Back to Journals » Patient Related Outcome Measures » Volume 14
Health-Related Quality of Life Instruments for Clinical Trials in AL Amyloidosis: Report from the Amyloidosis Forum HRQOL Working Group
Authors Rizio AA , White MK , D’Souza A, Hsu K, Schmitt P , Quock TP , Signorovitch J, Lousada I, Sanchorawala V
Received 21 December 2022
Accepted for publication 23 March 2023
Published 18 May 2023 Volume 2023:14 Pages 153—169
DOI https://doi.org/10.2147/PROM.S399658
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Robert Howland
Avery A Rizio,1 Michelle K White,1 Anita D’Souza,2 Kristen Hsu,3 Paula Schmitt,4 Tiffany P Quock,5 James Signorovitch,6 Isabelle Lousada,3 Vaishali Sanchorawala7
1QualityMetric Incorporated, LLC, Johnston, RI, USA; 2Froedtert & MCW Cancer Center, Medical College of Wisconsin, Milwaukee, WI, USA; 3Amyloidosis Research Consortium, Boston, MA, USA; 4Amyloidosis Support Groups, Inc, Wood Dale, IL, USA; 5Prothena Biosciences Inc, South San Francisco, CA, USA; 6Epidemiology and Biostatistics, Analysis Group, Inc, Boston, MA, USA; 7Amyloidosis Center, Boston University School of Medicine and Boston Medical Center, Boston, MA, USA
Correspondence: Isabelle Lousada, Amyloidosis Research Consortium, 320 Nevada Street, Suite 210, Newton, MA, 02460, USA, Tel +1 617 467 5170, Email [email protected]
Abstract: Systemic AL (light chain) amyloidosis is a rare protein misfolding disorder associated with plasma cell dyscrasia affecting various organs leading to organ dysfunction and failure. The Amyloidosis Forum is a public–private partnership between the Amyloidosis Research Consortium and the US Food and Drug Administration Center for Drug Evaluation and Research with the goal of accelerating the development of effective treatments for AL amyloidosis. In recognition of this goal, 6 individual working groups were formed to identify and/or provide recommendations related to various aspects of patient-relevant clinical trial endpoints. This review summarizes the methods, findings, and recommendations of the Health-Related Quality of Life (HRQOL) Working Group. The HRQOL Working Group sought to identify existing patient-reported outcome (PRO) assessments of HRQOL for use in clinical trials and practice deemed relevant across a broad spectrum of patients with AL amyloidosis. A systematic review of the AL amyloidosis literature identified 1) additional signs/symptoms not currently part of an existing conceptual model, and 2) relevant PRO instruments used to measure HRQOL. The Working Group mapped content from each identified instrument to areas of impact in the conceptual model to determine which instrument(s) provide coverage of relevant concepts. The SF-36v2® Health Survey (SF-36v2; QualityMetric Incorporated, LLC) and Patient-Reported Outcomes Measurement Information System-29 Profile (PROMIS-29; HealthMeasures) were identified as instruments relevant to patients with AL amyloidosis. Existing evidence of reliability and validity was evaluated with a recommendation for future work focused on estimating clinically meaningful within-patient change thresholds for these instruments. For sponsors, the context of use—including specific research objectives, trial population, and investigational product under study—should inherently drive selection of the appropriate PRO instrument and endpoint definitions to detect meaningful change and enable patient-focused drug development.
Keywords: endpoints, drug development, rare diseases, patient-reported outcome, SF-36v2, PROMIS-29
Introduction
AL Amyloidosis and Health-Related Quality of Life
Systemic AL (light chain) amyloidosis is a rare protein misfolding disorder associated with plasma cell dyscrasia affecting various organs leading to organ dysfunction and failure. Many vital organs such as the heart, kidneys, liver, nervous system, and gastrointestinal system can be affected.1 AL amyloidosis arises from a small B cell clone in the bone marrow producing thermodynamically and kinetically unstable light chains which interact with matrix protein to form soluble oligomers and deposition of insoluble amyloid fibrils in target organs.1,2 AL amyloidosis is the most common of the systemic amyloid disorders, with an incidence of 3–12.7 per million person-years and an annual prevalence of 20–58 per million person-years.1,3
The median age at diagnosis is approximately 65 years.4,5 However, due to the multi-systemic nature of AL amyloidosis and the myriad of presenting symptoms, diagnosis is often delayed. The effectiveness of plasma-cell directed therapies, such as chemotherapy, immunotherapy, and/or high-dose melphalan followed by autologous stem cell transplantation (HDM/SCT), may be limited due to a delayed diagnosis, subsequently contributing to negative outcomes.6–8 While many available treatment options for patients with AL amyloidosis use regimens originally designed for multiple myeloma, daratumumab hyaluronidase-fihj in combination with bortezomib, cyclophosphamide, and dexamethasone was approved by the US Food and Drug Administration (FDA) in 2021 specifically for patients newly diagnosed with AL amyloidosis.9
Both the disease process itself and the available therapeutic regimens have been shown to negatively impact patients’ subjective experiences of everyday life, including their physical, psychological, and social functioning which collectively comprise the broader concept referred to as health-related quality of life (HRQOL).10–15 The HRQOL impact of AL amyloidosis is often devastating to the patient. Data from patients and clinicians have documented the widespread burden of this disease, which has been shown to negatively impact physical functioning (eg, impaired mobility), mental health (including contributing to feelings of emotional distress), sleep, social relationships, and the ability to participate in desired activities or roles, such as those associated with one’s family, career, or hobbies.10–12,14,16,17 To help summarize the overall experience of AL amyloidosis, conceptual models have been developed to present an overview of the signs, symptoms, and impacts of the disease.12,13,16 Additional work published in 2021 identified major themes that impact the lives of those with AL amyloidosis, namely: disease diagnosis, living with the disease, symptom burden, and social roles (ie, the need for a support system).18
While the manifestations of the disease itself certainly contribute to the impacts experienced by those with AL amyloidosis, difficult to tolerate treatment may further impair HRQOL, calling attention to the need for new treatments that are effective in halting disease progression, reversing organ damage, and improving HRQOL, while also being associated with a side effect profile that does not further reduce HRQOL. Most investigational clinical trials in AL amyloidosis have been designed without evidence-based guidance on which patient-reported outcome (PRO) measures are best suited to evaluate HRQOL and capture the patient voice in the context of a clinical trial.
Patient-focused drug development (PFDD) guidances have been produced by the FDA with the purpose of describing the methodological considerations for incorporating the patient voice and experience into medical product development.19,20 To align with the goals of PFDD, selection of clinical outcome assessments (COAs), including PRO instruments, should assess concepts that are important from the patient perspective, as well as for a proposed clinical trial’s context of use and drug development goals.15,21 PRO instrument selection may include generic instruments, with a focus on domains of relevance and importance to a target patient population that are also expected to be able to change with treatment in the clinical trial context. Typically, these selected COAs reflect disease-related symptoms and/or proximal impacts of the disease that have the potential to change with treatment, are not biased, and reflect lived experiences. The end goal is to provide evidence to not only support regulatory approval, but also to inform clinical practice and reflect the patient experience. Clinical trials must therefore be designed with clinically meaningful endpoints; given this goal, COAs used in AL amyloidosis trials should be fit-for-purpose in order to capture impacts of treatment and improvements in HRQOL over time. Additionally, the evaluation of HRQOL can be useful for various stakeholder groups, including payers.
The Amyloidosis Forum and Approach to Evaluation of Endpoints for AL Amyloidosis Trials
The Amyloidosis Forum is a public–private partnership between the FDA Center for Drug Evaluation and Research (CDER) and the Amyloidosis Research Consortium (ARC; www.arci.org), a non-profit organization designed to improve patients’ lives through accelerating research in AL amyloidosis. The Amyloidosis Forum’s development history, group composition, and goals have been described previously.16
In 2020, the Amyloidosis Forum led the formation of 6 working groups with the overarching goal of developing a set of recommendations for 1) organ-specific patient-relevant endpoints that could be used in clinical trials in AL amyloidosis, 2) patient-reported measures of HRQOL to be incorporated into trial endpoints, and 3) approaches to composite endpoints and the statistical analysis of trial data. Figure 1 details each of the working groups and provides additional information on the process followed by the HRQOL Working Group, which included a patient representative, a statistician, and experts in AL amyloidosis and PRO measures, representing academia, industry, health outcomes research, and regulatory agencies. The HRQOL Working Group’s goal was to identify and review characteristics of PRO instruments for use in HRQOL-focused endpoints in AL amyloidosis clinical trials. The chairperson (VS) led a process consisting of literature reviews to identify candidate PRO instruments, an update of an existing conceptual model for AL amyloidosis,12 mapping the areas of impact identified in the conceptual model to the item-level content of each candidate PRO instrument, and an evaluation of the psychometric properties of a subset of candidate PRO instruments. A series of meetings occurred over a 5-month period, during which the HRQOL Working Group iteratively reviewed available information to identify PRO instruments that could be used to support HRQOL endpoints in clinical trials for AL amyloidosis.
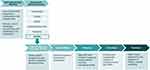 |
Figure 1 The Amyloidosis Forum set out to develop a novel, multi-domain, composite endpoint and/or analysis methods for use in clinical trials for AL amyloidosis. Specialized working groups identified condition-specific and HRQOL outcome measures; an additional working group focused on statistical approaches to analysis of clinical trial data. The HRQOL Working Group sought to identify existing PRO instruments, deemed relevant across a broad spectrum of AL amyloidosis patients, for use in clinical trials and practice to facilitate patient-focused drug development in AL amyloidosis. Figure adapted from one presented at the Amyloidosis Forum meeting on January 22, 2021.22 Abbreviations: Auto, autonomic; GI, gastrointestinal; HRQOL, health-related quality of life; PRO, patient-reported outcome. |
At a meeting of the Amyloidosis Forum held on 22 January 2021, the HRQOL Working Group reported preliminary findings and plans to develop a set of recommendations on the use of existing PRO measures to evaluate HRQOL and to consider whether development of a disease-specific instrument would be required to overcome gaps in measures for specific concepts. Forum proceedings are available at: https://amyloidosisforum.org.
Objective
The objective of the HRQOL Working Group was to identify and describe suitable instruments to evaluate HRQOL across a broad spectrum of AL amyloidosis patients and clinical trial designs. In identifying and reviewing HRQOL instruments that could be used to evaluate outcomes among patients with AL amyloidosis, this review seeks to provide a clear starting point for the incorporation of such outcome instruments in clinical trials.
Material and Methods
Literature Review of Patient-Reported Outcome Instruments in AL Amyloidosis
A literature search was conducted in PubMed (22 December 2020) to systematically document the type and breadth of PRO usage reported in published studies of AL amyloidosis. The search strategy included multiple disease terms (ie, primary amyloidosis, systemic amyloidosis, or light chain [AL] amyloidosis) and terms related to specified areas of interest (eg, physical function; mental health; variants of HRQOL, quality of life, and well-being; survey). Records were cross-referenced with articles provided by the Working Group members and a published systematic literature review17 to ensure completeness of the search results. Records returned by the search were evaluated using a 2-step process that included a round of title/abstract screening, followed by full text review of relevant articles. From these articles, a list of PRO instruments used to evaluate HRQOL in patients with AL amyloidosis was created.
Update to the Conceptual Model in AL Amyloidosis
Developing a comprehensive understanding of AL amyloidosis is the first step in incorporating patient-focused outcome measures in a clinical trial. A conceptual model is a diagram of the relationship among concepts related to a specific disease, characterizing the signs, symptoms, and impacts of a disease. Concepts of interest are aspects of an individual’s experience or state that an assessment is meant to capture. Concepts of interest must be selected specifically for each clinical trial, and must reflect aspects that are relevant to patients, are modifiable by the treatment under investigation, and can capture meaningful improvement in the time period of the trial.21 Once identified, concepts of interest are incorporated into endpoints of a clinical trial, which detail the specific assessments intended to measure each concept of interest. Therefore, the development of an accurate and complete conceptual model is an important first step to identifying a clinical trial endpoint strategy, as it provides information on which experiences or states could be included as concepts of interest, though the ultimate selection of concepts (and thus the endpoint strategy) may differ across trials. A conceptual model of AL amyloidosis has been developed based on evidence from literature review, clinician interviews, patient interviews, and online patient blogs.12 This model served as the basis for the Working Group’s conceptual model; 2 tasks were undertaken to explore whether updates to the model were needed.
As part of the Amyloidosis Forum initiative, patient representatives were invited to participate in each Forum meeting and Working Group. These representatives described the ways in which AL amyloidosis has impacted their lives; the information they provided was considered for inclusion in an updated conceptual model.
Along with solicitation of patient perspectives, the HRQOL Working Group performed a literature search to identify qualitative literature that would inform whether additional signs/symptoms or areas of impact should be added to the AL amyloidosis conceptual model. The search was conducted in PubMed (22 December 2020) using a strategy that incorporated multiple disease terms (ie, primary amyloidosis, systemic amyloidosis, or light chain [AL] amyloidosis) and terms related to qualitative research methodology (eg, focus group, interview). Records were screened for relevance, with potentially relevant articles undergoing full-text review. Concepts reported by either clinicians or patients with AL amyloidosis that relate to the signs, symptoms, or impacts of the disease were extracted from the articles. Extracted concepts were compared to those in the original model, and the members of the Working Group discussed whether additions to that model were needed.
Mapping Instrument Content to Areas of Impact
The individual items of each PRO instrument identified in the literature were compared to the concepts included in the updated conceptual model. This item-to-model mapping provided insight into the degree to which each PRO instrument measures concepts relevant to patients with AL amyloidosis and identified conceptual areas that were missing from the instruments, or content included in the instruments that was not relevant to patients with AL amyloidosis. Based on their correspondence to concepts included in the conceptual model, a subset of “highlighted PRO instruments” was selected for further evaluation.
Evaluation of Highlighted Health-Related Quality of Life Patient-Reported Outcome Instruments
Selected instruments were evaluated for possible inclusion in PRO-related endpoints for clinical trials of AL amyloidosis. Considerations included instrument characteristics (ie, item content, recall period, scoring, time to complete, modes of administration, and available translations), as well as documented evidence in AL amyloidosis of content validity, psychometric properties (ie, internal consistency reliability, test-retest reliability, convergent validity, known-groups validity, responsiveness), and score interpretation.
Results
Literature Review of Patient-Reported Outcome Instruments in AL Amyloidosis
PRO instruments have been used in prospective interventional clinical trials of AL amyloidosis to evaluate health status from the patient perspective (Table 1). The most frequently used PRO instruments in AL amyloidosis clinical trials have been the SF-36v2® Health Survey (SF-36v2; QualityMetric Incorporated, LLC; Johnston, RI USA) and the EuroQol-5D (EQ-5D; EuroQol, Rotterdam, The Netherlands).
PRO measures have also been used to evaluate HRQOL among patients with AL amyloidosis outside the space of clinical trials. Of the 146 articles identified through the search, 28 reported on the use of PRO measures (Figure 2A). While there were no published reports of AL amyloidosis disease-specific PRO instruments, 24 different PRO instruments have been described in the literature in the context of assessing patients with AL amyloidosis (Box 1). PRO instruments included as outcome measures in AL amyloidosis studies include condition-specific measures (eg, Kansas City Cardiomyopathy Questionnaire [KCCQ], Hematology Patient Reported Symptom Screen [HPRSS]), generic measures of HRQOL (SF-36v2, Patient-Reported Outcomes Measurement Information System [PROMIS] Global Health Scale, and PROMIS-29), and measures of mental health (eg, State-Trait Anxiety Inventory [STAI-Y], Center of Epidemiological Studies Depression Scale [CES-D]). PRO instruments were also used to evaluate work impacts (Work Productivity and Activity Impairment questionnaire), specific symptoms of AL amyloidosis (eg, PROMIS Fatigue, Epworth Sleepiness Score), and overall patient status using a single item (eg, Patient Global Impression of Severity). In addition to established PRO instruments, 3 manuscripts reported on the use of study-specific assessments developed by researchers to evaluate symptoms, treatment tolerance, quality of life, and/or the journey to diagnosis.11,16,23
 |
Box 1 List of Patient-Reported Outcome Instruments Used to Evaluate Health-Related Quality of Life in Published Papers of AL Amyloidosis |
 |
Table 1 Summary of Patient-Reported Outcome Instruments Incorporated into Secondary Endpoints in Recent Clinical Trials of AL Amyloidosis |
Update to the Conceptual Model in AL Amyloidosis
Every patient representative who participated in a Working Group described experiencing negative impacts on HRQOL. Examples of symptoms experienced by the representatives (not intended to capture all symptoms experienced) include overwhelming/debilitating fatigue, persistent nausea (even with supportive medications), and edema (with cardiac/kidney involvement). Patient representatives described needing assistive devices such as walkers, canes, and portable toilets. Common mental health issues described by patients include depression, anxiety, and the psychological impact of sexual dysfunction. The HRQOL Working Group patient representative (PS) noted patients frequently report physicians being less responsive to the psychological and emotional impacts of AL amyloidosis and stressed the negative impact of current treatments.
The literature review identified 4 articles that reported on clinician or patient-provided descriptors of the signs, symptoms, or impacts of AL amyloidosis (Figure 2B). A total of 12 signs/symptoms identified in the initial conceptual model were also described in at least 1 other source published since 2015.11,24,25 The qualitative literature review identified 2 signs/symptoms, carpal tunnel syndrome and fainting, that were not included in the original conceptual model. Convergence in the general areas of impact (physical functioning/daily activities, social functioning, emotional well-being, and sleep) was demonstrated between both the original published conceptual model and a second independent qualitative publication.12,25 Primary differences in the specific impacts documented in the original conceptual model and other publications were related to the language and detail with which impacts were described (eg, activities of daily living vs household chores).
Based on the strength of evidence, the HRQOL Working Group recommended updating the conceptual model to include fainting in the “Signs and Symptoms” section. Updates to the conceptual model are presented in Figure 3. It is important to note that pain and fatigue, which represent disease-related symptoms, are often incorporated into measures of HRQOL. As such, these 2 symptoms are included in the conceptual mapping described below.
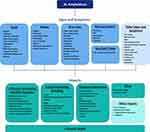 |
Figure 3 A conceptual model of AL amyloidosis was developed based on the work of Lin and colleagues,12 which incorporated information from patient interviews, clinician interviews, literature review, and online patient blogs. The HRQOL Working Group recommended updating the conceptual model based on the results of the literature review, which identified publications that reported on qualitative data from patients and/or clinicians on signs, symptoms, and impacts of AL amyloidosis. Concepts preceded by a “+” sign represent additions based on updated literature review (ie concepts that were not included in the original model). Adapted by permission of the publisher Taylor & Francis from Lin HM, Seldin D, Hui AM, Berg D, Dietrich CN, Flood E. The patient’s perspective on the symptom and everyday life impact of AL amyloidosis. Amyloid. 2015;22(4):244–251.12 |
Mapping Instrument Content to Areas of Impact
To assess conceptual coverage of relevant areas of impact experienced by patients with AL amyloidosis, the item content of each of the 19 multi-item PRO instruments identified through the literature review was mapped onto the areas of impact included in the updated conceptual model. Three generic measures of HRQOL—the SF-36v2, PROMIS-29, and PROMIS Global Health Scale—were identified as having the most overlap between item content and areas of impact identified in the conceptual model (Table 2; all other instrument mappings are shown in Supplemental Tables 1-3). The PROMIS Global Health Scale evaluated impacts at a “global” level but did not include many of the more specific impacts reported by patients with AL amyloidosis; thus, it was not recommended for further evaluation by the HRQOL Working Group. The SF-36v2 and PROMIS-29 were found to provide similar breadth of coverage for concepts relevant to patients with AL amyloidosis; differences in coverage within specified domains were noted.
 |
Table 2 Correspondence Between Concepts Measured by Generic Patient-Reported Outcome Instruments of Health-Related Quality of Life and Areas Impacted by AL Amyloidosis |
Overall, the SF-36v2 was found to include more items on concepts related to general health, physical functioning, and daily activities (20 items for SF-36v2 vs 6 items for PROMIS-29). The SF-36v2 produces individual domain scores for general health, physical functioning, and role limitations due to physical health. The PROMIS-29 also has a score for physical functioning; other items that relate to daily activities are part of the social functioning domain. Neither PRO instrument includes items on “reduction in physical strength”, although this concept could be measured by evaluating change from baseline over time.
Both the SF-36v2 and PROMIS-29 include 2 items clearly linked to social functioning/social interactions. With respect to evaluation of emotional well-being, both instruments include content that generally maps onto feelings of nervousness/anxiety and sadness/depression. The PROMIS-29, but not SF-36v2, specifically assesses worry and fear.
Neither the SF-36v2 nor the PROMIS-29 assess concepts such as anger, frustration, stress, difficulty coping, motivation, attractiveness, self-esteem, or cognition. Both PRO instruments assess pain interference and pain severity, though the PROMIS-29 has more items (5 items for PROMIS-29 vs 2 items for SF-36v2). Both instruments assess fatigue using 4 items, though in slightly different ways; only the PROMIS-29 assesses sleep. Neither instrument assesses loss of balance, appetite/food restrictions, or altered taste.
Highlighted Health-Related Quality of Life Patient-Reported Outcome Instruments
Summary characteristics of selected HRQOL measures are shown in Table 3. While the content mapping exercise provided insight to confirm the conceptual overlap between SF-36v2 and PROMIS-29 item content and the areas of impact identified in the conceptual model, additional evidence to support a recommendation for use in clinical trials was needed. The psychometric properties of SF-36v2 and PROMIS-29 were reviewed in the context of AL amyloidosis; properties are summarized in the following sections.
 |
Table 3 Summary of Patient-Reported Outcome Instruments of Health-Related Quality of Life to Be Considered for Use in Clinical Trials in AL Amyloidosis |
SF-36v2® Health Survey
The SF-36v2 is a PRO measure commonly used during all phases of medical product development.26 The instrument assesses 8 health domains (physical functioning, general health, role limitations due to physical health, bodily pain, social functioning, role limitations due to emotional health, vitality, mental health, and 1 health transition item) over a recall period of 4 weeks; an acute (1-week) recall option is also available. Each item has between 3 and 5 response options. The 8 health domains can also be summarized as a physical component summary (PCS) and mental component summary (MCS). Scores are benchmarked against a normative score of 50 and a standard deviation of 10, which reflects the average score of the US general population (or T-score of 50 for the average adult).
The literature review conducted by the HRQOL Working Group identified 12 published manuscripts that report on the use of the SF-36 (either SF-36v1 or SF-36v2) in patients with AL amyloidosis. These studies include both cross-sectional and longitudinal observational studies, a randomized control trial, and both retrospective and prospective evaluations of patients following HDM/SCT.
The content validity of the SF-36v2 in patients with AL amyloidosis has been demonstrated; qualitative interviews indicated the instrument is relevant, comprehensive, and understandable to this patient population.25 The psychometric properties of SF-36v2 in patients with AL amyloidosis were assessed using data from community-based (n = 341) and clinic-based (n = 1438) observational studies.27 Results indicated good internal consistency reliability (Cronbach’s alpha ≥0.76 for all domains), test–retest reliability across a 1-month period (intraclass correlation coefficients ≥0.73 for all domains), convergent validity, known-groups validity, and responsiveness.
Results from other studies in AL amyloidosis provide additional supportive evidence of the psychometric properties of the SF-36.14,23,25,27–31 All 8 domain scores, in addition to PCS, have been shown to correlate with B-type natriuretic peptide (BNP) levels. Evidence to support known-groups validity has been demonstrated when comparing across groups defined by numerous different patient characteristics, including time since diagnosis, cardiac involvement, and cardiac response to treatment.28 Providing evidence of the instrument’s predictive validity, initial SF-36v2 scores have been shown to be inversely associated with rates of emergency department visits and inpatient hospitalizations during a 12-month observation period. In addition, PCS scores are significantly related to 1- and 5-year mortality; Physical Functioning scores at baseline have been shown to be the leading factor in predicting survival after HDM/SCT.29
Additional evidence has also been published to support the instrument’s responsiveness (ie, ability to detect change), a property that is of particular importance in the context of clinical trials. Specifically, published data indicate that patients who achieved hematologic complete remission (CR) at 1-year post-HDM/SCT had significantly higher SF-36 scores than those who did not achieve CR.29 Taken together, the available literature supports the use of the SF-36v2 in patients with AL amyloidosis, including in clinical trials to evaluate improvement in HRQOL following therapeutic intervention.
PROMIS Instruments: PROMIS-29
The PROMIS-29 (HealthMeasures) consists of 28 items across 7 dimensions: depression, anxiety, physical function, pain interference, fatigue, sleep disturbance, and ability to participate in social roles and activities; each item has 5 response options. Pain intensity is measured as a separate item with a 0 (no pain) to 10 (worst pain imaginable) scale. The PROMIS-29 was designed to evaluate well-being over the prior 7-day period. Each domain is scored by T-score, which places an individual respondent’s score relative to the average score of the US general population (mean = 50, standard deviation = 10). Physical and mental health summary scores can also be calculated from the PROMIS-29.32
The literature review conducted by the HRQOL Working Group identified published reports that include data on PROMIS-29.18,31,33,34 Evidence to support the content validity or the test-retest reliability of the PROMIS-29 in patients with AL amyloidosis has not been published. Domains included in the PROMIS-29 have been shown to have good internal consistency reliability (Cronbach’s alpha for all domains ≥0.89) and convergent validity. Supporting the predictive validity of PROMIS-29, the majority of domain scores at baseline, after adjusting for amyloidosis stage, have been shown to be associated with 1-year mortality, including both physical and mental summary scores and all individual domains except pain interference and sleep disturbance.33 Limited evidence has been published to support known-groups validity: the physical functioning domain differed according to cardiac involvement; the physical functioning and social functioning domains differed according to disease severity (based on 2012 staging system) and number of organs involved; and the physical functioning, social functioning, and fatigue domains were lower than scores from the general population.34 Similarly, limited evidence has been published to support the responsiveness of select scales: change in physical function and depression domain scores were significantly associated with change in N-terminal pro B-type natriuretic peptide (NT-proBNP) and hematologic response.33
Given the available published literature, there is preliminary evidence to suggest that the PROMIS-29 is reliable and valid when used to evaluate HRQOL among patients with AL amyloidosis. Gaps in the available evidence (in particular, regarding content validity, test-retest reliability, known-groups validity, and responsiveness) may require further effort to establish suitability for use in clinical trials for AL amyloidosis.
Discussion
Considerations for Selection of Instruments to Evaluate Health-Related Quality of Life in AL Amyloidosis
The HRQOL Working Group evaluated multiple PRO instruments used previously in patients with AL amyloidosis. The majority of identified measures were excluded due to poor coverage of the full range of impacts experienced by patients with AL amyloidosis (as represented in the conceptual model). The content of both the SF-36v2 and PROMIS-29 instruments aligns with the areas of impact included in the AL amyloidosis conceptual model. Characteristics to support these PRO measures are summarized in Table 3.
Clinical trials that have used the SF-36v2 have generally incorporated the use of PCS score into an endpoint. Other domain scores may also be responsive to change and may also be considered for use in an endpoint. The duration of time across which change in a domain score should be assessed should account for trial-specific characteristics such as the trial design, mechanism of action of the intervention, and—relatedly—the expected time course for change on more distal properties such as HRQOL (which could differ across domains). As such properties are likely to differ across trials, the frequency of administration and time at which a meaningful change may be detected are expected to vary with the population and intervention under study.15
Other Patient-Reported Outcome Instruments Considered
The aim of the HRQOL Working Group was to identify a measure of HRQOL appropriate for use in clinical trials of patients with AL amyloidosis. To this end, it was important to consider the breadth of domains measured by the instrument (and its overlap with the areas of impact included in the conceptual model) and its relevance to patients regardless of specific organ involvement or symptoms. However, researchers may consider using additional PRO measures in a clinical trial; options for such additions are described briefly below. The decision to include any PRO measure in a clinical trial should have a clear rationale and be placed in the context of the current AL amyloidosis conceptual model, current knowledge of treatment benefit, the target product profile of the intervention, and the selection of trial endpoints. Researchers must also consider available evidence of the instrument’s psychometric properties in the specific patient population under evaluation.
Measures of Mental Health
AL amyloidosis is a debilitating condition, causing frustration, fear, anxiety, and depression.12,35 Measures of mental health—specifically the STAI-Y and the CES-D—provided coverage of specific aspects of mental well-being that are not assessed by other evaluated measures of HRQOL. The HRQOL Working Group agreed further evaluation is needed to assess whether there is a clear benefit to including a separate mental health–specific PRO instrument in AL amyloidosis clinical trials, or whether such an instrument would be better suited for clinicians to monitor the health of their patients in a health-care setting.
Measures of Specific Symptoms/Focused Areas of Impact
Additional PROs (PROMIS Fatigue, Epworth Sleepiness Score, STOP-Bang questionnaire for obstructive sleep apnea, Voice Handicap Index, Work Productivity Activity Impairment questionnaire) provide coverage of specific symptoms and areas of impact (eg, fatigue, sleepiness, work impairment) and may be applicable in certain trial designs. For example, a measure of sleep might be considered for pairing with the SF-36v2, which does not evaluate this domain. Researchers interested in exploring the use of such additional measures should carefully evaluate the correspondence between the outcomes measured by each symptom- or impact-specific item and the areas that are known to be impacted by AL amyloidosis to find the best match for the intended purpose.
Condition-Specific Measures
A variety of condition- or disease-specific measures have been used in patients with AL amyloidosis (eg, KCCQ, HPRSS). These instruments were generally not recommended by the HRQOL Working Group for a variety of reasons, including a lack of relevance across a broad spectrum of patients with AL amyloidosis. While data on the KCCQ in AL amyloidosis are limited, both the Cardiac and HRQOL Working Groups agreed that the KCCQ would be more useful to assess intermediate or late cardiac stage patients than earlier stage patients.36 Other organ-specific Working Groups have also reviewed the potential utility of symptom- or organ-specific HRQOL measures for use as tools in specific populations or in trials investigating the clinical benefit of drugs with an organ-targeted mechanism of action.
In general, when considering the inclusion of a disease-specific measure, attention should be paid to the patient population included in the trial to ensure the PRO instrument is relevant (as demonstrated through qualitative interviews to establish content validity). If administered in conjunction with a generic measure of HRQOL, the degree of content overlap between the measures should be examined; if a large amount of conceptual overlap exists, the inclusion of a second instrument may not be advantageous.
Summary, Future Directions, and Potential Limitations
Given the systemic, multi-organ, heterogeneous nature of AL amyloidosis, the Amyloidosis Forum is working toward identifying appropriate endpoints and analytical methodologies for use in clinical trials investigating novel therapies. HRQOL endpoints are critical components in the determination of clinical benefit for a given therapy. The HRQOL Working Group sought to identify PRO measures for HRQOL as the next step toward development of recommendations for endpoints in AL amyloidosis trials. The scope of the HRQOL Working Group also included a limited assessment of other condition-specific outcome measures with potential utility.
It is important to note that updating the conceptual model of AL amyloidosis and evaluating potential measures of HRQOL represent important initial steps in identifying appropriate endpoints for clinical trials. However, individual sponsors of clinical trials must use this information in light of their specific treatment’s mechanism of action and study design and build endpoint strategies accordingly. The conceptual model presented in this manuscript is only one piece of a full conceptual framework; the FDA provides additional guidance on tailoring endpoint selection to concept of use.21,37
The HRQOL Working Group conducted a systematic review of PRO measures in the AL amyloidosis literature, identified additional signs/symptoms and areas of impact for the conceptual model of AL amyloidosis, and mapped item content to areas of impact. The HRQOL Working Group recommended updates to the conceptual model to serve as a foundation for discussions and decisions related to the areas of HRQOL that should be assessed in patients with AL amyloidosis.
The group identified 2 PRO instruments, SF-36v2 and PROMIS-29, as the most relevant measures across the spectrum of AL amyloidosis patients and disease/treatment stages. Currently, there is no single instrument that comprehensively covers all areas of impact. SF-36v2 provides better coverage of impacts related to general health, physical functioning, and daily activities. The PROMIS-29 provides coverage of pain and sleep. However, both miss some specific important aspects of social functioning and mental health (eg, frustration, stress), though the domains themselves are assessed.
Based on currently available evidence, the HRQOL Working Group identified the SF-36v2 as a general PRO instrument which may address concepts important from the perspective of a patient with AL amyloidosis. Despite evidence to support the content validity and psychometric properties of the SF-36v2 in patients with AL amyloidosis, additional work to help support the use of the instrument in clinical trials may be warranted. Specifically, analyses are needed to estimate disease-specific thresholds to evaluate meaningful within-patient change (ie, responder definitions). Establishing a priori responder definitions for SF-36v2 domains will facilitate the evaluation of HRQOL in future clinical trials. Based on the target population and a treatment’s mechanism of action, specific domain scores of SF-36 (eg, physical function) may be easier to interpret than summary scores and should be considered. Similar work would also be beneficial to support future use of the PROMIS-29.
Many of the items in PROMIS metrics are derived from the SF-36 and other legacy measures. With additional AL amyloidosis–specific qualitative (eg, content validation) and quantitative evidence (eg, additional evaluation of measurement properties), the PROMIS-29 would likely also be a suitable candidate for use in clinical trials for AL amyloidosis. In general, both the SF-36v2 and PROMIS-29 offer potential for flexibility and reduction of patient burden through a modular approach to measurement (ie, different domains can be selected depending on the concept of interest) and the customization of measurement enabled by item banks.
Both the SF-36v2 and the PROMIS-29 are generic, rather than disease-specific instruments. To date, no AL amyloidosis-specific measures of HRQOL have been developed. Recommendation of generic PROs is in line with the report provided by the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) COA Emerging Good Practices Task Force; benefits to using generic PROs include the ability to make comparisons to the general population which may aid interpretation, availability of translations, and greater familiarity among regulatory bodies.38 Should future efforts be made to develop an AL amyloidosis–specific measure of HRQOL, the potential benefits of such an instrument (eg, broader conceptual coverage across relevant impacts) should be evaluated in light of the stated advantages of generic instruments.
The HRQOL Working Group was convened in recognition of the importance of evaluating HRQOL in clinical trials of AL amyloidosis, with the goal of aiding researchers in incorporating the voice of patients into medical product development. Providing guidance on which PRO measures to use in clinical trials is an important step in reaching this goal. Given the complexity and multi-systemic nature of AL amyloidosis and the intended context of use for a particular investigational product, sponsors should also seek to engage regulatory authorities to prospectively consider the acceptability of a general HRQOL outcome measure for use in a development program. It is also important to rank PRO-derived endpoints appropriately in the endpoint hierarchy and consider specific domains of interest (eg, disease-related symptoms and proximal impacts of the disease on functioning) that are most likely to demonstrate change in a clinical trial. Future efforts of the HRQOL Working Group may involve evaluation of available clinical trial datasets or exploration of one or more new disease-specific instruments to cover remaining gaps in concepts between the updated conceptual map and the existing instruments.
Conclusion
Measuring outcomes relevant to patients, such as HRQOL, should be recognized as a critical component of medical product development in AL amyloidosis. The SF-36v2 and PROMIS-29 were identified as instruments relevant to patients with AL amyloidosis and have demonstrated evidence of reliability and validity. As a result, these instruments may be considered for inclusion in clinical trials with this patient population. In developing trial endpoints that incorporate scores from these instruments, sponsors must take into account trial-specific considerations, such as the duration of the trial, the expected time course over which change in the endpoints are expected to occur, and how the specific domains of HRQOL will be prioritized in an endpoint strategy.
Abbreviations
AL (or LC), immunoglobulin light chain; ARC, Amyloidosis Research Consortium; BNP, B-type natriuretic peptide; CDER, Center for Drug Evaluation and Research; CES-D, Center of Epidemiological Studies Depression Scale; COA, clinical outcome assessments; CR, complete (hematologic) remission; EORTC QLQ-C30, European Organisation for Research and Treatment of Cancer Quality of Life Group Cancer Patients core questionnaire; EQ-5D, EuroQol-5 dimension; FACT-GOG-NTX, Functional Assessment of Cancer Therapy/Gynecological Oncology Group – Neurotoxicity; FDA, United States Food and Drug Administration; ISPOR, International Society for Pharmacoeconomics and Outcomes Research; HDM/SCT, high-dose melphalan followed by autologous stem cell transplantation; HPRSS, Hematology Patient Reported Symptom Screen; HRQOL, health-related quality of life; KCCQ, Kansas City Cardiomyopathy Questionnaire; MCS, Mental Component Summary; NT-proBNP, N-terminal pro B-type natriuretic peptide; PCS, Physical Component Summary; PFDD, patient-focused drug development; PRO, patient-reported outcome; PROMIS-29, Patient-Reported Outcome Measurement Information System-29 Profile; SF-36v2, SF-35v2® Health Survey; STAI-Y, State-Trait Anxiety Inventory.
Data Sharing Statement
Materials described in the manuscript, including all relevant raw data, will be freely available to any researcher wishing to use them for non-commercial purposes, without breaching participant confidentiality. All data generated or analyzed as part of the HRQOL Working Group’s efforts are included in this published article; supplemental tables have been provided.
The Amyloidosis Forum meetings are publicly available (https://amyloidosisforum.org/). Recordings from the 15 October 2020 meeting, Novel Endpoints and Analyses in Multisystemic Rare Disease Trials, are available at https://amyloidosisforum.org/novel-endpoint-and-analyses/. Recordings from the 22 January 2021 meeting, Considerations for Novel Endpoint Development in AL Amyloidosis, are available at https://amyloidosisforum.org/considerations-for-novel-endpoint-development-in-al-amyloidosis/.
Acknowledgments
The authors would like to recognize Paula Schmitt (Amyloidosis Support Groups) and all patient representatives for presenting powerful patient perspectives as part of the Amyloidosis Forum. Michelle Campbell and Elektra Papadopoulos (United States Food and Drug Administration) also participated in the Working Group.
Sarah Cairns-Smith and Robyn Himick of the Amyloidosis Research Consortium provided project management and administrative support. Kimberly Denis-Mize provided professional writing services in the preparation of this manuscript and was funded by the Amyloidosis Research Consortium.
This article reflects the views of the authors and should not be construed to represent views or policies of the US Food and Drug Administration.
Author Contributions
All authors made a significant contribution to the work reported, whether in the conception, study design, execution, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The Amyloidosis Forum is funded by ARC. ARC is funded through private/philanthropic donations and grants from for-profit pharmaceutical and biotechnology companies. ARC retains all influence, control, and autonomy over projects for which it has received external support. ARC received grants from AbbVie, Alexion, Caelum, GlaxoSmithKline, and Janssen in support of the Amyloidosis Forum Novel Endpoints and Analyses meeting series. ARC was responsible for designing the meeting series, co-developing each Amyloidosis Forum meeting agenda, establishing multi-stakeholder working groups, production of meeting materials, hosting each virtual meeting, and creating publications. Research reported in this publication was supported by the National Institute on Aging (Award Number R13AG071150), of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Disclosure
Avery A. Rizio is an employee of QualityMetric Incorporated, LLC and reports research funding from Amyloidosis Research Consortium. Michelle K. White is an employee of QualityMetric Incorporated, LLC. Anita D’Souza reports research funding from the National Heart, Lung, and Blood Institute (K23 HL141445, R01 HL166339); institutional research funding from AbbVie, Caelum, Celgene, Janssen, Novartis, Takeda, Sanofi, and TeneoBio; Advisory boards of Bristol Myers Squibb, Pfizer, and Janssen; Consultant for BMS/Celgene, Janssen and Prothena. Paula Schmitt reports personal fees from Alnylam Pharmaceuticals, outside the submitted work. Tiffany P. Quock is an employee of Prothena Biosciences Inc. and holds stock in Prothena Corporation plc group. James Signorovitch is an employee of Analysis Group Inc., which has received research funding from ARC. Vaishali Sanchorawala reports research funding from Prothena, Caelum, Millennium-Takeda, Takeda, Celgene, Janssen, Karyopharm, Sorrento, Oncopeptide, Alexion; Advisory boards for AbbVie, Proclara, Prothena, Caelum, Janssen, Regeneron, Protego, Pharmatrace, Telix, Alexion/AstraZeneca; Consultant for Pfizer, Janssen. The authors report no other conflicts of interest in this work.
References
1. Merlini G, Dispenzieri A, Sanchorawala V, et al. Systemic immunoglobulin light chain amyloidosis. Nat Rev Dis Primers. 2018;4(1):38. doi:10.1038/s41572-018-0034-3
2. Gertz MA, Comenzo R, Falk RH, et al. Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): a consensus opinion from the 10th International symposium on amyloid and amyloidosis. Am J Hematol. 2005;79(4):319–328. doi:10.1002/ajh.20381
3. Quock TP, Yan T, Chang E, Guthrie S, Broder MS. Epidemiology of AL amyloidosis: a real-world study using US claims data. Blood Adv. 2018;2(10):1046–1053. doi:10.1182/bloodadvances.2018016402
4. Desport E, Bridoux F, Sirac C, et al. AL amyloidosis. Orphanet J Rare Dis. 2012;7:54. doi:10.1186/1750-1172-7-54
5. Muchtar E, Gertz MA, Kyle RA, et al. A modern primer on light chain amyloidosis in 592 patients with mass spectrometry-verified typing. Mayo Clin Proc. 2019;94(3):472–483. doi:10.1016/j.mayocp.2018.08.006
6. Schulman A, Connors LH, Weinberg J, et al. Patient outcomes in light chain (AL) amyloidosis: the clock is ticking from symptoms to diagnosis. Eur J Haematol. 2020;105(4):495–501. doi:10.1111/ejh.13472
7. Varga C, Comenzo RL. High-dose melphalan and stem cell transplantation in systemic AL amyloidosis in the era of novel anti-plasma cell therapy: a comprehensive review. Bone Marrow Transpl. 2019;54(4):508–518. doi:10.1038/s41409-018-0284-4
8. Sanchorawala V. High-dose melphalan and autologous peripheral blood stem cell transplantation in AL amyloidosis. Acta Haematol. 2020;143(4):381–387. doi:10.1159/000506498
9. Kastritis E, Palladini G, Minnema MC, et al. Daratumumab-based treatment for immunoglobulin light-chain amyloidosis. N Engl J Med. 2021;385(1):46–58. doi:10.1056/NEJMoa2028631
10. Lousada I, Comenzo RL, Landau H, Guthrie S, Merlini G. Light chain amyloidosis: patient experience survey from the Amyloidosis Research Consortium. Adv Ther. 2015;32(10):920–928. doi:10.1007/s12325-015-0250-0
11. McCausland KL, White MK, Guthrie SD, et al. Light chain (AL) amyloidosis: the journey to diagnosis. Patient. 2018;11(2):207–216. doi:10.1007/s40271-017-0273-5
12. Lin HM, Seldin D, Hui AM, Berg D, Dietrich CN, Flood E. The patient’s perspective on the symptom and everyday life impact of AL amyloidosis. Amyloid. 2015;22(4):244–251. doi:10.3109/13506129.2015.1102131
13. Wilson IB, Cleary PD. Linking clinical variables with health-related quality of life. A conceptual model of patient outcomes. JAMA. 1995;273(1):59–65. doi:10.1001/jama.1995.03520250075037
14. Bayliss M, McCausland KL, Guthrie SD, White MK. The burden of amyloid light chain amyloidosis on health-related quality of life. Orphanet J Rare Dis. 2017;12(1):15. doi:10.1186/s13023-016-0564-2
15. Food and Drug Administration. Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. Guidance for Industry. Food and Drug Administration (US); 2009.
16. Inaugural Amyloidosis Forum P, Lousada I. The amyloidosis forum: a public private partnership to advance drug development in AL amyloidosis. Orphanet J Rare Dis. 2020;15(1):268. doi:10.1186/s13023-020-01525-2
17. Lin HM, Gao X, Cooke CE, et al. Disease burden of systemic light-chain amyloidosis: a systematic literature review. Curr Med Res Opin. 2017;33(6):1017–1031. doi:10.1080/03007995.2017.1297930
18. D’Souza A, Myers J, Cusatis R, et al. Development of a conceptual model of patient-reported outcomes in light chain amyloidosis: a qualitative study. Qual Life Res. 2022;32:1083–1092. doi:10.1007/s11136-021-02943-w
19. Food and Drug Administration. Patient-Focused Drug Development: Methods to Identify What is Important to Patients. Guidance for Industry. Food and Drug Administration (US); 2022.
20. Food and Drug Administration. Patient-Focused Drug Development: Collecting Comprehensive and Representative Input. Guidance for Industry. Food and Drug Administration (US); 2020.
21. Food and Drug Administration. Patient-focused drug development: Selecting, developing, or modifying fit-for-purpose clinical outcome assessments. Guidance for Industry [draft]. Food and Drug Administration (US): 2022.
22. Lousada I. Considerations for Novel Endpoint Development in AL Amyloidosis.
23. Rizio AA, White MK, McCausland KL, et al. Treatment tolerability in patients with immunoglobulin light-chain amyloidosis. Am Health Drug Benefits. 2018;11(8):430–437.
24. Lousada I, Boedicker M. The impact of al amyloidosis: the patient experience. Hematol Oncol Clin North Am. 2020;34(6):1193–1203. doi:10.1016/j.hoc.2020.08.003
25. White MK, Bayliss MS, Guthrie SD, Raymond KP, Rizio AA, McCausland KL. Content validation of the SF-36v2® health survey with AL amyloidosis patients. J Patient Rep Outcomes. 2017;1(1):13. doi:10.1186/s41687-017-0020-7
26. Maruish ME, Ed. User’s Manual for the SF-36v2 Health Survey.
27. White MK, McCausland KL, Sanchorawala V, Guthrie SD, Bayliss MS. Psychometric validation of the SF-36 health survey in light chain amyloidosis: results from community-based and clinic-based samples. Patient Relat Outcome Meas. 2017;8:157–167. doi:10.2147/PROM.S146849
28. McCausland KL, Quock TP, Rizio AA, et al. Cardiac biomarkers and health-related quality of life in patients with light chain (AL) amyloidosis. Br J Haematol. 2019;185(5):998–1001. doi:10.1111/bjh.15693
29. Seldin DC, Anderson JJ, Sanchorawala V, et al. Improvement in quality of life of patients with AL amyloidosis treated with high-dose melphalan and autologous stem cell transplantation. Blood. 2004;104(6):1888–1893. doi:10.1182/blood-2004-01-0089
30. Sanchorawala V, McCausland KL, White MK, et al. A longitudinal evaluation of health-related quality of life in patients with AL amyloidosis: associations with health outcomes over time. Br J Haematol. 2017;179(3):461–470. doi:10.1111/bjh.14889
31. D’Souza A, Szabo A, Flynn KE, et al. Adjuvant doxycycline to enhance anti-amyloid effects: results from the dual Phase 2 trial. eClinicalMedicine. 2020;2:23.
32. Hays RD, Spritzer KL, Schalet BD, Cella D. Promis®-29 v2.0 profile physical and mental health summary scores. Qual Life Res. 2018;27(7):1885–1891. doi:10.1007/s11136-018-1842-3
33. D’Souza A, Brazauskas R, Dispenzieri A, Panepinto J, Flynn KE. Changes in patient-reported outcomes in light chain amyloidosis in the first year after diagnosis and relationship to NT-proBNP change. Blood Cancer J. 2021;11(2):29. doi:10.1038/s41408-021-00412-8
34. D’Souza A, Magnus BE, Myers J, Dispenzieri A, Flynn KE. The use of PROMIS patient-reported outcomes (PROs) to inform light chain (AL) amyloid disease severity at diagnosis. Amyloid. 2020;27(2):111–118. doi:10.1080/13506129.2020.1713743
35. Shu J, Lo S, Phillips M, et al. Depression and anxiety in patients with AL amyloidosis as assessed by the SF-36 questionnaire: experience in 1226 patients. Amyloid. 2016;23(3):188–193. doi:10.1080/13506129.2016.1208081
36. Maurer MS, Dunnmon P, Fontana M, et al. Proposed cardiac end points for clinical trials in immunoglobulin light chain amyloidosis: report from the Amyloidosis Forum Cardiac Working Group. Circ Heart Fail. 2022;15(6):e009038. doi:10.1161/CIRCHEARTFAILURE.121.009038
37. Food and Drug Administration. Patient-focused drug development: Incorporating clinical outcome assessments into endpoints for regulatory decision-making. Guidance for Industry [draft]. Food and Drug Administration (US): 2023
38. Benjamin K, Vernon MK, Patrick DL, Perfetto E, Nestler-Parr S, Burke L. Patient-reported outcome and observer-reported outcome assessment in rare disease clinical trials: an ISPOR COA Emerging Good Practices Task Force Report. Value Health. 2017;20(7):838–855. doi:10.1016/j.jval.2017.05.015
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

