Back to Journals » Patient Preference and Adherence » Volume 12
Health-related quality of life in type 2 diabetes mellitus patients with different risk for obstructive sleep apnea
Authors Gabric K , Matetic A, Vilovic M, Ticinovic Kurir T, Rusic D, Galic T, Jonjic I, Bozic J
Received 10 February 2018
Accepted for publication 23 March 2018
Published 9 May 2018 Volume 2018:12 Pages 765—773
DOI https://doi.org/10.2147/PPA.S165203
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Kresimir Gabric,1,2,* Andrija Matetic,1,* Marino Vilovic,1 Tina Ticinovic Kurir,1 Doris Rusic,3 Tea Galic,4 Ivana Jonjic,2 Josko Bozic1
1Department of Pathophysiology, University of Split School of Medicine, Split, Croatia; 2University Eye Hospital Svjetlost, Zagreb, Croatia; 3Department of Pharmacy, University of Split School of Medicine, Split, Croatia; 4Study of Dental Medicine, University of Split School of Medicine, Split, Croatia
*These authors contributed equally to the work
Purpose: Our study primarily aimed to investigate health-related quality of life (HRQoL) in type 2 diabetes mellitus (T2DM) patients with different risk for obstructive sleep apnea (OSA).
Patients and methods: This cross-sectional, questionnaire-based study included 466 adult patients with T2DM on regular visit to Center for Diabetes of University Hospital of Split from April to September 2017. All subjects underwent detailed anamnestical evaluation and physical examination with anthropometric measurements. Additionally, all subjects completed STOP (Snoring, Tiredness, Observed apnea, and high blood Pressure) questionnaire to assess risk for OSA, Epworth Sleepiness Scale to assess daytime sleepiness, and Medical Outcomes Study Short Form-36 (SF-36) instrument to evaluate HRQoL.
Results: Most subjects (N=312, 67.0%) represented high-risk OSA group based on STOP questionnaire (STOP score ≥2). Statistically significantly lower HRQoL scores in all SF-36 dimensions were found in T2DM patients with high risk for OSA compared to low-risk group (P<0.001). STOP score showed statistically significant negative correlation with all SF-36 dimensions (P<0.001). In multiple linear regression analysis, STOP score was confirmed as statistically significant independent predictor for all SF-36 components, adjusted for body mass index, age, glycated hemoglobin, and T2DM duration (P<0.001).
Conclusion: Our study found that high proportion of patients with T2DM are at high risk for OSA. Furthermore, we showed that group of T2DM patients with high risk for OSA has lower HRQoL in all SF-36 dimensions compared to low-risk patients.
Keywords: quality of life, type 2 diabetes mellitus, obstructive sleep apnea, surveys and questionnaires
Plain language summary
It is estimated that type 2 diabetes mellitus (T2DM) affects 422 million adults worldwide representing significant global health burden. Chronic therapy, disease burden, and well-known complications of T2DM affect all aspects of everyday life. Moreover, T2DM is often associated with multiple comorbidities which impair quality of life.
One such comorbidity is obstructive sleep apnea (OSA) which is a well-established disease playing a major role in the pathophysiology of metabolic disorders. It has been already shown that the prevalence of OSA is higher among patients with T2DM compared to general population and that OSA influences glucose regulation. However, the impact of OSA on quality of life in T2DM patients was not thoroughly investigated.
Our questionnaire-based study aimed to investigate differences in quality of life among T2DM patients with different risk for OSA. Since full-night polysomnography, which is a gold standard for OSA diagnosis, is a time-consuming and an expensive method, and available questionnaires offer acceptable sensitivity and specificity for OSA prediction, it is reasonable to use such tools.
T2DM patients from our study with high risk for OSA exhibited lower values in all aspects of quality of life. This finding was confirmed after analysis adjustments for several confounding variables such as body mass index and HbA1c which are well-known influential factors.
Our study emphasizes the importance of routine measurements of quality of life among T2DM patients, especially when associated with comorbidities such as OSA.
Introduction
Type 2 diabetes mellitus (T2DM) and obstructive sleep apnea (OSA) are highly prevalent chronic diseases with numerous complications and significant morbidity. It is estimated that almost every 12th adult in the world suffers from diabetes, while at least 14% of male and 5% of female population have OSA.1,2 Furthermore, specific subgroups have even higher prevalence based on the presence of acclaimed risk factors.3 Due to increasing prevalence, adverse health impact, and enormous economic burden, T2DM and OSA represent burgeoning global health issue.1,2
OSA is a most common sleep-related breathing disorder characterized by reversible upper airway collapse leading to intermittent airflow cessation. Major pathophysiological effects of such events are intermittent hypoxia, sleep fragmentation, cortical arousals, and sympathetic excitation, which make patients with OSA prone to development of numerous long-term complications. Except cardiovascular and cerebrovascular disorders, metabolic dysfunction is a well-known complication of OSA.4,5 Previous studies have showed increased prevalence and incidence of T2DM in OSA patients compared to general population.6,7 Notably, in a large multiethnic study, prevalence of T2DM increased with worsening OSA severity.8 In fact, it seems that autonomic neuropathy can affect respiratory control and upper airway patency while asleep, leading to bidirectional effects of OSA and T2DM.9
Aforementioned findings indicate that patients with T2DM should be screened for OSA symptoms. Consequently, it has been shown that most of the primary care diabetic patients have high risk for OSA.10 Available questionnaires for OSA risk such as STOP (Snoring, Tiredness, Observed apnea, and high blood Pressure) questionnaire offer sufficient strength in predicting OSA.11 Accordingly, polysomnographically based studies confirm increased prevalence of OSA among T2DM population.12 Moreover, these findings are highlighted by the fact that even in the general population most of the individuals with OSA are undiagnosed.10 Finally, available studies support the evidence for independent association of increasing OSA severity with higher glycated hemoglobin (HbA1c) values.8,13
Health-related quality of life (HRQoL) in patients with chronic disease is an essential clinical outcome with significant health implications. Disease chronicity, numerous complications, and long-term therapy have significant physical and psychological consequences affecting individual’s everyday functioning and well-being.14
Previous studies have showed adverse effects of OSA on HRQoL.15 OSA-related symptoms such as excessive daytime sleepiness, nocturia, morning headaches, cognitive dysfunction, and impotence disturb daily activities and welfare.4 Importantly, occupational and traffic accidents are common in OSA.15
Likewise, numerous studies have observed lower overall HRQoL in diabetic patients.16 Widely familiar diabetes-related symptoms, negative pathophysiologic effects, and multiple diabetes-related complications along with multiple comorbidities have significant everyday consequences.1 Accordingly, it has been shown that patients with worse glycemic control exhibit decreased HRQoL, yet available studies are inconsistent.17 Importantly, decreased HRQoL is associated with increased mortality in patients with T2DM.18
Additionally, both OSA and T2DM patients have increased prevalence and incidence of multiple comorbidities which certainly deteriorate HRQoL, such as depression, cerebrovascular, and cardiovascular disorders.4,19,20 Moreover, obesity, which is a major risk factor for OSA and T2DM, impairs HRQoL.21
Important determinant of HRQoL is quality of sleep.22 It seems that sleep quality plays an important role in “circulus vitiosus” of T2DM and sleep-disordered breathing. Previous studies suggest that most diabetic patients have impaired sleep quality.23 Moreover, there are evidences that decreased sleep amount and impaired sleep quality deteriorate glycemic control.24 Since sleep quality markedly contributes to HRQoL, it is reasonable that concomitant OSA lowers HRQoL in diabetic patients.12,22 However, whether the presence of high risk for OSA has aggravational impact on HRQoL in diabetic patients still remains to be elucidated.
Therefore, main goal of our study was to evaluate risk for OSA in addition to HRQoL in T2DM patients.
Patients and methods
Study design
This cross-sectional, questionnaire-based study was conducted in Center for Diabetes of University Hospital of Split over a period from April to September 2017. All participants were informed about procedures, course, and aim of this study.
Ethical considerations
Written informed consent was obtained from all individual participants included in the study. The study protocol was approved by the Ethics Committee of the University of Split School of Medicine. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Subjects
Study sample of 466 patients on regular visit to diabetologist were enrolled in the study. Inclusion criteria were having T2DM, aged 18–75 years, and receiving stable medication therapy for 3 months prior to the research. Exclusion criteria were prior diagnosis of OSA or other sleep-related breathing disorder; type 1 diabetes mellitus; gestational diabetes or other type of diabetes mellitus; severe cardiovascular, pulmonary, renal, neurologic, or psychiatric disease; active malignant disease; and alcohol or substance abuse.
Clinical assessment
Anthropometric measurements were proceeded according to the conventional medical standards. All patients on regular visit brought results of HbA1c values that are not older than 7 days. Detailed medical history was obtained from anamnestical data, medical records, and self-reports. After regular clinical evaluation, all patients were asked to complete questionnaires for assessment of OSA-related variables and HRQoL.
OSA-related variables
Risk for OSA was determined with STOP questionnaire, while Epworth Sleepiness Scale (ESS) was used to assess daytime sleepiness.
ESS comprises 8 questions related to likelihood of falling asleep in ordinary daily situations which patients score on a scale from 0 (never) to 3 (high likelihood). Higher sum of 8-question score, ranging from 0 to 24, indicates increased propensity to sleep.25 Subjects were classified into 3 groups based on ESS score (≤6 normal; 7 and 8 moderate; ≥9 severe). Validated Croatian version of the ESS had been used.11
STOP questionnaire is a special 4-item survey primarily designed to assess the risk for OSA in surgical patients.26 It contains 4 questions related to snoring, daytime tiredness, observed apnea, and blood pressure in yes/no format. Patients with 1 positive answer were classified as having low risk for OSA and patients with 2 or more positive answers were classified as having high risk. Validated Croatian version of the STOP questionnaire with sensitivity of 96% and specificity of 83% had been used.11 Additional questions directed at presence of hypertension, depression, asthma, and gastroesophageal reflux disease (GERD) were added in the questionnaire.
HRQoL assessment
HRQoL was measured by Medical Outcomes Study Short Form-36 (SF-36) questionnaire which has been a well established instrument for HRQoL in OSA patients.27 SF-36 is a multifunctional, non-disease specific, 36-question health survey that evaluates 8 domains of health providing an overall assessment of HRQoL. Health aspects that are being valued are physical functioning, physical role limitations, bodily pain, general health perception, social functioning, emotional role limitations, emotional well-being, and vitality. Each domain is scored on a 0 to 100 scale, with higher score indicating better HRQoL.28 Validated Croatian version of the SF-36 questionnaire had been used.29
Statistical analysis
Statistical software MedCalc (Mariakerke, Belgium; version 11.5.1.0) for Windows was used for statistical data analysis. Data were expressed as mean ± standard deviation for continuous variables and as whole numbers and percentage for categorical variables. Kolmogorov–Smirnov test has been used for normality of data distribution. Student’s t-test was used for comparison of independent continuous data between groups with different risk for OSA. Chi-square test has been used for analysis of gender differences and comorbidites presence between groups with different risk for OSA. Pearson correlation served for assessment of correlation between SF-36 variables and other variables. Multiple linear regression analysis with stepwise selection algorithm was used to determine the relative importance of independent variables (STOP score, body mass index [BMI], age, HbA1c, and T2DM duration) in prediction of SF-36 parameters. Variables that did not reach statistical significance were excluded from the model. The statistical significance was defined as P<0.05.
Results
Sample characteristics
Our sample comprised a total of 466 subjects; of them, 47.8% were male subjects. Mean age of our population was 66.3±7.7 years, while mean BMI was 28.7±2.0 kg/m2. Furthermore, mean HbA1c value of our study sample was 7.9%±1.8% and mean T2DM duration was 13.2±9.4 years (Table 1).
Mean STOP score of our sample was 2.1±1.1. Therefore, most subjects (N=312, 67.0%) represented high-risk OSA group based on STOP questionnaire. High-risk OSA group exhibited higher BMI (29.6±5.1 vs 27.0±4.4 kg/m2, P<0.001), neck circumference (38.2±5.0 vs 37.1±3.7 cm, P=0.003), waist circumference (103.7±13.8 vs 98.2±12.2 cm, P<0.001), ESS score (6.5±4.5 vs 4.0±3.3, P<0.001), and duration of T2DM (14.3±9.2 vs 11.0±5.0 years, P<0.001). There was no difference in other variables between groups (Table 1).
Comorbidities
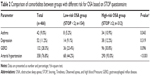
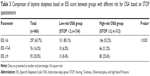
Prevalence of arterial hypertension and asthma was significantly higher in subjects with high risk for OSA based on STOP questionnaire (93.0%, N=290 vs 44.2%, N=68, P<0.001 and 10.9%, N=34 vs 5.2%, N=8, P=0.043, respectively). Furthermore, group with high risk for OSA exhibited higher prevalence of depression and GERD (12.2%, N=38 vs 9.1%, N=14, P=0.319 and 30.8%, N=96 vs 23.4%, N=36, P=0.096, respectively), but without statistical significance (Table 2).
Daytime sleepiness
Most patients represented low/normal daytime sleepiness (63.7%), followed by severe (20.0%) and finally moderate daytime sleepiness group (16.3%). Among high-risk OSA group, there were statistically significantly more subjects with ESS ≥7 (P<0.001) (Table 3).
HRQoL analysis
High-risk OSA group exhibited significantly lower SF-36 scores in all health dimensions compared to low-risk OSA group of our sample based on STOP questionnaire (P<0.001) (Table 4).
Furthermore, STOP score and BMI showed statistically significant negative correlation with all SF-36 dimensions (P<0.001). Moreover, age was in statistically significant negative correlation with physical functioning (r=−0.30, P<0.001), physical role limitations (r=−0.33, P<0.001), bodily pain (r=−0.31, P<0.001), emotional role limitations (r=−0.12, P=0.012), and vitality (r=−0.29, P<0.001). In addition, ESS score exhibited significant negative correlation with general health perception (r=−0.20, P<0.001) and social functioning (r=−0.13, P=0.005). Finally, HbA1c significantly correlated only with vitality domain (r=−0.19, P<0.001), while T2DM duration and insulin therapy duration showed statistically significant correlation with most SF-36 components except emotional role limitations and social functioning, as well as physical role limitations, emotional role limitations, and general health perception (Table 5).
Based on multiple linear regression model, STOP score was confirmed as statistically significant independent predictor for all SF-36 components, adjusted for BMI, age, HbA1c, and T2DM duration (P<0.001). Furthermore, STOP score was the strongest predictor of social functioning, emotional role limitations, and emotional well-being (Table 6).
Discussion
Findings from our study demonstrate that high proportion of T2DM patients have high risk for OSA. Furthermore, we observed that group with high risk for OSA has lower HRQoL in all SF-36 dimensions compared to low-risk patients. While it has been previously shown that diabetic patients with comorbidities such as depression and cardiovascular disorders have impaired HRQoL, limited evidence exists regarding the influence of concomitant OSA on HRQoL in T2DM population.30 As far as we know, to this date only one recent polysomnography-assisted study questioned HRQoL in T2DM subjects with concurrent OSA.12 However, whether high risk for OSA represents aggravating factor in HRQoL was unknown. To our knowledge, this is the first questionnaire-based study examining the influence of OSA risk on HRQoL in adult subjects with T2DM.
HRQoL imposes as an important clinical outcome in therapeutic approach to patients with T2DM as suggested in recent guidelines.31 Available studies report that most individuals with T2DM have impaired HRQoL.16 Furthermore, sleep quality, which is a significant predictor of HRQoL, is poorer in diabetic population.23 Study by Luyster and Dunbar-Jacob showed that poor sleep quality unfavorably alters all health dimensions of SF-36.22 It has been previously established that main features of OSA adversely affect individual’s sleep quality and HRQoL.24 However, available studies do not provide consistent results regarding the influence of OSA severity on HRQoL.24,32 Still, recent related study on T2DM patients by Bironneau et al showed lower vitality, physical role functioning, and social functioning components of SF-36 with increasing OSA severity in recruited population.12 Our findings are comparable with aforementioned study; however, we obtained significantly lower SF-36 scores in all health dimensions in high-risk OSA group. Additionally, we found significant negative correlation of STOP score with all SF-36 dimension scores. After adjustment for BMI, age, HbA1c, and T2DM duration in multiple regression model, STOP score maintained statistical significance as an independent predictor of SF-36 parameters. Having in mind that diabetic patients have impaired HRQoL per se, consequences of these HRQoL deteriorations are yet to be recognized.16
There are evidences that better glycemic control improves HRQoL.17 However, HbA1c values from our study did not show statistically significant negative correlation with most of the SF-36 dimensions, except with vitality. Furthermore, Kent et al showed that concomitant OSA impairs glycemic control in T2DM patients.8 Similarly, Aronsohn et al demonstrated higher HbA1c values among T2DM population with concomitant OSA compared to other diabetic patients.13 However, in our multiple regression model, HbA1c lacked statistical significance as an independent predictor of SF-36 parameters, except for physical role limitations. Nonetheless, we did not find statistically significant difference in HbA1c values between different OSA risk groups.
Important to notice is that there was no statistically significant difference in depression prevalence between high-risk and low-risk OSA groups. In a study by Wexler et al, depression was the strongest correlate associated with reduced HRQoL in T2DM patients.20 Similarly, there was no significant difference in GERD prevalence between our high-risk and low-risk OSA groups. However, total prevalence was still higher than expected in general population.33 On contrary, coexistence of arterial hypertension and asthma, which are a well-established comorbidities of both T2DM and OSA, was significantly higher among high-risk OSA group in our study. Most available studies provide evidence about negative effects of arterial hypertension on HRQoL in diabetic patients, especially after the development of well-known complications.16 However, some authors did not find significant correlation of arterial hypertension with HRQoL.20
Furthermore, a previous study had shown that sleepiness may lead to significant difficulties in everyday functioning and HRQoL.15 Group with high risk for OSA from our study exhibited significantly higher daytime sleepiness compared to low-risk group. Even though Silva et al showed that daytime sleepiness is an important predictor of HRQoL, our analysis revealed negative correlation of ESS score only with general health perception and social functioning components of SF-36.27
It has been formerly shown that aging impairs HRQoL in T2DM patients.20 Consistently, we found significant negative correlation of age with physical functioning, physical role limitations, bodily pain, emotional role limitations, and vitality of SF-36 questionnaire. Moreover, multiple regression model confirmed age as a statistically significant predictor of mentioned SF-36 variables. In fact, age was the strongest predictor of abovementioned variables except for emotional role limitations. These results suggest higher correlation of age with physical dimensions of HRQoL which is reasonable mainly due to physiologic changes, as well as longer disease duration in most patients. Similar findings were described by Klein et al while there are evidences about inverse correlation of HRQoL with age.34,35 Latter could be due to skeptical approach to chronic disease in younger patients. However, most subjects from our study were older than 60 years and therefore could have more mature view on the disease, allowing for the physical difficulties to mainly affect HRQoL. Moreover, there are evidences about sex differences in HRQoL.36 Importantly, our sample was evenly representated with both sex and age and there was no statistically significant difference in age and gender between high-risk and low-risk OSA group.
High-risk OSA group from our sample exhibited significantly higher BMI values compared to low-risk group. Obesity has been already shown to have independent adverse effect on HRQoL.21 Likewise, our analysis showed significant negative correlation of BMI with all SF-36 categories. However, in multiple regression analysis, BMI lacked statistical significance as predictor of emotional role limitations and emotional well-being components of SF-36. Interestingly, Wexler et al did not find a relationship between obesity and decreased HRQoL.20
Groups with high and low risk for OSA from our study statistically significantly differed in T2DM duration. Moreover, T2DM duration showed significant negative correlation with most SF-36 components, except social functioning and emotional role limitations. Similar influence of T2DM duration on HRQoL expressed by Health Utility Index-III was obtained by Wexler et al.20 However, in our multiple regression analysis, T2DM duration lacked statistical significance in prediction of SF-36 components, except in weak prediction of general health perception. Furthermore, insulin use has mostly been reported as a negative factor in HRQoL of diabetic patients.20 Similarly, our analysis revealed negative correlation with several components of SF-36. However, groups with high and low risk for OSA from our study did not significantly differ in insulin use or in duration of insulin therapy.
Together with preceding findings, present analysis confirmed that high proportion of patients with T2DM have high risk for OSA. As much as 67% of our sample stands for high-risk OSA group based on STOP questionnaire. It has been well-established that patients with T2DM have increased risk for OSA, as well as increased prevalence of OSA, compared to general population. Previous questionnaire-based studies examining the risk for OSA in T2DM patients reported high prevalence of high-risk OSA group in T2DM population.10 These data are supported by multitude polysomnography-assisted research which reassure increased OSA prevalence in T2DM population. One such study by Bironneau et al even reported OSA prevalence of 69% in diabetic patients.12 Our findings are consistent with available studies confirming that a significant proportion of T2DM patients account for high-risk OSA group, which is much higher than expected in general population.
Additionally, it is interesting to remark that our sample had lower HRQoL in all SF-36 components in comparison with Croatian SF-36 norms, except for emotional well-being and vitality which were higher in our sample.29
Unrecognized significance of these results by health care profeşsionals is emphasized by Lecomte et al who showed that only 35% of T2DM patients with OSA-related symptoms have been already diagnostically evaluated for OSA.37 Likewise, Heffner et al retrospectively detected considerable mismatch in actually diagnosed and expected OSA prevalence in more than 16,000 T2DM patients.38 This highlights the importance of implementation of routine HRQoL assessment in specific populations, such as diabetic patients, which could highlight the patients with occult symptoms for further diagnostic procedures. Essentially, lower HRQoL in T2DM patients is associated with higher total mortality, independently of sex or age.18 Most importantly, a recent meta-analysis by Kuhn et al demonstrated that CPAP therapy improves HRQoL, indicating the need for prompt screening, diagnosis, and management of OSA in diabetic patients.39
Similar to any other research study, this study has some limitations. Organization of this study was cross-sectional, so it was not possible to establish any causal relationship. Furthermore, it was a questionnaire-based study, which is feasible for response bias. Moreover, the questionnaire used for HRQoL was not disease specific but rather a generic instrument (SF-36). Therefore, combination of generic with disease-specific questionnaire could be more comprehensive.
Conclusion
Our study has shown that T2DM patients with high risk for OSA have lower HRQoL compared to low-risk group, as examined by SF-36 questionnaire. Additionally, we have confirmed that among diabetic population, there is high proportion of patients with high risk for OSA.
Disclosure
The authors report no conflicts of interest in this work.
References
NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016;387(10027):1513–1530. | ||
Peppard P, Young T, Barnet J, Palta M, Hagen E, Hla K. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177:1006–1014. | ||
Franklin KA, Lindberg E. Obstructive sleep apnea is a common disorder in the population-a review on the epidemiology of sleep apnea. J Thorac Dis. 2015;7(8):1311–1322. | ||
Galic T, Bozic J, Pecotic R, Ivkovic N, Valic M, Dogas Z. Improvement of cognitive and psychomotor performance in patients with mild to moderate obstructive sleep apnea treated with mandibular advancement device: a prospective 1-year study. J Clin Sleep Med. 2016;12(02):177–186. | ||
Bozic J, Galic T, Supe-Domic D, et al. Morning cortisol levels and glucose metabolism parameters in moderate and severe obstructive sleep apnea patients. Endocrine. 2016;53(3):730–739. | ||
Reichmuth KJ, Austin D, Skatrud JB, Young T. Association of sleep apnea and type II diabetes: a population-based study. Am J Respir Crit Care Med. 2005;172(12):1590–1595. | ||
Wang X, Bi Y, Zhang Q, Pan F. Obstructive sleep apnoea and the risk of type 2 diabetes: a meta-analysis of prospective cohort studies. Respirology. 2012;18(1):140–146. | ||
Kent B, Grote L, Ryan S, et al. Diabetes mellitus prevalence and control in sleep-disordered breathing. Chest. 2014;146(4):982–990. | ||
Bottini P, Redolfi S, Dottorini M, Tantucci C. Autonomic neuropathy increases the risk of obstructive sleep apnea in obese diabetics. Respiration. 2008;75(3):265–271. | ||
West SD, Nicoll DJ, Stradling JR. Prevalence of obstructive sleep apnoea in men with type 2 diabetes. Thorax. 2006;61(11):945–950. | ||
Pecotic R, Dodig I, Valic M, Ivkovic N, Dogas Z. The evaluation of the Croatian version of the Epworth sleepiness scale and STOP questionnaire as screening tools for obstructive sleep apnea syndrome. Sleep Breath. 2011;16(3):793–802. | ||
Bironneau V, Goupil F, Ducluzeau P, et al. Association between obstructive sleep apnea severity and endothelial dysfunction in patients with type 2 diabetes. Cardiovasc Diabetol. 2017;16(1):39. | ||
Aronsohn R, Whitmore H, Van Cauter E, Tasali E. Impact of untreated obstructive sleep apnea on glucose control in type 2 diabetes. Am J Respir Crit Care Med. 2010;181(5):507–513. | ||
Labrador Barba E, Rodriguez de Miguel M, Hernández Mijares A, et al. Medication adherence and persistence in type 2 diabetes mellitus: perspectives of patients, physicians and pharmacists on the Spanish health care system. Patient Prefer Adherence. 2017;11:707–718. | ||
Moyer C, Sonnad S, Garetz S, Helman J, Chervin R. Quality of life in obstructive sleep apnea: a systematic review of the literature. Sleep Med. 2001;2(6):477–491. | ||
Trikkalinou A, Papazafiropoulou A, Melidonis A. Type 2 diabetes and quality of life. World J Diabetes. 2017;8(4):120. | ||
Kamarul Imran M, Ismail AA, Naing L, Wan Mohamad WB. Type 2 diabetes mellitus patients with poor glycaemic control have lower quality of life scores as measured by the Short Form-36. Singapore Med J. 2010;51(2):157–162. | ||
Landman G, van Hateren K, Kleefstra N, Groenier K, Gans R, Bilo H. Health-related quality of life and mortality in a general and elderly population of patients with type 2 diabetes (ZODIAC-18). Diabetes Care. 2010;33(11):2378–2382. | ||
Hayes A, Arima H, Woodward M, et al. Changes in quality of life associated with complications of diabetes: results from the ADVANCE study. Value Health. 2016;19(1):36–41. | ||
Wexler D, Grant R, Wittenberg E, et al. Correlates of health-related quality of life in type 2 diabetes. Diabetologia. 2006;49(7):1489–1497. | ||
Ul-Haq Z, Mackay D, Fenwick E, Pell J. Meta-analysis of the association between body mass index and health-related quality of life among adults, assessed by the SF-36. Obesity. 2013;21(3):E322–E327. | ||
Luyster F, Dunbar-Jacob J. Sleep quality and quality of life in adults with type 2 diabetes. Diabetes Educ. 2011;37(3):347–355. | ||
Cunha M, Zanetti M, Hass V. Sleep quality in type 2 diabetics. Rev Latino-Am Enfermagem. 2008;16(5):850–855. | ||
Lee S, Ng K, Chin W. The impact of sleep amount and sleep quality on glycemic control in type 2 diabetes: a systematic review and meta-analysis. Sleep Med Rev. 2017;31:91–101. | ||
Johns M. Daytime sleepiness, snoring, and obstructive sleep apnea. Chest. 1993;103(1):30–36. | ||
Chung F, Yegneswaran B, Liao P, et al. STOP Questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108(5):812–821. | ||
Silva G, Goodwin J, Vana K, Quan S. Obstructive sleep apnea and quality of life: comparison of the SAQLI, FOSQ, and SF-36 questionnaires. Southwest J Pulm Crit Care. 2016;13(3):137–149. | ||
Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. | ||
Maslic Sersic D, Vuletic G. Psychometric evaluation and establishing norms of Croatian SF-36 health survey: framework for subjective health research. Croat Med J. 2006;47(1):95–102. | ||
Wändell P. Quality of life of patients with diabetes mellitus: an overview of research in primary health care in the Nordic countries. Scand J Prim Health Care. 2005;23(2):68–74. | ||
American Diabetes Association. Comprehensive medical evaluation and assessment of comorbidities. Diabetes Care. 2016;40(Suppl 1):S25–S32. | ||
Dutt N, Janmeja A, Mohapatra P, Singh A. Quality of life impairment in patients of obstructive sleep apnea and its relation with the severity of disease. Lung India. 2013;30(4):289. | ||
Dent J. Epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2005;54(5):710–717. | ||
Klein B, Klein R, Moss S. Self-rated health and diabetes of long duration: the Wisconsin Epidemiologic Study of Diabetic Retinopathy. Diabetes Care. 1998;21(2):236–240. | ||
Wang H, Yeh M. The quality of life of adults with type 2 diabetes in a hospital care clinic in Taiwan. Qual Life Res. 2012;22(3):577–584. | ||
Luscombe F. Health-related quality of life measurement in type 2 diabetes. Value Health. 2000;3(Suppl 1):S15–S28. | ||
Lecomte P, Criniere L, Fagot-Campagna A, Druet C, Fuhrman C. Underdiagnosis of obstructive sleep apnoea syndrome in patients with type 2 diabetes in France: ENTRED 2007. Diabetes Metab. 2013;39(2):139–147. | ||
Heffner J, Rozenfeld Y, Kai M, Stephens E, Brown L. Prevalence of diagnosed sleep apnea among patients with type 2 diabetes in primary care. Chest. 2012;141(6):1414–1421. | ||
Kuhn E, Schwarz E, Bratton D, Rossi V, Kohler M. Effects of CPAP and mandibular advancement devices on health-related quality of life in OSA. Chest. 2017;151(4):786–794. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.