Back to Journals » Cancer Management and Research » Volume 12
Heavy Water Affects Vital Parameters of Human Melanoma Cells in vitro
Authors Kleemann J, Reichenbach G, Zöller N, Jäger M, Kaufmann R, Meissner M , Kippenberger S
Received 13 September 2019
Accepted for publication 24 January 2020
Published 17 February 2020 Volume 2020:12 Pages 1199—1209
DOI https://doi.org/10.2147/CMAR.S230985
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Johannes Kleemann,* Gabi Reichenbach,* Nadja Zöller, Manuel Jäger, Roland Kaufmann, Markus Meissner, Stefan Kippenberger
Department of Dermatology, Venereology and Allergy, Johann Wolfgang Goethe University, Frankfurt/Main, Germany
*These authors contributed equally to this work
Correspondence: Stefan Kippenberger
Department Of Dermatology, Venereology and Allergy, Johann Wolfgang Goethe University, Theodor-Stern-Kai 7, Frankfurt/Main D-60590, Germany
Email [email protected]
Purpose: Although regular water is composed of two hydrogens and one oxygen, referred to as H2O, a small amount of water on this planet contains alternative forms of elements with different molecular weights because of the addition of neutrons. The present study was dedicated to studying the effect of heavy water (D2O), in which the two hydrogens become substituted by deuterium, on the cell physiology of different human cells with particular focus on malignant melanoma cells.
Methods: Cells were cultured in regular medium in which the content of H2O was gradually substituted by D2O or deuterium-depleted water (DDW). Following this, the changes of basic cellular parameters, such as morphology, migration, proliferation, cell cycle, apoptosis and microtubule integrity were examined.
Results: It was found that raising the D2O content above the standard levels led to a concentration-dependent decrease in proliferation. Lowering the D2O levels below this level had no effect. Likewise, elevated D2O levels hampered migration. Moreover, cell-cycle analysis showed an increase of sub-G1 cells. Corroboratively, markers for apoptosis were induced (histone-associated DNA fragments, Bax, and PARP). In regard to microtubule integrity, only very high levels of D2O (75%) caused partial filament condensation.
Conclusion: D2O, although chemically identical with H2O, shows proapoptotic and antiproliferative effects on melanoma cells. These findings give a closer look of this interesting compound.
Keywords: deuterium oxide, heavy water, melanoma, apoptosis, isotypes
Introduction
Although water is omnipresent in all physiological processes, it is mostly considered as an unavoidable medium for enzymatic reactions and not as a promising therapeutic target. In the following, we introduce an idea of how to interfere with cellular water and how to use this for therapeutic purposes.
Regular water is a molecule composed of two hydrogens and one oxygen referred to as H2O. In addition to this, a small amount is composed of stable isotopes, which are alternative forms of elements with different atomic weights because of the addition of neutrons. For hydrogen (1H) which has a relative abundance of 99.985%, there exists the heavier isotope 2H, known as deuterium, with an abundance of 0.015%. For regular oxygen (16O) the relative abundance is 99.759%, the two stable isotopes, 17O and 18O, have an abundance of 0.037% and 0.204%, respectively.1 In the following, we focus on the role of deuterium, which can substitute one or both hydrogens in the water molecule (HDO, D2O). The content of deuterium in surface water from different regions varies slightly from 0.0130% in arctic surface water to 0.0162% in the river Nile in Egypt.2,3 Consequently, a small amount of deuterium-containing water is present in living matter. Although isotopes are chemically identical, they differ in their physical properties. Of note, it was observed that changes in the natural content of deuterization exert physiological responses. Some organisms such as some algae, bacteria, yeasts and molds can be propagated in media consisting of almost 100% D2O, however with lower growth rates and phenotypic alterations. In contrast, higher plants and animals do not survive a full exchange of hydrogen by deuterium.4 Rats given a regular diet with 50% D2O in tap water showed severe generalized symptoms after 10 days. At this time the amount of D2O in the body water was 15%. At exchange rates higher than 30% rats become comatose.5 Besides rats, a body water deuterization of 30–40% was shown to be lethal also in mice and dogs.6 However, several studies revealed that a moderate body deuterization had little or no adverse effects and was well tolerated by mice and rats.7
In humans, the amount of total body water can change inter-individually with age, body weight, obesity and sex.8 As a rough estimate, an amount of 50 to 60% of the total body weight can be assumed.9 Humans supplied with 140–250g D2O, corresponding to 0.5% of total body water, show only temporary symptoms such as vertigo, maybe due to perturbations in the vestibular fluid by heavy water.6 In a more recent paper using D2O as a tracer to study muscle metabolism, humans were given boluses of D2O to achieve an amount of 0.4 to 0.8% within the body water.10 No side effects were reported.
Because of its growth inhibitory effects, D2O seems to be an interesting compound in tumor therapy. In this paper, we focus on melanoma, which is still a therapeutically challenging condition despite the progress in treatment emerging in the recent years. In an in vitro attempt, we evaluated the susceptibility of melanoma cells on different amounts of D2O in the culture medium.
Materials and Methods
Ethics Statement
This study was conducted according to the Declaration of Helsinki Principles and in agreement with the Local Ethic Commission of the faculty of Medicine of the Johann Wolfgang Goethe University (Frankfurt am Main, Germany). The Local Ethic Commission waived the need for consent.
Cell Culture and Application of D2O
The malignant melanoma cell lines A375, SK-Mel-28 and SK-Mel-30 (all from ATCC) and normal human skin fibroblasts were cultured in Dulbecco’s modified Eagle’s medium (DMEM, high glucose, Gibco, Karlsruhe, Germany) with 5% fetal calf serum (FCS, Gibco, Karlsruhe Germany) and 1% penicillin–streptomycin solution (Biochrom KG, Berlin, Germany) and 1% Asp (L-ascorbic acid 2-phosphate, Sigma-Aldrich, Taufkirchen, Germany). Normal human melanocytes isolated from foreskins were propagated in DermaLife M medium (CellSystem, Troisdorf, Germany). All cells were held at 37°C in a 5% CO2 atmosphere. The medium was renewed twice a week. In order to study the effect of heavy water, powdered DMEM medium was reconstituted using deuterium dioxide (D2O, 99.9%) or deuterium-depleted water (DDW, D2O ≤ 1ppm), both from Sigma-Aldrich (Taufkirchen, Germany), to the indicated concentrations.
Cell Proliferation Assay
Cells were seeded in 96-microwell plates at a density of 1.0×104 cells/per well. After 24 h the media were exchanged to the indicated D2O concentrations. For the last 16 hrs, cells were pulsed with 5-bromo-2ʹ-deoxyuridine (BrdU). Subsequently, the incorporation rate of BrdU was determined using a commercial enzyme-linked immunosorbent assay (ELISA) kit (Roche, Mannheim, Germany). Briefly, cells were fixed and immune complexes were formed using peroxidase-coupled BrdU-antibodies. A colorimetric reaction with tetramethylbenzidine (TMB) as a substrate gave rise to a reaction product measured at 450 nm in a scanning multiwell spectrophotometer (ELISA-Reader ASYS Expert 96, Deelux Labortechnik, Gödenstorf, Germany).
Immunocytochemistry
Coverslips were placed into 12-well plates and cells were seeded at a density of 6×104 cells per well in 1.5mL DMEM high glucose for 24 hrs at 37°C and 5% CO2. Cells were treated with different D2O concentrations for 24 hrs at 37°C and 5% CO2. For displaying microtubules, cells were fixed with 4% paraformaldehyde (PFA) for 10 mins at room temperature. After removal of PFA, cells were washed 3 times with cold PBS for 5 mins each and then permeabilized with 0.1% Triton-X-100 in PBS (10 mins, RT). Unspecific antibody-binding sites were blocked with 1% BSA in 0.1% Tween 20 in PBS for 30 mins at RT. Microtubule antibody anti-human alpha-tubulin (DM1A, Cell Signaling, Frankfurt, Germany) was diluted 1:1000 in phosphate-buffered saline (PBS) with 1% bovine serum albumin (BSA) and applied to the cells over night at 4°C. After three washes with PBS, cells were incubated with alexa 546-coupled goat anti-mouse (life technologies, Darmstadt Germany, 1:100 in PBS) for 60 mins at RT. Nuclei were stained with DAPI for 1 min. Indirect immunofluorescence was examined with the Axioskop (Zeiss, Jena, Germany).
Morphologic Analysis and Migration Assay
Cells were seeded at a cell density of 3×105 cells/mL in 12-well plates. Consecutively, the medium was replaced with the indicated D2O concentrations. Live cell microscopy of the scratch closure was performed every 2 hrs for up to 24 hrs with an incubator microscope unit (IncuCyte ZOOM, EssenBioScience, Hertfordshire, UK). In order to study the migration of cells standardized scratches were applied to confluent cultures by using pipettor tips. The closure of the cell-free space was analyzed using the ImageJ software.11
Histone-Associated DNA Fragments
Apoptosis was quantified on the basis of cytoplasmic histone-associated DNA fragments using the Cell Death Detection ELISA (Roche, Mannheim, Germany) according to the manufacturer’s manual. In brief, cells were cultured in microwell plates (2×104 cells per 0.33cm2) and treated with the indicated D2O concentrations. After 24 h cytosolic fraction (200 g supernatant) was used as antigen source in a sandwich enzyme-linked immunosorbent assay with primary anti-histone antibody coated to a microtiter plate and secondary anti-DNA antibody coupled to peroxidase. Optical density was measured at 530nm in an ELISA reader (ELISA-Reader ASYS Expert 96, Deelux Labortechnik, Gödenstorf, Germany).
Western Blot Analysis
Protein extracts of cells treated with D2O for 48 hrs were separated by SDS-polyacrylamide gels and electroblotted as described.12 Membranes were incubated with the following primary antibodies: Bcl-2, Bcl-XL, Bax, AIF, cleaved PARP, surviving (all purchased from Cell Signaling, Frankfurt, Germany) and actin (Heidelberg, Santa Cruz). Primary antibody application was followed by incubation with horseradish peroxidase-conjugated secondary antibodies (anti-mouse and anti-rabbit IgG, Amersham, Uppsala, Sweden). Blots were developed using an enhanced chemiluminescence detection system (ECL) (Amersham) according to the manufacturer’s instructions.
Annexin-V-Fluorescein Isothiocyanate/Propidium Iodide (AV-FITC/PI) Double-Staining Assay
9×104 cells per were seeded in a 12-well plate for 24 hrs at 37°C and 5% CO2 and treated with D2O as indicated for 24 hrs. Early apoptosis and late apoptosis were detected by using TACS Annexin V-FITC Kit (Trevigen, Gaithersburg, MD, USA) according to the manufacturer´s protocol. Briefly, all cells were collected by centrifugation at 300g for 5 mins at RT and pellet was washed once with 500µL cold PBS and the centrifugation step was repeated. 100µL Annexin V/propidium iodide incubation reagent was applied to the cell pellet for 15 mins in the dark at RT and 400µL 1× binding buffer was applied. Analysis of Annexin V/propidium iodide was assessed by a BD FACScan Cytometer (Becton Dickinson, Franklin Lakes, NJ, USA) within 1 hr.
Fluorescence-Activated Cell Sorting Analysis
Cells were incubated with D2O as indicated for 24 hrs. Nocodazole (1µg/mL, applied for 2 hrs) was used as positive control to induce G2/M arrest. After fixation in ice-cold 70% ethanol cells were incubated in PBS containing 40g/mL RNase A for 30 mins at 37°C and resuspended in PBS containing 50g/mL propidium iodide. Analysis of cell cycle was assessed by a FACScan Cytometer (Becton Dickinson, Franklin Lakes, NJ, USA) as described.13
Statistical Analysis
All data are presented as mean values ± standard deviation. Statistical significance of the data was calculated by Mann–Whitney-Rank Sum test (BIAS, Frankfurt, Germany). Each set of data was related to the referring control or as indicated in the legends.
Results
In order to study the effect of D2O on melanoma cells, a set of culture media were tested which differ in their D2O content. Medium with a content of 0.014% D2O represents control condition.
In Figure 1 the effect of different D2O contents on cell morphology of A375 melanoma cells is displayed after 24, 48 and 72 hrs. Besides control conditions culture medium with ≤1ppm D2O, also referred as deuterium-depleted water (DDW), with 50% D2O and 100% D2O were tested. Cells cultured in DDW show a similar morphology over the recorded timespan as their counterparts cultured under control conditions with 0.014% D2O. Cells held in 50% D2O show already after 24 hrs a decrease in cell count and changes in cell morphology. In particular, a considerable part of the cells became spherical and detached from the support. This effect became more distinct in cultures held in 100% D2O. After 24 hrs a small amount of cells shows the typical morphology of vital A375 cells, the rest becomes spherical and detached. After 48 and 72 hrs no viable cells were observed.
Consecutively, the effect of D2O on DNA synthesis was tested in different melanoma cells (Figure 2). Lowering the amount of D2O shows no significant effect on DNA synthesis as measured by incorporation of 5-bromo-2´-deoxyuridine (BrdU) in the DNA in A375 cells (Figure 2A), SK-Mel-28 (Figure 2B) and SK-Mel-30 (Figure 2C). In contrast, increased levels of D2O above the normal range offer inhibition of DNA synthesis. In A375 cells already 5% D2O showed a significant decrease in DNA synthesis. Cultivation in 100% D2O decreased the level to 18% (EC50: 41.3% D2O, calculated according to14). In SK-Mel-28 75% D2O showed a significant inhibition; at 100% D2O the DNA-synthesis was inhibited to 74% (EC50: 44.6% D2O). In SK-Mel-30 cells a slight but significant reduction was present at 75% D2O; at 100% D2O DNA-synthesis was reduced to 25% (EC50: 79.9% D2O). In order to capture D2O effects on non-malignant cells normal human melanocytes and fibroblasts were tested (Figure S1). It was found that D2O also offers anti-proliferative effects in fibroblasts (Figure S1A; EC50: 45.6% D2O). Interestingly, in melanocytes D2O shows no anti-proliferative effect (Figure S1B; EC50: 171.5% D2O).
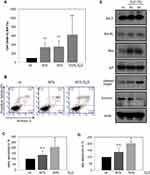
From the above-described experiments, it became evident that D2O inhibits DNA-synthesis and also leads to dramatic morphological changes. These findings indicate that D2O triggers processes in the context of cell death. Therefore, A375 melanoma cells, which show the clearest effect in response to D2O, were tested on apoptosis (Figure 3). It was found that D2O containing medium induced cytoplasmic histone-associated DNA fragments (Figure 3A) which indicates advanced apoptosis.15 In 40% D2O the amount of cytoplasmic histone-associated DNA fragments was increased to 344%, in 50% D2O to 355% and in 100% D2O to 625%. In order to discriminate between vital, necrotic, and apoptotic cells, cells were labelled with fluorochrome-labeled Annexin V and propidium iodide and analyzed by FACS (Figure 3B). In this presentation the cell count of the lower right quadrant represents early apoptotic cells; cell count of the upper right quadrant represents late apoptotic cells. The summary of 4 independent experiments regarding early apoptosis shows an increase of 135% (40% D2O) and 208% (50% D2O), respectively (Figure 3C). For late apoptosis an increase of 138% (40% D2O) and 203% (50% D2O) was detected (Figure 3D). In order to learn more about the molecular processes involved in D2O-stimulated apoptosis Western blot analysis was performed using a panel of pro- and anti-apoptotic markers (Figure 3E). Compared to control conditions with 0.014% D2O cells cultured with 40 and 50% D2O feature higher levels anti-apoptotic Bcl-2 and Bcl-XL but also lower levels of survivin. Moreover, pro-apoptotic Bax and cleaved PARP were elevated. The level of caspase-independent AIF was unchanged. In sum, these results speak for a complex regulation of pro-apoptotic and also counter-regulating effectors.
After giving proof for the induction of apoptosis it was investigated whether increased levels of D2O have also impact on the cell cycle (Figure 4). Analysis of cell cycle distribution was investigated by FACS using propidium iodide staining to determine the cellular DNA content (Figure 4A–D). Summarized results given in Figure 4E show no significant alterations in the amount of cells in the S- and G2/M-phase by D2O (40% and 50%) compared to control conditions (0.014% D2O). However, the amount of G1/G0 cells decreased with a concomitant increase of sub-G1 cells. The latter finding is in concert with the above described pro-apoptotic action of D2O.
In order to study a possible effect of D2O on tumor dissemination, a migration assay was performed (Figure 5). After application of standardized scratches closure of the cell-free space was monitored by live cell microscopy for 24 hrs (Figure 5A). Quantitative measurements of gap closure demonstrated an inhibition to circa 20% by D2O (40% and 50%) compared to control conditions (0.014% D2O) (Figure 5B).
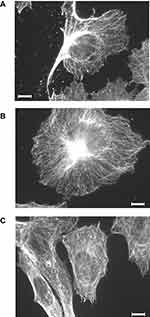
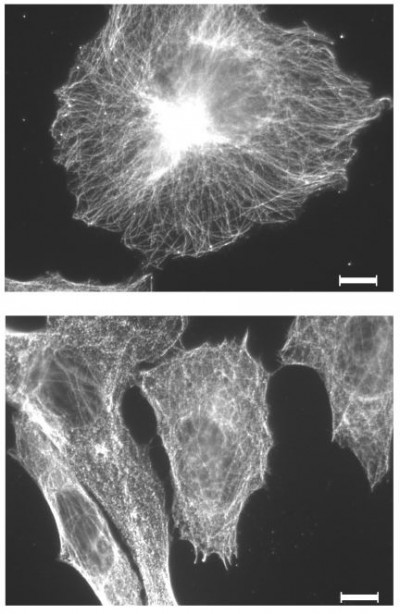
Several studies describe an effect of D2O on microtubule stability in cell-free systems. Therefore, the effect of D2O on the microtubule filament was investigated in in vitro cultured melanoma cells. Indirect immunofluorescence against alpha-tubulin displayed a finely woven maze under control conditions (0.014% D2O) (Figure 6A). Treatment with 50% D2O for 24 hrs had no visible impact on the microtubule organization (Figure 6B). An increase to 75% D2O lead to a partially blurred picture indicating microtubule disintegration with small anti-tubulin reactive aggregates (Figure 6C).
Discussion
In the present study, we investigated the effect of D2O on basal physiological parameters in melanoma cells. Increasing the D2O concentration above the normal range causes a massive decrease of cells at 50% D2O already after 24 hrs as detected by live microscopy; a total replacement of regular H2O by D2O leads to complete cell disruption. The growth inhibitory effect of D2O was further substantiated by showing a concentration-dependent decrease of DNA synthesis. This suits well to the anti-tumor effect of D2O which was detected in a colon and SCC xenograft tumor model in mice. Here the drinking water was replaced by 30% D2O, which reduced the tumor growth in a range from 22% to 65% compared to the controls after approximately 30 days. The D2O concentration in the serum was 16%.16 Also, a protective effect of D2O on tumor growth was described. Mice which were supplied with 30% D2O drinking water for 7 days and then inoculated with pancreatic cancer cells developed smaller tumors.17 However, this effect was only transient.
Interestingly, also a decrease of the D2O concentration below the normal range using DDW was shown to have anti-tumor effects.18–20 It is thought that DDW diminishes the amount of deuterium of sugar-phosphates in the DNA backbone stabilizing the hydrogen bond network and therefore protecting against aneuploidy and strand breaks.21 In our model using melanoma cells, we found no effects of DDW in regard to cell morphology and DNA synthesis. Therefore, we focused on the following only on effects caused by elevated D2O levels. In order to characterize growth inhibition by D2O cell cycle distribution and apoptosis were analyzed. In the former case, no specific cell cycle arrest was found. Others found cell accumulation in the G2/M phase in a model using other malignant cells22,23 speaking for a cell-specific mode of action. Confirmatory, an upregulation of apoptosis was found in some cell lines and in others not.23 However, in our model using A375 cells a significant increase of cytoplasmic histone-associated DNA fragments, which is typical for advanced apoptosis, was found. Also, Annexin V, an early apoptosis marker, was increased by D2O. However, besides the upregulation of pro-apoptotic markers (Bax, cleaved PARP) and the downregulation of an anti-apoptotic marker (Survivin) also some anti-apoptotic markers became slightly upregulated (Bcl-2, Bcl-XL) which speaks for a complex regulation of pro- and anti-apoptotic cascades which in sum shifted the equilibrium towards apoptosis.
In order to specify the anti-tumor effect of D2O cell migration, which plays an important role in tumor dissemination, was regarded. Utilizing a scratching assay shows a significantly reduced cell migration. This finding is in concert with others who found a suppression of cell locomotion by D2O in mouse fibroblasts.24 Cell shape and cell migration are controlled by filaments of the cytoskeleton. Besides the microfilament and the intermediate filament also microtubules contribute to polarized cell migration.25 Microtubules are of particular interest in this context as it was found that D2O enhances tubulin polymerization in a cell-free system.26 In cultured cells, it was found that D2O promotes the assembly of microtubules from tubulin and stabilized them.27 Moreover, also stabilization of the spindle apparatus was found in the eggs of the sea urchin resulting in a mitosis halt.27,28 In general, D2O seems to stabilize microtubule and make them more resistant towards exogenous noxa reversing effects of compounds interacting with microtubule stability (demecolcine, depolymerization; taxol, microtubule stabilization).29,30 The sensitivity of microtubules against D2O might explain the overall resistance of prokaryotes. Our results with melanoma cells also display a well-ordered microtubule network at 50% D2O, which may be more rigid than under control conditions explaining the decreased migratory activity. However, a further increase in the D2O concentration up to 75% caused a partial disruption of the fine woven microtubule filament. The manifestation of anti-tubulin reactive aggregates speaks for a general toxic effect.
In sum, our results demonstrate that a D2O, a chemically identical isotope of H2O exerts massive effects on pivotal cell parameters such as growth and apoptosis. However, the sensitivity towards D2O differed markedly between the different melanoma cells tested. Surprisingly, D2O shows, in contrast to the melanoma cells tested, no anti-proliferative effect in normal human melanocytes. If D2O is in general more effective in malignant cells should be investigated in future studies. However, experiments with fibroblasts speak against a tumor-specific action of D2O. Hence, possible adverse health effects have to be carefully evaluated before considering a clinical application. It is an interesting issue to identify cellular determinants that correspond to the sensitivity against D2O. First indications suggest that Aquaporin-11 is relevant for the transmission of the effect.31 In sum, in tumors with a high sensitivity towards D2O an exchange of normal water by D2O may be therapeutic option particularly in combination with other measures.
Conclusion
Commonly most active substances are developed on the basis of chemical structures. Here we show that a compound, chemically identical with regular water, but different on the physical level, has an impact on melanoma cell physiology. These findings suggest a closer look at stable isotypes. The replacement of regular atoms in a lead compound by one or more stable isotypes may lead to new and more effective molecules. Although the mechanism of the D2O effect is not clear, it could be speculated that the deuterium ion interferes with the Akt hydrogen network.32
Disclosure
The authors report no conflicts of interest in this work.
References
1. Eheringer JR, Rundel PW. Stable isotopes: units, history and instrumention. In: Rundel PW, Eheringer JR, Nagy KA, editors. Stable Isotopes in Ecological Research. New York: Springer-Verlag; 1989:1–16.
2. Miller AI. Heavy water: a manufacturers’ guide for the hydrogen century. Can Nucl Soc Bull. 2001;22(1):1–14.
3. Craig H. Standard for reporting concentrations of deuterium and Oxygen-18 in natural waters. Science. 1961;133(3467):1833–1834. doi:10.1126/science.133.3467.1833
4. Katz JJ, Crespi HL. Deuterated organisms: cultivation and uses. Science. 1966;151(3715):1187–1194. doi:10.1126/science.151.3715.1187
5. Thomson JF. Physiological effects of D2O in mammals. Ann N Y Acad Sci. 1960;84(16):736–744. doi:10.1111/j.1749-6632.1960.tb39105.x
6. Jones PJ, Leatherdale ST. Stable isotopes in clinical research: safety reaffirmed. Clin Sci (Lond). 1991;80(4):277–280. doi:10.1042/cs0800277
7. Somlyai G, Jancso G, Jakli G, et al. Naturally occurring deuterium is essential for the normal growth rate of cells. FEBS Lett. 1993;317(1–2):1–4. doi:10.1016/0014-5793(93)81479-J
8. Watson PE, Watson ID, Batt RD. Total body water volumes for adult males and females estimated from simple anthropometric measurements. Am J Clin Nutr. 1980;33(1):27–39. doi:10.1093/ajcn/33.1.27
9. Schoeller DA. Changes in total body water with age. Am J Clin Nutr. 1989;50(5Suppl):
10. Lambert BS, Shimkus KL, Fluckey JD, et al. Anabolic responses to acute and chronic resistance exercise are enhanced when combined with aquatic treadmill exercise. Am J Physiol Endocrinol Metab. 2015;308(3):E192–E200. doi:10.1152/ajpendo.00689.2013
11. Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671–675. doi:10.1038/nmeth.2089
12. Kippenberger S, Muller J, Schultz M, et al. Oligonucleotides suppress PKB/Akt and act as superinductors of apoptosis in human keratinocytes. Nucleic Acids Res. 2009;37(12):3850–3864. doi:10.1093/nar/gkp252
13. Kaluzki I, Hrgovic I, Hailemariam-Jahn T, et al. Dimethylfumarate inhibits melanoma cell proliferation via p21 and p53 induction and bcl-2 and cyclin B1 downregulation. Tumour Biol. 2016;37(10):13627–13635. doi:10.1007/s13277-016-5285-6
14. Alexander B, Browse DJ, Reading SJ, Benjamin IS. A simple and accurate mathematical method for calculation of the EC50. J Pharmacol Toxicol Methods. 1999;41(2–3):55–58. doi:10.1016/S1056-8719(98)00038-0
15. Salgame P, Varadhachary AS, Primiano LL, Fincke JE, Muller S, Monestier M. An ELISA for detection of apoptosis. Nucleic Acids Res. 1997;25(3):680–681. doi:10.1093/nar/25.3.680
16. Altermatt HJ, Gebbers JO, Laissue JA. Heavy water delays growth of human carcinoma in nude mice. Cancer. 1988;62(3):462–466.
17. Takeda H, Nio Y, Omori H, et al. Mechanisms of cytotoxic effects of heavy water (deuterium oxide: D2O) on cancer cells. Anticancer Drugs. 1998;9(8):715–725. doi:10.1097/00001813-199809000-00007
18. Gyongyi Z, Budan F, Szabo I, et al. Deuterium depleted water effects on survival of lung cancer patients and expression of Kras, Bcl2, and Myc genes in mouse lung. Nutr Cancer. 2013;65(2):240–246. doi:10.1080/01635581.2013.756533
19. Krempels K, Somlyai I, Somlyai G. A retrospective evaluation of the effects of deuterium depleted water consumption on 4 patients with brain metastases from lung cancer. Integr Cancer Ther. 2008;7(3):172–181. doi:10.1177/1534735408322851
20. Somlyai G, Molnar M, Laskay G, et al. A természetben megtalálható deutérium biológiai jelentősége: a deutériumdepletio daganatellenes hatása [Biological significance of naturally occurring deuterium: the antitumor effect of deuterium depletion]. Orv Hetil. 2010;151(36):1455–1460. doi:10.1556/OH.2010.28865
21. Boros LG, D’Agostino DP, Katz HE, Roth JP, Meuillet EJ, Somlyai G. Submolecular regulation of cell transformation by deuterium depleting water exchange reactions in the tricarboxylic acid substrate cycle. Med Hypotheses. 2016;87:69–74. doi:10.1016/j.mehy.2015.11.016
22. Uemura T, Moritake K, Akiyama Y, Kimura Y, Shingu T, Yamasaki T. Experimental validation of deuterium oxide-mediated antitumoral activity as it relates to apoptosis in murine malignant astrocytoma cells. J Neurosurg. 2002;96(5):900–908. doi:10.3171/jns.2002.96.5.0900
23. Hartmann J, Bader Y, Horvath Z, et al. Effects of heavy water (D2O) on human pancreatic tumor cells. Anticancer Res. 2005;25(5):3407–3411.
24. Omori H, Kuroda M, Naora H, et al. Deuterium oxide (heavy water) accelerates actin assembly in vitro and changes microfilament distribution in cultured cells. Eur J Cell Biol. 1997;74(3):273–280.
25. Etienne-Manneville S. Microtubules in cell migration. Annu Rev Cell Dev Biol. 2013;29:471–499. doi:10.1146/annurev-cellbio-101011-155711
26. Itoh TJ, Sato H. The effects of deuterium oxide (2H2O) on the polymerization of tubulin in vitro. Biochim Biophys Acta. 1984;800(1):21–27. doi:10.1016/0304-4165(84)90089-8
27. Inoue S, Sato H. Cell motility by labile association of molecules. The nature of mitotic spindle fibers and their role in chromosome movement. J Gen Physiol. 1967;50(6):259–292. doi:10.1085/jgp.50.6.259
28. Marsland D, Zimmerman AM. Structural stabilization of the mitotic apparatus by heavy water in the cleaving eggs of Arabica punctulata increased resistance to pressure-induced disorganization. Exp Cell Res. 1965;38:306–313. doi:10.1016/0014-4827(65)90406-4
29. Marsland D, Tilney LG, Hirshfield M. Stabilizing effects of D2O on the microtubular components and needle-like form of heliozoan axopods: a pressure-temperature analysis. J Cell Physiol. 1971;77(2):187–194. doi:10.1002/(ISSN)1097-4652
30. Urata C, Watanabe A, Ogawa Y, et al. Effect of deuterium oxide (D2O) on the IgE-mediated Ca2+ influx, arachidonic acid and histamine release in rat basophilic leukemia cells. Arerugi. 1989;38(3):285–295.
31. Lee PJ, Park HJ, Cho N, Kim HP. Aquaporin 11-dependent inhibition of proliferation by deuterium oxide in activated hepatic stellate cells. Molecules. 2018;23(12):3209. doi:10.3390/molecules23123209
32. Radisavljevic Z. AKT as locus of hydrogen bond network in cancer. J Cell Biochem. 2018;119(1):130–133. doi:10.1002/jcb.26193
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.