Back to Journals » ClinicoEconomics and Outcomes Research » Volume 12
Healthcare Resource Utilization Pre- and Post-Initiation of Eslicarbazepine Acetate Among Pediatric Patients with Focal Seizure: Evidence from Routine Clinical Practice
Authors Mehta D , Davis M , Epstein AJ , Williams GR
Received 9 May 2020
Accepted for publication 3 July 2020
Published 23 July 2020 Volume 2020:12 Pages 379—387
DOI https://doi.org/10.2147/CEOR.S261960
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Giorgio Colombo
Darshan Mehta,1 Matthew Davis,2 Andrew J Epstein,2 G Rhys Williams1
1Health Economics and Outcomes Research, Sunovion Pharmaceuticals Inc., Marlborough, MA, USA; 2Health Economics and Outcomes Research, Medicus Economics, LLC, Milton, MA, USA
Correspondence: Darshan Mehta
Health Economics and Outcomes Research, 84 Waterford Drive, Marlborough, MA 01752, USA
Tel +1-774-369-7913
Email [email protected]
Objective: To examine the impact of initiating treatment with eslicarbazepine acetate (ESL) on healthcare resource utilization (HCRU) among pediatric patients with focal seizures (FS).
Methods: This retrospective study used Symphony Health’s Integrated Dataverse® claims data. Patients aged 4 to 17 years with a diagnosis of FS and a new prescription for ESL between April 2015 and June 2018 were included and defined as the overall patient population. Index date was the first dispensed claim for ESL. Baseline period was the 90-day block immediately prior to the index date. The follow-up period comprised up to 4 consecutive 90-day blocks immediately following the index date. Subgroups were defined based on the presence (DP+) or absence (DP−) of developmental and/or psychiatric disorders at baseline. All-cause and FS-related inpatient (IP), emergency room (ER), outpatient (OP) hospital, and office (OF) visits were measured during the follow-up period. Reduction in HCRU per block in the post-ESL period was assessed using fixed-effects linear regression models.
Results: A total of 234 patients were included in the overall study population, of whom 86 (36.8%) were DP+ and 148 (63.2%) were DP−. Relative to the baseline period, significant reductions were observed in the overall population for all-cause ER (P=0.001), OP (P< 0.001), and OF (P< 0.001) visits and FS-related IP (P=0.037) and OF (P< 0.001) visits in the follow-up period. Among DP+ and DP− patients, significant reductions were observed for all-cause ER (DP+: P=0.024; DP−: P=0.017), OP (DP+: P< 0.001; DP−: P=0.035), and OF (DP+: P=0.004; DP−: P=0.001) visits during the follow-up period. No significant differences were observed between DP+ and DP− patients in the change in all-cause or FS-related HCRU from baseline to the follow-up period.
Conclusion: Pediatric patients with FS (DP+ and DP-) who initiated ESL had significant reductions in all-cause ER, OP, and OF visits and FS-related IP and OF visits.
Keywords: eslicarbazepine acetate, focal seizure, health resource utilization, pediatric
Introduction
Epilepsy is a common pediatric neurological disorder in the United States (US), affecting 470,000 persons aged 0–17 years, with a prevalence of 1.2%.1 The majority (60%) of pediatric patients with epilepsy suffer from focal (partial-onset) seizures (FS).2
Epilepsy poses substantial individual, societal, and economic burdens. In the US, the annual aggregate cost of pediatric epilepsy is estimated at $5.8 billion (2016 US Dollar [USD]), with the majority of direct costs accounted for by inpatient, home healthcare, outpatient, and medication costs.3 Mean annual healthcare expenditures among pediatric patients with epilepsy are approximately 6 times higher than those in the general pediatric population ($12,577 vs. $2,024; 2016 USD).3 Healthcare resource utilization (HCRU) and spending are particularly high for the estimated 19.4% of pediatric patients with uncontrolled epilepsy,4 who experience more hospitalizations (30.1% vs. 12.0%; P<0.001) and higher all-cause ($30,343 vs. $18,206; P<0.001) and epilepsy-related ($16,894 vs. $7,979; P<0.001) spending than children with stable disease.4
Comorbid conditions are common and variable among pediatric patients with epilepsy, and often more disabling than the seizures themselves.5 In the US prospective, community-based Connecticut Study of Epilepsy, 30% of patients with newly diagnosed pediatric epilepsy had 1 chronic comorbidity and 31% had ≥2 comorbidities.6 Prevalence of neurodevelopmental spectrum disorder, psychiatric disorders and chronic medical illness was 39%, 26%, and 24%, respectively.6 Data extracted from a nationwide healthcare claims database of pediatric patients with newly diagnosed epilepsy revealed neurobehavioral disorders were 2.5 times more prevalent in pediatric patients with epilepsy than those without epilepsy.7 The US National Survey of Children’s Health (2007) demonstrated that learning disability (56% vs. 7%), developmental delay (51% vs. 3%), attention deficit hyperactivity disorder (ADHD; 23% vs. 6%), anxiety (17% vs. 3%), conduct problems (16% vs. 3%), autism spectrum disorder (ASD; 16% vs. 1%), and depression (8% vs. 2%) were significantly more common among pediatric patients with epilepsy than in the general pediatric population.8 Developmental and/or psychiatric disorders in pediatric patients with epilepsy are associated with a substantial economic burden. Data from the Medical Expenditure Panel Survey-Household Component (MEPS-HC) (2003 to 2014) showed that total healthcare expenditures for pediatric patients with epilepsy with developmental disorders were $29,227 (2016 USD) vs. $11,974 (2016 USD) among pediatric patients with epilepsy alone.3 This difference was largely driven by higher expenditures for home healthcare.3
Seizure control is the goal of treatment in epilepsy, and is attained primarily through the use of antiepileptic drugs (AEDs), which can be classified into 3 generations. First-generation barbiturate-derived agents are associated with poor tolerability profiles, particularly among patients with comorbid psychiatric conditions.9,10 Some second- and third-generation AEDs may be associated with lower rates of psychiatric and behavioral side effects.11–13 The choice of AED depends on patient-specific and AED-specific variables, including age, comorbidities and AED adverse events (AEs), with the goal of seizure control while avoiding worsening of existing comorbidities.14,15 The potentially improved safety and tolerability profile of newer AEDs may be particularly valuable for patients with comorbid developmental and/or psychiatric disorders.16
Eslicarbazepine acetate (ESL), a third-generation AED, is approved by the US Food and Drug Administration for the treatment of partial-onset seizures (FS) in patients ≥4 years of age.17 ESL received approval in the pediatric patient population ages 4 years and older based upon extrapolation of efficacy data obtained from adequate and well-controlled studies in adult patients with FS, pharmacokinetic data from adult and pediatric patients, and safety data from clinical studies in pediatric patients’ ages from 4 to 17 years.17 Real-world outcomes, specifically those related to HCRU following initiation of ESL for patients aged 4–17 years, are unknown. The main objective of this retrospective study was to examine the impact of initiating treatment with ESL on HCRU among pediatric patients with treated FS. A subgroup analysis of patients with FS stratified by the presence or absence of developmental and/or psychiatric disorders was also performed.
Methods
Data Source
This retrospective, longitudinal cohort analysis used Symphony Health’s Integrated Dataverse (IDV®) open-source claims database that tracks 274 million active patients in the US. Data from April 1, 2015 to June 30, 2018 on patient demographics, medical resource use, and prescription drugs were studied. IDV® data are de-identified in compliance with the Health Insurance Portability and Accountability Act; therefore, this study did not constitute Human Subjects Research, and review by an institutional review board was not required.18
Study Design
A pre-post analysis was conducted to evaluate the effects of ESL initiation on HCRU. A longitudinal panel data approach was used, and the unit of analysis was a person-specific “block” of 90 consecutive days. The index date was defined as the first dispensed claim for ESL. The baseline period was defined as the 90-day block immediately prior to the index date. The follow-up period was defined as the 4 consecutive 90-day blocks immediately following the index date (Figure 1).
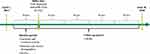 |
Figure 1 Study design. Abbreviations: ESL, eslicarbazepine acetate; HCRU, healthcare resource utilization. |
Patient Population
Patients were included in the analysis if they met the following inclusion criteria: 1) residence in the US; 2) ≥1 medical claim with a diagnosis of FS (International Classification of Diseases, 9th Revision [ICD-9], Clinical Modification codes 345.4x or 345.5x or ICD-10 codes G40.1x or G40.2x); 3) ≥1 dispensed pharmacy claim for an AED; 4) ≥1 dispensed pharmacy claim for ESL; 5) aged 4–17 on the index date; 6) continuous data availability for 90 days prior to and following the index date; and 7) ≥1 medical and pharmacy claim after the index date (Figure 2). Thus, the overall study population included pediatric patients (aged 4–17 years on index date) with a diagnosis of FS who received a new prescription for ESL and had at least one 90-day block before and after the index date.
Patients in the overall population were assigned to 2 subgroups: those with FS with developmental and/or psychiatric disorders (DP+) or without (DP−). Patients were classified into the DP+ subgroup if they had FS and any of ADHD, anxiety, autism, behavioral/emotional disorders (excluding ADHD), cognitive impairment, depression, intellectual disability, disorders of psychological development (including autism), schizophrenia, or unspecified developmental delay during the baseline period. All other patients with FS were included in the DP− subgroup.
Study Measures and Statistical Analyses
Baseline Characteristics
During the baseline period, patient demographic and clinical characteristics were measured for the overall population, including the patient subgroups. Demographic characteristics included age, gender, and payer type (i.e. commercial, Medicaid, and cash/assistance programs). Clinical characteristics included common medical, neurological, and psychiatric comorbidities observed among pediatric patients with epilepsy.7,19
AED characteristics were recorded on the index date and included AED therapy characteristics such as monotherapy vs. adjunctive therapy and prior AED experience. Patients were considered to be on adjunctive ESL therapy if they had a claim for another AED on the index date, or had remaining days’ supply from a prior AED claim on the index date and a subsequent claim for the same AED within 30 days of exhausting that days’ supply. All other patients prescribed ESL who did not meet the criteria for adjunctive therapy were classified as receiving ESL monotherapy. Prior AED experience included number of distinct AEDs before ESL initiation. The number of claims for branded and generic AEDs was also studied.
HCRU in the Baseline and Follow-Up Periods
All-cause and FS-related inpatient (IP), emergency room (ER), outpatient (OP) hospital, and office (OF) visits were measured for each patient-block during the baseline and follow-up periods (i.e. pre- and post-ESL initiation). Claims were categorized by place of service. All-cause HCRU was defined as HCRU due to any cause. FS-related HCRU was defined as HCRU with a diagnosis of FS in any diagnosis position associated with that claim. Each HCRU category was presented as the proportion of patients with ≥1 HCRU visit of each type.
Statistical Analyses
For baseline clinical and demographic characteristics, continuous variables were expressed as means and standard deviations (SDs). Dichotomous and categorical variables were expressed as counts and percentages. To assess differences between the subgroups at baseline, means were compared with t-tests and percentage distributions were compared with χ2 tests.
Linear regression models with person-specific fixed effects were estimated to assess within-person changes in HCRU between baseline and follow-up. A separate model was run for each of the all-cause and FS-related HCRU categories, and for the overall population and the patient subgroups. The primary exposure was a binary indicator for whether the person-block was from the baseline or the follow-up period. The fitted coefficient on the exposure was interpreted as the within-person percentage point change from baseline to follow-up in HCRU per 90-day block. Model standard errors were adjusted to be made robust to heteroskedasticity of unknown form and to account for the clustering of multiple 90-day blocks within patients.
Statistical analyses were performed using SAS software version 9.4 (SAS Institute, Cary, NC) and Stata MP software version 16 (StataCorp, LLC, College Station TX). Two-sided statistical tests were used and P<0.05 was considered significant.
Results
Baseline Characteristics
234 patients met the inclusion criteria and were included in the overall population. Forty-four (18.8%) patients had one 90-day follow-up block; 36 (15.4%) patients had two blocks; 16 (6.8%) patients had three blocks; and the remaining 138 (59.0%) patients had at least four blocks. Patients in the overall population had a mean (SD) age of 12.7 (±3.9) years, and there were equal proportions of males and females. A majority of the patients were enrolled in commercial (51%) or Medicaid (44%) plans. Most (56%) patients received ESL as adjunctive therapy. The mean number of AEDs prior to ESL initiation was 1.8 (±1.5). 43%, 38% and 37% of patients had medical, neurological or psychiatric and/or developmental disorders at baseline, respectively (Table 1).
 |
Table 1 Baseline Characteristics of Patients in the Overall Population |
Among patients in the overall population, 86 patients had FS with developmental and/or psychiatric disorders (DP+), and 148 patients had FS only (DP−) (Table S1). Compared to DP− patients, DP+ patients received a significantly higher mean number of prior AEDs (2.02 [±1.44] vs. 1.59 [±1.45]; P=0.027), and a significantly larger proportion had ESL as adjunctive therapy (vs. monotherapy) (66% vs. 49%; P=0.012). The prevalence of medical disorders (55% vs. 36%; P=0.005) and neurological disorders (48% vs. 32%, P=0.015) was significantly higher among DP+ patients compared to DP− patients.
HCRU in the Baseline and Follow-Up Periods
All-cause and FS-related HCRU in the baseline and follow-up periods for patients in the overall population are presented in Figure 3. There were statistically significant reductions in all-cause ER (−11.3 percentage points [ppts]; P=0.001), OP (−15.1 ppts; P<0.001), and OF (−14.9 ppts; P<0.001) visits, as well as reductions in FS-related IP (−4.7 ppts; P=0.037) and OF (−13.7 ppts; P<0.001) visits in the follow-up period compared to baseline. Numerical, but statistically non-significant reductions were observed for all-cause IP visits (Figure 3A) and FS-related ER and OP visits (Figure 3B).
All-cause and FS-related HCRU in the baseline and follow-up periods for DP+ and DP− patients are presented in Figure 4. In both subgroups, there were statistically significant reductions in all-cause ER (DP+: −11.9 ppts, P=0.024; DP−: −10.9 ppts, P=0.017), OP (DP+: −23.5 ppts, P<0.001; DP−: −10.3 ppts, P=0.035), and OF (DP+: −14.4 ppts, P=0.004; DP−: −15.2 ppts, P=0.001) visits, as well as reductions in FS-related OF visits (DP+: −12.5 ppts, P=0.019; DP−: −14.3 ppts, P<0.001) in the follow-up period compared to baseline. In both subgroups, there were numerical, but statistically non-significant reductions in all-cause IP visits (Figure 4A) and FS-related IP, ER, and OP visits (Figure 4B). Reductions in HCRU in the follow-up period compared to baseline were similar across the DP+ and DP− subgroups.
Discussion
The results from this retrospective real-world study of national claims data suggest ESL initiation was associated with statistically significant reductions in all-cause ER, OP, and OF visits and FS-related IP and OF visits in pediatric patients with FS. Similar results were observed among adults with FS initiating ESL as adjunctive or monotherapy.20 Reductions in all-cause ER and OP visits, and all-cause IP visits and epilepsy-related ER and OP visits were observed in adults initiating ESL monotherapy.20 Previous studies assessed HCRU in pediatric patients with FS who initiated treatment with a third-generation AED.21,22 Consistent with our findings, a statistically significant reduction in all-cause and epilepsy-related IP hospitalization risk was observed following initiation of perampanel in patients with FS aged 4−11 years covered by commercial or government-sponsored insurance plans or those covered specifically by a Medicaid health plan.21,22
Subgroup analyses demonstrated statistically significant reductions in all-cause ER, OP, and OF visits and FS-related OF visits following ESL initiation in pediatric patients with FS with and without developmental and/or psychiatric disorders. Reductions in HCRU were similar for both patient subgroups, which is consistent with data showing that the clinical efficacy of ESL in patients with FS with developmental and/or psychiatric disorders is similar to that in patients with FS only.23,24
The reductions in HCRU following ESL initiation in pediatric patients with FS may be driven by the clinical efficacy, safety and tolerability of ESL, as described in recent studies of adjunctive ESL therapy in pediatric patients with refractory FS.25–28 A retrospective chart review of pediatric patients with pharmacologically intractable epilepsy treated with ESL concluded ESL was well-tolerated and had a good response rate.28 A Phase II study aimed to evaluate the neurocognitive effects of ESL in pediatric patients with refractory FS showed ESL was effective at reducing seizure frequency and overall, had no significant effects on neurocognitive behavior.25 Other studies evaluating the safety and tolerability of ESL as adjunctive therapy in pediatric patients with refractory FS found ESL was well-tolerated, and a greater relative reduction in standardized seizure frequency was observed for patients ≥6 years of age compared to placebo.26,27
Pediatric patients with epilepsy with developmental or psychiatric comorbidities may have higher HCRU compared to pediatric patients with epilepsy alone.29 In a Canadian health administrative database study evaluating HCRU among pediatric patients with epilepsy with comorbidities including depression, anxiety, learning disability, ADHD and ASD, the frequency of ER visits was higher for all comorbidities, with learning disability having the largest difference.30 The present study showed that ESL initiation was associated with similar reductions in HCRU in pediatric patients with FS alone or FS with developmental and/or psychiatric disorders. These data are in line with studies showing psychiatric AEs are uncommon in patients with FS treated with ESL.23,31
Strengths and Limitations
This study has several strengths. A large claims database was used that captures claims data from multiple US insurance and payer types, supporting the generalizability of the study findings to the US population of pediatric patients with FS. Fixed-effects models were employed in the statistical analysis, with patients serving as their own controls, to hold constant all time-invariant patient characteristics.
This study has several limitations. The Symphony IDV® is an open-source database and may not capture all claims for a patient, resulting in incomplete data. However, the IDV® database captures nearly 97% of pharmacy claims in the US. The pre-post design and fixed-effects models do not eliminate confounding by other within-patient, time-varying factors. FS may be subject to under-coding that results in lower FS-related HCRU estimates; however, all-cause HCRU analysis may have captured these missing data. This was not a comparative study, and there were no parallel controls. Patients were classified as DP+ or DP− based on the presence of specific ICD-9/ICD-10 diagnosis codes that may be subject to under-coding leading to lower prevalence estimates of DP+ patients as compared to other studies. These diagnosis codes were used previously to identify these comorbidities.7 Patients with either psychiatric and/or developmental disorders could not be further analyzed as separate groups due to small sample size.
Conclusion
Results from this real-world study of pediatric patients with FS found that ESL initiation was associated with statistically significant reductions in all-cause ER, OP, and OF visits and FS-related IP and OF visits. Initiation of ESL reduced HCRU among patients with FS with developmental and/or psychiatric disorders. Further studies examining the impact of ESL initiation on healthcare expenditures in pediatric patients with FS are warranted.
Highlights
- Most ESL use was adjunctive among patients who had tried 2 prior AEDs.
- ESL reduced all-cause ER, OP, and OF visits in pediatric patients with FS.
- ESL reduced FS-related IP and OF visits in pediatric patients with FS.
- HCRU reduction with or without developmental and/or psychiatric disorders was similar.
Acknowledgments
Medical writing support provided by Joanna Dembowy, PhD and editorial support provided by Jane Kondejewski, PhD of SNELL Medical Communication Inc.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
Darshan Mehta is an employee of Sunovion Pharmaceuticals Inc. Matthew Davis is an employee of Medicus Economics, LLC, which received funding from Sunovion to participate in this research. Andrew J Epstein is an employee of Medicus Economics, LLC, which received funding from Sunovion to participate in this research. G Rhys Williams is an employee of Sunovion Pharmaceuticals Inc. The authors report no other conflicts of interest in this work.
References
1. Zack MM, Kobau R. National and state estimates of the numbers of adults and children with active epilepsy—United States, 2015. MMWR Morb Mortal Wkly Rep. 2017;66(31):821–825. doi:10.15585/mmwr.mm6631a1
2. Sillanpää M, Jalava M, Shinnar S. Epilepsy syndromes in patients with childhood-onset seizures in Finland. Pediatr Neurol. 1999;21(2):533–537. doi:10.1016/s0887-8994(99)00031-4
3. Lekoubou A, Bishu KG, Ovbiagele B. The direct cost of epilepsy in children: evidence from the medical expenditure panel survey, 2003–2014. Epilepsy Behav. 2018;83:103–107. doi:10.1016/j.yebeh.2018.03.020
4. Cramer JA, Wang ZJ, Chang E, et al. Healthcare utilization and costs in children with stable and uncontrolled epilepsy. Epilepsy Behav. 2014;32:135–141. doi:10.1016/j.yebeh.2014.01.016
5. Wei SH, Lee WT. Comorbidity of childhood epilepsy. J Formos Med Assoc. 2015;114(11):1031–1038. doi:10.1016/j.jfma.2015.07.015
6. Baca CB, Vickrey BG, Caplan R, Vassar SD, Berg AT. Psychiatric and medical comorbidity and quality of life outcomes in childhood-onset epilepsy. Pediatrics. 2011;128(6):e1532–e1543. doi:10.1542/peds.2011-0245
7. Oh A, Thurman DJ, Kim H. Comorbidities and risk factors associated with newly diagnosed epilepsy in the U.S. pediatric population. Epilepsy Behav. 2017;75:230–236. doi:10.1016/j.yebeh.2017.07.040
8. Russ SA, Larson K, Halfon N. A national profile of childhood epilepsy and seizure disorder. Pediatrics. 2012;129(2):256–264. doi:10.1542/peds.2010-1371
9. Mula M. Treatment-emergent psychiatric adverse events of antiepileptic drugs in epilepsy: how can we avoid them? Neuropsychiatry. 2011;1(4):371–376. doi:10.2217/npy.11.29
10. Mula M, Sander JW. Negative effects of antiepileptic drugs on mood in patients with epilepsy. Drug Saf. 2007;30(7):555–567. doi:10.2165/00002018-200730070-00001
11. Chen B, Detyniecki K, Choi H, et al. Psychiatric and behavioral side effects of anti-epileptic drugs in adolescents and children with epilepsy. Eur J Paediatr Neurol. 2017a;21(3):441–449. doi:10.1016/j.ejpn.2017.02.003
12. Chen B, Choi H, Hirsch LJ, et al. Psychiatric and behavioral side effects of antiepileptic drugs in adults with epilepsy. Epilepsy Behav. 2017b;76:24–31. doi:10.1016/j.yebeh.2017.08.039
13. Weintraub D, Buchsbaum R, Resor SR, Hirsch LJ. Psychiatric and behavioral side effects of the newer antiepileptic drugs in adults with epilepsy. Epilepsy Behav. 2007;10(1):105–110. doi:10.1016/j.yebeh.2006.08.008
14. Glauser T, Ben-Menachem E, Bourgeois B, et al. ILAE treatment guidelines: evidence-based analysis of antiepileptic drug efficacy and effectiveness as initial monotherapy for epileptic seizures and syndromes. Epilepsia. 2006;47(7):1094–1120. doi:10.1111/j.1528-1167.2006.00585.x
15. Snead OC, Donner EJ. A new generation of anticonvulsants for the treatment of epilepsy in children. Paediatr Child Health. 2007;12(9):741–744. doi:10.1093/pch/12.9.741
16. Moavero R, Santarone ME, Galasso C, Curatolo P. Cognitive and behavioral effects of new antiepileptic drugs in pediatric epilepsy. Brain Dev. 2017;39(6):464–469. doi:10.1016/j.braindev.2017.01.006
17. APTIOM® Prescribing Information. Retrieved January 18, 2020. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/022416s000lbl.pdf.
18. US Department of Health & Human Services. Federal policy for the protection of human subjects (‘common rule’). Available from: https://www.hhs.gov/ohrp/regulations-and-policy/regulations/common-rule/index.html.
19. Aaberg KM, Bakken IJ, Lossius MI, et al. Comorbidity and childhood epilepsy: a nationwide registry study. Pediatrics. 2016;138(3):e20160921–e20160921. doi:10.1542/peds.2016-0921
20. Mehta D, Lee A, Simeone J, Nordstrom B Characteristics of adult patients with focal seizure receiving eslicarbazepine acetate therapy in routine clinical practice: evidence from a large US commercial claims database [abstract]. American Epilepsy Society Annual Meeting 2018. 2018. Available from: https://www.aesnet.org/meetings_events/annual_meeting_abstracts/view/500810.
21. Faught E, Li X, Choi J, Malhotra M, Knoth R. Inpatient hospitalization risk in patients with epilepsy before and after perampanel treatment [abstract]. Neurology. 2019;92(15Supplement):
22. Chatterjee D, Li X, Malhotra M, Choi J Inpatient hospitalization risk in medicaid patients with epilepsy before and after perampanel treatment [abstract]. American Academy of Neurology Annual Meeting 2019. 2019. Available from: http://indexsmart.mirasmart.com/AAN2019/PDFfiles/AAN2019-001535.pdf.
23. Doherty CP, Rheims S, Assenza G, et al. Eslicarbazepine acetate in epilepsy patients with psychiatric comorbidities and intellectual disability: clinical practice findings from the Euro-Esli study. J Neurol Sci. 2019;402:88–99. doi:10.1016/j.jns.2019.04.040
24. Shankar R, Henley W, Allard J, et al. Comparing response of eslicarbazepine acetate between people with intellectual disability (ID) and general population – results from the Ep-ID UK register [abstract]. American Epilepsy Society Meeting 2019. 2019. Available from: https://www.aesnet.org/meetings_events/annual_meeting_abstracts/view/2422217.
25. Jóźwiak S, Veggiotti P, Moreira J, Gama H, Rocha F, Soares-da-Silva P. Effects of adjunctive eslicarbazepine acetate on neurocognitive functioning in children with refractory focal-onset seizures. Epilepsy Behav. 2018;81:1–11. doi:10.1016/j.yebeh.2018.01.029
26. Kirkham F, Auvin S, Moreira J, et al. Efficacy and safety of eslicarbazepine acetate as adjunctive therapy for refractory focal-onset seizures in children: a double-blind, randomized, placebo-controlled, parallel-group, multicenter, phase-III clinical trial. Epilepsy Behav. 2020;105:106962. doi:10.1016/j.yebeh.2020.106962
27. Mintz M, Pina-Garza JE, Wolf SM, et al. Safety and tolerability of adjunctive eslicarbazepine acetate in pediatric patients (aged 4–17 years) with focal seizures. J Child Neurol. 2020;35(4):265–273. doi:10.1177/0883073819890997
28. Tanritanir A, Wang X, Loddenkemper T. Experience with eslicarbazepine acetate treatment at a pediatric epilepsy center [abstract]. Neurology. 2019;92(15Supplement):
29. Lekoubou A, Bishu KG, Ovbiagele B. Nationwide healthcare utilization among children with epilepsy in the United States: 2003–2014. Epilepsy Res. 2018b;141:90–94. doi:10.1016/j.eplepsyres.2018.02.012
30. Puka K, Smith ML, Moineddin R, Snead OC, Widjaja E. Health resource utilization varies by comorbidities in children with epilepsy. Epilepsy Behav. 2016;57(Pt A):151–154. doi:10.1016/j.yebeh.2016.02.011
31. Jalihal V, Shankar R, Henley W, et al. Eslicarbazepine acetate as a replacement for levetiracetam in people with epilepsy developing behavioral adverse events. Epilepsy Behav. 2018;80:365–369. doi:10.1016/j.yebeh.2018.01.020
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



