Back to Journals » Journal of Hepatocellular Carcinoma » Volume 4
Healthcare costs of transarterial chemoembolization in the treatment of hepatocellular carcinoma
Authors Fateen W, Khan F, O’Neill RJ, James MW, Ryder SD , Aithal GP
Received 15 June 2017
Accepted for publication 28 August 2017
Published 16 October 2017 Volume 2017:4 Pages 123—130
DOI https://doi.org/10.2147/JHC.S144068
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ahmed Kaseb
Waleed Fateen,1,2 Farooq Khan,1 Richard J O’Neill,3 Martin W James,1 Stephen D Ryder,1 Guruprasad P Aithal1,2
1NIHR Nottingham Biomedical Research Centre, Nottingham University Hospitals NHS Trust and University of Nottingham, 2Nottingham Digestive Diseases Centre, University of Nottingham, 3Department of Radiology, Nottingham University Hospitals, NHS Trust, Nottingham, UK
Background: A meta-analysis comparing drug-eluting beads transarterial chemoembolization (DEB-TACE) with conventional transarterial chemoembolization (cTACE) has recently been published. On balance, no significant differences were found in terms of objective response and overall survival. The impact on healthcare costs had been studied in small series based on a hypothetical model and was in favor of DEB-TACE. We aimed to evaluate and compare healthcare costs and effectiveness of both modalities in a cohort of patients from Nottingham, UK.
Methods: Using a dedicated radiology database, we identified all patients who had undergone cTACE or DEB-TACE between 2006 and 2012 at a single tertiary referral center based in Nottingham. We collected clinical data, including treatment response, postprocedure complications and 30-day mortality. Costing models were constructed to present both our local hospital perspective as well as the national health service position.
Results: During our study period, 101 procedures were performed on 43 patients (76 cTACE procedures on 26 patients and 25 DEB-TACE procedures on 17 patients). Overall, 11/26 in cTACE and 5/17 in DEB-TACE group had progressive disease (p=0.52). Adverse events were seen in 6/76 cTACE compared with 7/25 DEB-TACE group (p=0.16). Based on the predetermined standard pathway there was an unadjusted average cost difference of £3770.30 (TACE =£9070.44, DEB-TACE =£5300.14) in favor of the DEB-TACE. Results from our costing models indicated a £2715.33 (95% CI £580.88–4849.77) cost difference in favor of the same procedure.
Conclusions: Even when the extra costs of DEB-TACE were considered, the overall treatment costs per patient were lower in relation to cTACE.
Keywords: hepatocellular carcinoma, transarterial chemoembolization, healthcare costs, drug-eluting beads, objective response
Introduction
Hepatocellular carcinoma (HCC) is the most common primary malignancy of the liver and the second leading cause of all cancer-related mortality globally.1,2 The majority of cases are diagnosed late at advanced stages and deemed unsuitable for curative treatments.3 Transarterial chemoembolization (TACE) is commonly used for the management of unresectable primary liver cancer.4 It works by blocking the tumor feeding arterial supply using an embolizing agent and enabling a local injection of chemotherapy directly into the tumor. Multiple TACE sessions may be required to achieve a desirable outcome.
Response to TACE is commonly judged according to time-to-event, for example, progression as well as overall survival.4 The Modified Response Evaluation Criteria in Solid Tumors (mRECIST) is commonly used to objectively measure tumor response or progression. It is based on a radiological assessment of tumor size specifically relating to contrast enhancement.5 Sustainable objective response is achieved in ~35% of patients.6 The benefits of this treatment on 2-year survival have been established by meta-analysis of randomized controlled trials (RCTs) (odds ratio, 0.54; 95% CI: 0.33, 0.89; p=0.015).7
TACE with drug-eluting beads (DEB-TACE) as opposed to conventional TACE (cTACE) involves the injection of beads into hepatic artery branches offering simultaneous embolization with sustained and controlled drug release. Consequently, DEB-TACE offers a favorable pharmacokinetic profile and lower peak plasma concentrations of chemotherapy.8 DEB-TACE is associated with 50% 4-year survival based on a retrospective and non-comparative study.9
Five meta-analyses comparing the safety and efficacy of DEB-TACE in comparison with cTACE have been published within the last 2 years.10–14 The aforementioned studies had different selection criteria and methodologies and therefore report varying comparative outcomes on the safety and efficacy of both procedures. Cost comparisons between cTACE and DEB-TACE were performed in 2 studies. Vadot et al retrospectively analyzed healthcare costs within the French infrastructure and found a significantly shorter mean hospital admission duration for patients who received DEB-TACE. This led to a subsequently better reported economic profile in France.15 The results of the study were limited to France and further studies were required to confirm generalizability to other national healthcare systems. Cucchetti et al constructed a hypothetical cost effectiveness Markov simulation model on published studies investigating a total number of 1860 patients and found a lower incremental cost of DEB-TACE.16 We have evaluated the safety, efficacy and costs of DEB-TACE versus cTACE from real-life patients from a large UK-based tertiary care institution. We aimed to first, validate the results published from France and review their generalizability. Second, validate the results published on a large hypothetical cohort using Markov simulation. With the widespread economic concerns, this may have a quick translation impact on healthcare systems.
Methods
Ethics and consent statement
The study is registered by the Digestive Diseases and Thoracic Directorate of the Nottingham University Hospitals NHS Trust as an audit. Ethical approval and patient consent were not required for this retrospective study which was performed as a part of our service evaluation.
Patient selection
We performed a retrospective analysis of patients who had cTACE or DEB-TACE between January 2006 and December 2012 for the treatment of HCC in a tertiary referral center. We identified the patients using a dedicated radiology database that is used to store and report procedural details as part of routine clinical practice. Clinical notes as well as electronic record information were used for data collection. We collected: 1) patient variables, including demographics, etiology of liver disease, Child–Pugh score and UK model for end-stage liver disease; 2) tumor variables, including size, location and number; 3) procedure variables, including number of TACE sessions required, length of hospital stay, postprocedure complications and 30-day mortality. Patients were excluded if they: 1) had not had follow-up in our unit; 2) had TACE for indications other than HCC; 3) had both cTACE and DEB-TACE on different occasions. Patients were followed up until discharge from clinic, including death or until the date of the last available cross-sectional imaging, if still under follow-up. Computerized tomography scans pre- and postprocedure were used to objectively assess tumor response using mRECIST. The images were reviewed by an expert radiologist as a part of the study. Cumulative tumor size of the 2 largest measurable lesions per liver was used where there was more than 1 lesion.5
Statistical analysis
Differences between groups of continuous variables were assessed by unpaired t-tests (parametric data) or Mann–Whitney U (non-parametric data) tests. A p-value <0.05 was considered statistically significant. Possible predictors of objective response were fitted into a proportional ordered logistic regression model. This analysis was performed on R statistical software using R packages mass and ggplot2 (R core team, Vienna, Austria). TACE procedures were counted twice for the same patient if the interval between both procedures was more than 4 months and both were assessed by postprocedure cross-sectional imaging independently. Covariates were limited by data availability and included the following:
- Interval in weeks from TACE to cross-sectional imaging
- Tumor size at baseline
- Vascular invasion and/or extra-hepatic disease, including porta-hepatis lymph nodes.
Costs
In order to obtain per patient cumulative cost figures, a reference treatment pathway was constructed with the assumption that it would be followed by all patients during the study’s time. Follow-up procedures were modeled as shortened versions of the first procedure. Two different costing models were constructed in order to present both the local and the national perspectives. The local cost of the beads was added to both models since the national equivalent could not be identified. Details on the price weights can be found in Table 1. National Health Service (NHS) schedule of reference cost 2012–2013 was used to obtain the cost data.
Cost analysis
To address the non-normality of distributions, costs were analyzed using generalized linear models (GLM) as described by Dunn et al.17 Regression on raw costs using ordinary least squares (OLS) was used for comparison. Covariates were chosen from the available dataset with the aim of maximizing the model’s explanatory power. To objectively measure clinical effectiveness, patient age, tumor size at presentation and last known mRECIST score were added to the model along with total costs and the treatment variable. Lowest Akaike information criterion and the modified Park test as described by Manning and Mullahy18 were used to evaluate the model’s goodness of fit. Three patients were excluded from the cost analysis due to lack of availability of sufficient data. Re-admissions were accounted for in our cost analysis.
Results
Patient outcomes
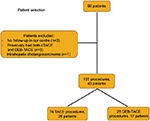
Fifty patients in total were identified. After exclusions, we analyzed 101 procedures (cTACE=76, DEB-TACE=25) performed on 43 patients (cTACE=26, DEB-TACE=17) as described in Figure 1. Descriptive statistics are outlined in Table 2.
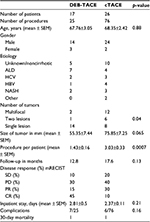
The majority of patients were male in both groups, with similar ages. Included patients had variable etiologies as outlined in Table 2. Both groups were followed up for more than 1 year on average. Patients in the DEB-TACE group had a significantly smaller number of nodules within their livers (p=0.04). Despite this, the cumulative tumor size of all nodules per liver added together was not significantly different between both groups. DEB-TACE group required significantly less sessions (median=1) in comparison with cTACE (median=2.5) per patient (p<0.005).
Mortality and complications
One patient in the DEB-TACE group died from biliary sepsis on day 14 of the same admission. In the cTACE group, there was a single 30-day mortality from hepatic decompensation; on day 2, postprocedure, the patient was transferred to a specialist palliative care facility. Severe transaminitis (>20× the normal) and hospital-acquired pneumonia occurred postprocedure in 1 patient in the cTACE group which prolonged admission in hospital by 5 days. One of the DEB-TACE cases had re-admission 6 days after the discharge from hospital with left hepatic artery dissection complicated by hospital-acquired pneumonia. The second admission had a duration of 7 days. There were no other notable severe complications otherwise, apart from limited transaminitis and postembolization syndrome (e.g., fever, nausea, vomiting or pain).
Objective response
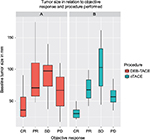
The method of TACE delivery (cTACE vs DEB-TACE) was not found to be independent predictor of objective response. Tumor size predicted objective response independent of all other variables as demonstrated in Figure 2 (p=0.0004, OR=1.035, 95% CI 1.017–1.057).
Costs
The analysis of the cost data took into account 40 out of the 43 patients for whom there was a complete dataset. The cTACE and DEB-TACE arms differed in the added cost of the beads for the DEB-TACE and the average number of follow-up procedures per patient. Using the aforementioned pathway, the 2 procedures had an unadjusted average cost difference of £3770.30 (cTACE=£9070.44, DEB-TACE=£5300.14) based on the NHS costing and of £3253.68 (cTACE=£9033.19, DEB-TACE=£5222.89) based on local costing, in favor of the DEB-TACE arm. Results are summarized in Table S1. Upon comparing the output of both models, there was no significant benefit from the use of GLMs while the Park test did not highlight a specific model as the best fit. On the OLS regression model, the cost difference was at £2715.33 based on the national NHS costing and at £2746.58 based on our local costing. Treatment type was found to predict total costs independent of all other included covariates (p=0.014).
Discussion and conclusions
We have demonstrated that overall treatment costs of DEB-TACE are lower than that of cTACE for the treatment of HCC. Our analysis is based on a real-life scenario. In the UK, all patients with HCC are managed through a regional cancer network with treatment decisions made by multidisciplinary teams (MDTs) based on predetermined pathways. Our economic model was based on the predetermined standard pathway which included the patient’s journey from time of referral to the tertiary center until the time of discharge, death or last follow-up. It included the costs of outpatient appointments, MDT discussion, inpatient stay, blood tests, TACE procedure and cross-sectional imaging. This economic modeling has shown an unadjusted average cost benefit of £3770.30 for patients receiving DEB-TACE compared with those who received cTACE. Patients undergoing DEB-TACE have required less number of procedures over the observed study period. The data suggest that contribution to the cost comparison is in favor of DEB-TACE with potential benefits to healthcare costs. Although neither the OLS regression nor any GLM had a significant explanatory power, there is an indication that the DEB-TACE arm can be less costly in the medium term. This is consistent with recently published data by French and Italian groups.15,16
Our limitations include the retrospective study design and possible selection bias, including the fact that patients treated by DEB-TACE had significantly fewer target liver lesions. The cost of beads reported in our study may lack generalizability as our analysis was based on our local purchase costs. However, the commissioning of health services is determined based on these estimates; so, these influence the design and delivery of services in the British NHS. The use of cTACE had been well established in our unit for many years. In contrast, DEB-TACE had been introduced as a relatively new technique. The choice of patients having DEB-TACE was mainly influenced by the time of introduction of the new service rather than specific clinical features. Similarly, classically, we will protocol cross-sectional imaging after 2 sessions of cTACE. However, as DEB-TACE was relatively new at the time of introduction, we protocoled cross-sectional imaging after each session of DEB-TACE in the majority of cases. Cross-sectional imaging after each session of cTACE may have led to different results. The study also highlights that, judging by objective response, we demonstrated that DEB-TACE is similar to cTACE. It also affirms the likelihood that its medico-economic consequences are more favorable in the medium term in comparison with cTACE.
There is some controversy in the literature, however, about the effect of DEB-TACE on objective tumor shrinkage.19 We therefore fitted our data into a logistic regression model and confirmed lack of direct effect from DEB-TACE on improved objective tumor response within our cohort. We found tumor size to be an independent predictor of objective response to TACE as would be expected. Interestingly, progressive disease (PD) did not seem to be limited by tumor size, that is, patients who had PD did not necessarily have larger tumors (Figure 2). This highlights the seemingly unstoppable role of tumor biology in dictating behavior and prognosis.
Based on the recent meta-analyses, DEB-TACE has no statistically significant impact on survival or objectively evaluated treatment response according to RCTs. In contrast, the largest meta-analysis to date that included non-RCT prospective and retrospective studies, demonstrated that DEB-TACE was associated with survival benefit.13 Another large meta-analysis including 4 RCTs and 6 retrospective studies found significant differences in the incidence of post-embolization syndrome or liver dysfunction (Table 3).14 Meta-analysis limited to RCTs found DEB-TACE to be safer, specifically in terms of myelosuppression and alopecia.11 Xie et al, reported significant improvement in objective response in the DEB-TACE group. The meta-analysis was subsequently heavily criticized by Kodama et al who reported no difference in objective response upon reproducing the results.12,19
It is worth noting that there are several limitations of reviewing studies based on meta-analysis. First, the number of TACE sessions required and practically performed was not compared. The studies did not report if less sessions were required by DEB-TACE to achieve the same outcome. Second, the size of beads and dosage of chemotherapy was variable. Third, the timing and criteria used for radiological assessments were heterogeneous. Finally, the patient populations, for example, model for end-stage liver disease score and stage of fibrosis was not unified. Better efficacy and safety remain a theoretical possibility based on the aforementioned results.
We conclude that DEB-TACE, a newer technique for the treatment of unresectable HCC, is an effective modality and our cost analysis shows it to have cost benefits when compared with cTACE. Prospective head-to-head trials on selected patient populations are needed to evaluate its efficacy as well as cost benefits to the healthcare system.
Data sharing statement
The dataset is available from the corresponding author at ([email protected]). Consent was not obtained from participants but the presented data are anonymized and risk of identification is low.
Acknowledgments
The authors would like to thank all study patients and research staff at Nottingham Digestive Disease Centre for their participation in this study. The authors would also like to acknowledge the help of Dr Zergham Zia, Department of Radiology, Nottingham University Hospitals NHS Trust, Nottingham, UK for his help with radiological assessments; Mr Eleftherios Floros, Department of Health Sciences, University of Nottingham, Nottingham, UK for his help with cost analysis; and Mrs Gurpreet Mahal, Financial Deputy Manager at Nottingham University Hospital NHS Trust in providing relevant financial information. The research was funded by the National Institute of Health Research (NIHR) Nottingham Digestive Diseases Biomedical Research Unit. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
The abstract of this paper was presented at the 65th Annual Meeting of the American Association for the Study of Liver Diseases: The Liver Meeting 2014 as a poster presentation with interim findings. The poster’s abstract has been published.20
Author contributions
W Fateen and F Khan contributed equally to this work. All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Ferlay J, Shin H-R, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J cancer. 2010;127(12):2893–2917. | ||
Ferlay J, Soerjomataram I I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2014;136(5):E359–E386. | ||
Fateen W, Ryder S. Screening for hepatocellular carcinoma: patient selection and perspectives. J Hepatocell Carcinoma. 2017; 4:71–79. | ||
Llovet JM, Di Bisceglie AM, Bruix J, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100(10):698–711. | ||
Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60. | ||
Llovet JM, Real MI, Montaña X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359(9319):1734–1739. | ||
Cammà C, Schepis F, Orlando A, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology. 2002;224(1):47–54. | ||
Varela M, Real MI, Burrel M, et al. Chemoembolization of hepatocellular carcinoma with drug eluting beads: Efficacy and doxorubicin pharmacokinetics. J Hepatol. 2007;46(3):474–481. | ||
Burrel M, Reig M, Forner A, et al. Survival of patients with hepatocellular carcinoma treated by transarterial chemoembolisation (TACE) using drug eluting beads. Implications for clinical practice and trial design. J Hepatol. 2012;56(6):1330–1335. | ||
Zhou X, Tang Z, Wang J, et al. Doxorubicin-eluting beads versus conventional transarterialchemoembolization for the treatment of hepatocellular carcinoma: a meta-analysis. Int J Clin Exp Med. 2014; 7(11):3892–3903. | ||
Hui Y, Ruihua T, Jing L, et al. Meta-analysis of doxorubicin-eluting beads via transcatheter arterial chemoembolization in the treatment of unresectable hepatocellular carcinoma. Hepatogastroenterology. 2015; 62(140):1002–1006. | ||
Xie Z-B, Wang X-B, Peng Y-C, et al. Systematic review comparing the safety and efficacy of conventional and drug-eluting bead transarterial chemoembolization for inoperable hepatocellular carcinoma. Hepatol Res. 2015;45(2):190–200. | ||
Chen P, Yuan P, Chen B, Sun J, Shen H, Qian Y. Evaluation of drug-eluting beads versus conventional transcatheter arterial chemoembolization in patients with unresectable hepatocellular carcinoma: a systematic review and meta-analysis. Clin Res Hepatol Gastroenterol. 2017;41(1):75–85. | ||
Facciorusso A, Di Maso M, Muscatiello N. Drug-eluting beads versus conventional chemoembolization for the treatment of unresectable hepatocellular carcinoma: A meta-analysis. Dig Liver Dis. 2016;48(6):571–577. | ||
Vadot L, Boulin M, Guiu B, et al. Clinical and economic impact of drug eluting beads in transarterial chemoembolization for hepatocellular carcinoma. J Clin Pharm Ther. 2015;40(1):83–90. | ||
Cucchetti A, Trevisani F, Cappelli A, et al. Cost-effectiveness of doxorubicin-eluting beads versus conventional transarterial chemo-embolization for hepatocellular carcinoma. Dig Liver Dis. 2016;48(7):798–805. | ||
Dunn G, Mirandola M, Amaddeo F, Tansella M. Describing, explaining or predicting mental health care costs: a guide to regression models. Methodological review. Br J Psychiatry. 2003;183:398–404. | ||
Manning WG, Mullahy J. Estimating log models: to transform or not to transform? J Health Econ. 2001;20(4):461–494. | ||
Kodama Y, Matsui T, Tsuji K, et al. Is drug-eluting bead transcatheter arterial chemoembolization (TACE) associated with better tumor response than conventional TACE in meta-analysis? Hepatol Res. 2015;45(12):1258–1259. | ||
Poster presentations. Poster sessions. Hepatology. 2014;60(S1):92A–196A. Available from: http://onlinelibrary.wiley.com/doi/10.1002/hep.27415/full. Accessed September 16, 2017. |
Supplementary material
 © 2017 The Author(s). This work is published by Dove Medical Press Limited, and licensed under a Creative Commons Attribution License.
The full terms of the License are available at http://creativecommons.org/licenses/by/4.0/.
The license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
© 2017 The Author(s). This work is published by Dove Medical Press Limited, and licensed under a Creative Commons Attribution License.
The full terms of the License are available at http://creativecommons.org/licenses/by/4.0/.
The license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.