced arthritis. J Med Chem. 2013;56(24):9982–10002. doi:10.1021/jm401251p
80. Harnett W, Harnett MM, Leung BP, et al. The anti-inflammatory potential of the filarial nematode secreted product, ES-62. Curr Top Med Chem. 2004;4(5):553–559. doi:10.2174/1568026043451212
81. Smits HH, Hammad H, van Nimwegen M, et al. Protective effect of Schistosoma mansoni infection on allergic airway inflammation depends on the intensity and chronicity of infection. J Allergy Clin Immunol. 2007;120(4):932–940. doi:10.1016/j.jaci.2007.06.009
82. Satoguina J, Mempel M, Larbi J, et al. Antigen-specific T regulatory-1 cells are associated with immunosuppression in a chronic helminth infection (onchocerciasis). Microbes Infect. 2002;4(13):1291–1300. doi:10.1016/S1286-4579(02)00014-X
83. Jackson JA, Friberg IM, Little S, et al. Review series on helminths, immune modulation and the hygiene hypothesis: immunity against helminths and immunological phenomena in modern human populations: coevolutionary legacies? Immunology. 2009;126(1):18–27. doi:10.1111/j.1365-2567.2008.03010.x
84. Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32(5):593–604. doi:10.1016/j.immuni.2010.05.007
85. Popovic PJ, Zeh HJ
86. Choi BS, Martinez‐Falero IC, Corset C, et al. Differential impact of l‐arginine deprivation on the activation and effector functions of T cells and macrophages. J Leukoc Biol. 2009;85(2):268–277. doi:10.1189/jlb.0508310
87. Lynch NR, Hagel IA, Palenque ME, et al. Relationship between helminthic infection and IgE response in atopic and nonatopic children in a tropical environment. J Allergy Clin Immunol. 1998;101(2):217–221. doi:10.1016/S0091-6749(98)70386-0
88. Rieger A, Bar-Or A. B-cell-derived interleukin-10 in autoimmune disease: regulating the regulators. Nat Rev Immunol. 2008;8(6):486–487. doi:10.1038/nri2315-c1
89. Wördemann M, Junco Diaz R, Menocal Heredia L, et al. Association of Atopy, Allergic Rhinoconjunctivitis, Atopic Dermatitis and Intestinal Helminth Infections in Cuban Children. 2008.
90. Janson PC, Winerdal ME, Marits P, et al. FOXP3 promoter demethylation reveals the committed treg population in humans. PLoS One. 2008;3(2):e1612. doi:10.1371/journal.pone.0001612
91. Cooper P. Toxocara canis infection: an important and neglected environmental risk factor for asthma? This editorial discusses the findings of the paper in this issue. Clin Exp Allergy. 2008;38(4):551–553. doi:10.1111/j.1365-2222.2008.02934.x
92. Hankin CS, Cox L, Lang D, et al. Allergy immunotherapy among medicaid-enrolled children with allergic rhinitis: patterns of care, resource use, and costs. J Allergy Clin Immunol. 2008;121(1):227–232. doi:10.1016/j.jaci.2007.10.026
93. Pinart M, Albang R, Maier D, et al. Systematic review on the definition of allergic diseases in children: the MeDALL study. Int Arch Allergy Immunol. 2015;168(2):110–121. doi:10.1159/000442414
94. Stiemsma LT, Reynolds LA, Turvey SE, et al. The hygiene hypothesis: current perspectives and future therapies. ImmunoTargets Ther. 2015;4:143. doi:10.2147/ITT.S61528
95. Weinstock JV, Summers RW, Elliott DE. Role of helminths in regulating mucosal inflammation. In: Springer Seminars in Immunopathology. Springer; 2005:249–271.
96. Tan LD, Schaeffer B, Alismail A. Parasitic (helminthic) infection while on asthma biologic treatment: not everything is what it seems. J Asthma Allergy. 2019;12:415. doi:10.2147/JAA.S223402
97. Khan AR, Fallon PG. Helminth therapies: translating the unknown unknowns to known knowns. Int J Parasitol. 2013;43(3–4):293–299. doi:10.1016/j.ijpara.2012.12.002
98. Bager P, Arnved J, Rønborg S, et al. Trichuris suis ova therapy for allergic rhinitis: a randomized, double-blind, placebo-controlled clinical trial. J Allergy Clin Immunol. 2010;125(1):123–130e123. doi:10.1016/j.jaci.2009.08.006
99. Navarro S, Pickering DA, Ferreira IB, et al. Hookworm recombinant protein promotes regulatory T cell responses that suppress experimental asthma. Sci Transl Med. 2016;8(362):362ra143–362ra143. doi:10.1126/scitranslmed.aaf8807
100. Khosravi M, Mirsamadi ES, Mirjalali H, et al. Isolation and functions of extracellular vesicles derived from parasites: the promise of a new era in immunotherapy, vaccination, and diagnosis. Int J Nanomedicine. 2020;15:2957. doi:10.2147/IJN.S250993
101. Everts B, Perona-Wright G, Smits HH, et al. Omega-1, a glycoprotein secreted by Schistosoma mansoni eggs, drives Th2 responses. Journal of Experimental Medicine. 2009;206(8):1673–1680.
102. Bell KS, Al-Riyami L, Lumb FE, et al. The role of individual protein kinase C isoforms in mouse mast cell function and their targeting by the immunomodulatory parasitic worm product, ES-62. Immunol Lett. 2015;168(1):31–40. doi:10.1016/j.imlet.2015.09.001
103. Wang X, Wang J, Liang Y, et al. Schistosoma japonicum HSP60-derived peptide SJMHE1 suppresses delayed-type hypersensitivity in a murine model. Parasit Vectors. 2016;9(1):147.
104. Falcón CR, Masih D, Gatti G, et al. Fasciola hepatica kunitz type molecule decreases dendritic cell activation and their ability to induce inflammatory responses. PLoS One. 2014;9(12):e114505. doi:10.1371/journal.pone.0114505
105. Croese J, O’neil J, Masson J, et al. A proof of concept study establishing necator americanus in Crohn’s patients and reservoir donors. Gut. 2006;55(1):136–137. doi:10.1136/gut.2005.079129
106. Flohr C, Tuyen L, Quinnell R, et al. Reduced helminth burden increases allergen skin sensitization but not clinical allergy: a randomized, double‐blind, placebo‐controlled trial in Vietnam. Clin Exp Allergy. 2010;40(1):131–142.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.