Back to Journals » Journal of Asthma and Allergy » Volume 16
Health-Related Quality of Life Impairment Among Patients with Severe Chronic Rhinosinusitis with Nasal Polyps in the SINUS-24 Trial
Authors Maspero JF, Khan AH , Philpott C , Hellings PW, Hopkins C, Wagenmann M , Siddiqui S , Msihid J , Nash S, Chuang CC, Kamat S , Rowe PJ, Deniz Y, Jacob-Nara JA
Received 29 June 2022
Accepted for publication 8 March 2023
Published 29 March 2023 Volume 2023:16 Pages 323—332
DOI https://doi.org/10.2147/JAA.S372598
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Luis Garcia-Marcos
Jorge F Maspero,1 Asif H Khan,2 Carl Philpott,3 Peter W Hellings,4 Claire Hopkins,5 Martin Wagenmann,6 Shahid Siddiqui,7 Jérôme Msihid,8 Scott Nash,7 Chien-Chia Chuang,9 Siddhesh Kamat,7 Paul J Rowe,10 Yamo Deniz,7 Juby A Jacob-Nara10
1Allergy & Respiratory Research Unit, Fundación CIDEA, Buenos Aires, Argentina; 2Global Medical Affairs, Sanofi, Chilly-Mazarin, France; 3Rhinology and ENT Research Group, Norwich Medical School, University of East Anglia, Norwich, UK; 4Department of Otorhinolaryngology – Head and Neck Surgery, University Hospitals Leuven, Leuven, Belgium; 5Department of Otorhinolaryngology, King’s College London, London, UK; 6Department of Otorhinolaryngology, Düsseldorf University Hospital (UKD), Düsseldorf, Germany; 7Medical Affairs, Regeneron Pharmaceuticals Inc., Tarrytown, NY, USA; 8Health Economics and Value Assessment, Sanofi, Chilly-Mazarin, France; 9Health Economics and Value Assessment, Sanofi, Cambridge, MA, USA; 10Global Medical Affairs, Sanofi, Bridgewater, NJ, USA
Correspondence: Jorge F Maspero, Allergy & Respiratory Research Unit, Fundación CIDEA, Paraguay 2035, 2SS, Buenos Aires, Argentina, Tel +54 9 11 4183-7294, Email [email protected]
Purpose: Chronic rhinosinusitis with nasal polyps (CRSwNP) is a predominantly type 2 inflammatory disease with a high symptom burden. Data are lacking on the comparative health status of patients with CRSwNP. This analysis compared baseline physical and mental health-related quality of life (HRQoL) and overall health status of patients with severe CRSwNP enrolled in a Phase 3 clinical trial with general population norms and with other chronic diseases.
Methods: In this post hoc cross-sectional analysis of baseline data from the SINUS-24 study (NCT02912468), HRQoL was measured using the 36-item Short Form (SF-36) questionnaire and general health status was measured using the EuroQol-5 Dimension visual analog scale (EQ-VAS). Analyses included the intention-to-treat (ITT) population and subgroups defined by prior sinonasal surgery, systemic corticosteroid use, and coexisting asthma or non-steroidal anti-inflammatory drug-exacerbated respiratory disease (NSAID-ERD). Scores were compared with published values for population norms (50 for SF-36 physical component summary (PCS) and mental component summary (MCS), 70.4− 83.3 for EQ-VAS) and for rheumatoid arthritis, type 2 diabetes, and asthma.
Results: In the ITT population (n=276), mean SF-36 physical component summary (PCS), SF-36 mental component summary (MCS), and EQ-VAS scores were below general population norms (46.4, 48.6, and 66.0, respectively). Mean SF-36 PCS and EQ-VAS scores were below population norms across all subgroups; mean SF-36 MCS scores were below the population norm in all subgroups except no prior surgery. SF-36 PCS and MCS scores from SINUS-24 were generally similar to other chronic diseases, except SF-36 PCS which was lower in rheumatoid arthritis. EQ-VAS scores in SINUS-24 were lower than in other chronic diseases. HRQoL scores weakly correlated with objective measures of disease severity.
Conclusion: In patients with severe CRSwNP, including those with coexisting asthma/NSAID-ERD, HRQoL was worse than population norms and as burdensome as diseases such as type 2 diabetes, asthma, and rheumatoid arthritis.
Graphical Abstract:
Keywords: chronic rhinosinusitis with nasal polyps, health-related quality of life, symptom burden
Graphical Abstract:
Plain Language Summary
Chronic rhinosinusitis with nasal polyps (CRSwNP) affects 1–4% of the US population. The main symptoms are blocked and runny nose and loss of smell. Many people with CRSwNP suffer these and other symptoms continually and this badly affects their everyday lives; they may find it difficult to sleep, go to work, and enjoy social activities. The researchers in this study wanted to know how the quality of life (QoL) of people with CRSwNP compares with (1) the general population and (2) other common long-lasting diseases. The study involved 276 patients with severe CRSwNP (disease not controlled by standard treatments). Patients completed questionnaires about different aspects of their QoL – physical aspects (e.g., pain and ability to be active), mental aspects (e.g., emotion and mental health), and general well-being. The results showed firstly that the patients had worse QoL (physical and mental) than the general population. Secondly, the impact on QoL was similar to that of people with asthma, diabetes, or rheumatoid arthritis. New treatments that effectively reduce the symptoms of CRSwNP should also improve the QoL of people with CRSwNP.
Introduction
Chronic rhinosinusitis with nasal polyps (CRSwNP) is a predominantly type 2-mediated inflammatory disease of the sinuses.1 The symptoms of CRSwNP negatively impact patients’ physical and mental health-related quality of life (HRQoL)1–3 and productivity.2 Patients with CRSwNP report nasal congestion (NC), loss of smell (LoS), and rhinorrhea among their most troublesome and severe symptoms.4 A real-life study found that around half of patients with CRS experience symptoms daily and have uncontrolled disease despite standard of care.5 Consequently, patients with CRSwNP often become frustrated with their symptoms, particularly LoS.6–8 Patients with CRSwNP experience a high clinical burden,1–3 associated with a greater number of physician visits, comorbidities, and increased healthcare costs than the general population.9 The annual US healthcare cost of CRSwNP is estimated to be $5.7 billion.9
Type 2 inflammation is central to multiple diseases, including CRSwNP, asthma, allergic rhinitis,10 and non-steroidal anti-inflammatory drug-exacerbated respiratory disease (NSAID-ERD).3 Up to 87% of patients with CRSwNP have type 2-mediated disease11 and frequently have other coexisting type 2 inflammatory diseases, leading to greater disease burden and HRQoL impairment.10 Previous studies have shown that patients with CRSwNP have significantly lower physical and mental HRQoL scores compared with population norms.2,12 The reduced HRQoL observed in patients with CRSwNP is comparable to that of other chronic diseases, such as chronic obstructive pulmonary disease, asthma, and diabetes.2
Dupilumab is a fully human VelocImmune®-derived monoclonal antibody that blocks the shared receptor component for interleukin (IL)-4 and IL-13, which are key and central drivers of type 2 inflammation in CRSwNP and other diseases.13–15 In the Phase 3 SINUS-24 (NCT02912468) and SINUS-52 (NCT02898454) studies, dupilumab added to standard of care significantly improved endoscopic nasal polyp score (NPS) and patient-reported nasal congestion/obstruction (NC) scores compared with placebo in patients with severe CRSwNP.16 A subsequent post hoc analysis of the SINUS-24 and SINUS-52 study populations showed that dupilumab treatment significantly improved disease-specific HRQoL and general health status.17 Disease-specific HRQoL was also improved with dupilumab in patients from the SINUS studies who had clinical features of obstructive pulmonary disease.18
The SINUS study populations had severe, uncontrolled disease at baseline despite standard of care,19 and a substantial proportion of participants had coexisting asthma and/or NSAID-ERD. There is a paucity of recent data on the comparative health status in such patients, which would allow comparisons with other diseases. In SINUS-24, physical and mental HRQoL was evaluated using the 36-item Short Form questionnaire version 2 (SF-36 V2). SF-36 was captured only at baseline and was not assessed over the study period.20 Improvement on SF-36 with dupilumab treatment was observed in a previous proof of concept study.21 Overall health status was assessed with the EuroQoL-5 Dimension visual analog scale (EQ-VAS). The aim of this analysis was to compare baseline results for HRQoL and overall health status of patients with severe CRSwNP, pooling data randomized in placebo and dupilumab groups from SINUS-24, with general population norms for these scales and data from other chronic diseases. A second aim was to evaluate correlations between SF-36 scores and CRSwNP disease characteristics and general health status at baseline, to determine if use of a disease-specific HRQoL measure would provide a good assessment of general HRQoL. This is the first study reporting the health status of patients from a Phase 3 study for the assessment of biologics in CRSwNP.
Methods
This was a post hoc cross-sectional analysis of baseline data from patients enrolled in the SINUS-24 study;16 dupilumab treatment effects were not assessed. HRQoL was measured using the SF-36 V2.22 SF-36 is a self-reported measure of health and wellbeing with a recall period of 4 weeks, from which 8 domains (physical functioning, role-physical, bodily pain, general health, vitality, social functioning, role-emotional, and mental health), and 2 component summary scores (physical component score [PCS] and mental component score [MCS]) can be derived.23–26 SF-36 PCS and MCS scores range from 0 to 100, with lower scores indicating worse HRQoL. Health status was measured using the EQ-VAS (range 0–100), a standardized measure assessing patient-reported health state on the day of assessment.27 It is one component of the EuroQoL-5 Dimension (EQ-5D) questionnaire, a simple measure for clinical and economic appraisal.28 Lower EQ-VAS scores indicate worse HRQoL. EQ-VAS scores were derived from reported values.
Analyses were conducted in the intention-to-treat (ITT) population and in patient subgroups: with/without prior nasal polyps (NP) surgery; previous systemic corticosteroid (SCS) use (within the last 2 years) and prior surgery, or previous SCS use and no prior NP surgery; with/without coexisting asthma; with/without coexisting NSAID-ERD, and with/without anosmia (anosmia being defined by a score <19 on University of Pennsylvania Smell Identification Test [UPSIT]). Results were compared with population norms (SF-36 [USA] 50;29 international EQ-VAS scores which ranged from 70.4 to 83.3).30 SF-36 and EQ-VAS scores from SINUS-24 were also compared with published scores for rheumatoid arthritis (PCS 32.5, MCS 55.6, EQ-VAS 75.5), type 2 diabetes (PCS 46.5, MCS 50.8, EQ-VAS 75.9), and asthma (PCS 45.5, MCS 48.6, EQ-VAS 76.3).24–26,31 The published SF-36 scores were determined for rheumatoid arthritis in Norwegian patients with late-stage disease according to duration (>3 to <20 years), for type 2 diabetes in a nationwide cross-section of patients from the Swedish National Diabetes Register, and for asthma in patients with asthma (any severity) enrolled in the European Community Respiratory Health Survey.24–26 The published EQ-VAS scores for rheumatoid arthritis, type 2 diabetes, and asthma were determined using data from US patients with any level of disease severity.31 The purpose of these analyses was to investigate comparative disease burden of patients with CRSwNP in SINUS-24 relative to population norms and to rheumatoid arthritis, type 2 diabetes, and asthma; all comparisons are descriptive and no statistical testing was performed.
Associations between SF-36 scores and CRSwNP disease characteristics (NPS, NC, Lund-Mackay computed tomography [LMK-CT], UPSIT, total symptom score, daily loss of smell [LoS], the 22-item CRSwNP-specific HRQoL instrument Sino-Nasal Outcome Test [SNOT-22 – total score and individual scores for the ear/facial, emotion, function, nasal, and sleep domains],32 and Rhinosinusitis visual analog scale) and general health status (EQ-VAS) at baseline were determined using Spearman correlation. Correlation coefficients were interpreted as follows: 0 to <0.2 = very weak, 0.2 to <0.4 = weak, 0.4 to <0.6 = moderate, 0.6 to <0.8 = strong, and 0.8 to 1.0 = very strong.33
Results
Overall Population
There were 276 patients in the ITT population from the SINUS-24 study. The cohort was representative of a population with severe CRSwNP, including 72% of patients with prior sinonasal surgery, 38% with prior sinonasal surgery and SCS use in the preceding 2 years, 58% with coexisting asthma, 30% with coexisting NSAID-ERD, and 76% with anosmia (Table 1).
 |
Table 1 Demographics and Baseline Disease Characteristics in the SINUS-24 Study (ITT Population) |
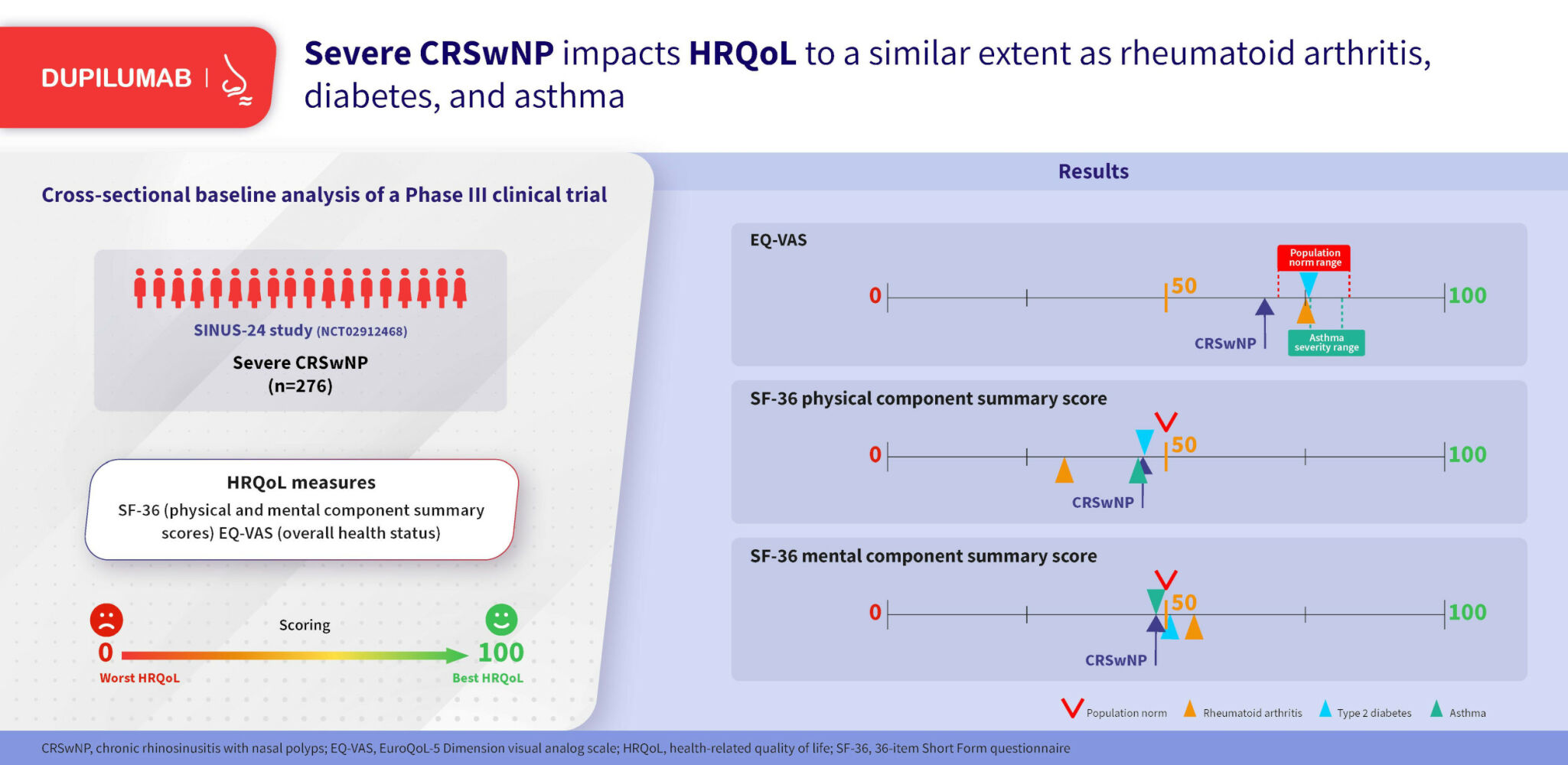
Mean SF-36 PCS and MCS scores in the overall population were 46.4 and 48.6, which are below the population norms of 50 for each summary scale (Table 2). Mean EQ-VAS score was 66.0, which is below the range of published population norm scores, which ranged between 70.4 and 83.3. Comparison with EQ-VAS scores for other chronic diseases showed that patients in SINUS-24 had lower scores than patients with rheumatoid arthritis, type 2 diabetes, and asthma (Figure 1). SF-36 PCS and MCS scores in SINUS-24 were similar to scores for other chronic diseases, except for SF-36 PCS in rheumatoid arthritis which was markedly lower (32.5).
 |
Table 2 SF-36 and EQ-VAS Scores of Patients with Severe CRSwNP in SINUS-24 (ITT Population) |
 |
Figure 1 SF-36 and EQ-VAS scores for patients with CRSwNP from SINUS-24 vs patients with other chronic diseases [references: SF-36;24–26 EQ-VAS31]. Abbreviations: CRSwNP, chronic rhinosinusitis with nasal polyps; EQ-VAS, EuroQoL-5 Dimension visual analog scale; GINA, Global Initiative for Asthma; MCS, mental component summary; PCS, physical component summary; SF-36, 36-item Short Form questionnaire. Notes: Populations: CRSwNP (SINUS-24, A–C), international patients with NPS of at least 5 (of a maximum 8) despite treatment with SCS in the preceding 2 years (or a medical contraindication or intolerance to SCS) or previous sinonasal surgery; rheumatoid arthritis (A and B), Norwegian real-world population with late-stage disease according to duration (>3 – <20 years), (C) US real-world population with any level of disease severity; type 2 diabetes (A and B), patients from the Swedish National Diabetes Register, (C) US real-world population with any level of disease severity; asthma (A and B), European patients with diagnosis of asthma confirmed by a doctor and at least one respiratory symptom (wheeze, nocturnal chest tightness, attack of breathlessness after activity; at rest or at night) or asthma attack or use of inhaled/oral medicines because of breathing problem in the last 12 months, (C) US real-world population with any level of disease severity. Higher GINA category indicates more severe disease. |
Subgroups
There were 161 patients (58.3%) with CRSwNP and coexisting asthma. Mean SF-36 PCS and MCS scores in the asthma subgroup were 44.5 and 48.1, which are below the population norms of 50 for each summary scale (Table 2). Mean EQ-VAS score in the asthma subgroup was 64.8, which is below the range of published population norm scores. Comparison of EQ-VAS score in the asthma subgroup with other chronic diseases, based on the selected publications, showed that patients in SINUS-24 with CRSwNP and coexisting asthma had lower scores than patients with rheumatoid arthritis, type 2 diabetes, and asthma. HRQoL and general health status scores in the subgroup with NSAID-ERD (n=84) were marginally worse than the subgroup with coexisting asthma (44.4, 48.1, and 63.9 for SF-36 PCS, SF-36 MCS, and EQ-VAS, respectively).
In the subgroup with anosmia (n=208), HRQoL and general health status were impacted to a similar degree as in the subgroup with coexisting asthma; with mean SF-36 PCS, SF-36 MCS, and EQ-VAS scores of 45.4, 48.3, and 63.9, respectively (Table 2). The subgroups with prior surgery (n=198), and with previous SCS and prior surgery (n=106) also had HRQoL and general health status scores below population norms.
Associations Between Scores and Disease Characteristics
In the SINUS-24 study, the SF-36 component summary scores were moderately (PCS) and weakly (MCS) correlated with EQ-VAS score (Table 3). SF-36 scores, both MCS and PCS, generally correlated very weakly with objective measures of disease severity (NPS, LMK-CT, and UPSIT) (Table 3). This was also true for most of the patient-reported clinical measures, including NC, LoS, total symptom score, and Rhinosinusitis VAS. The exception was SNOT-22, where there were moderate correlations with SNOT-22 total score for both the SF-36 MCS and PCS. Individual SNOT-22 domains showed moderate correlations with SF-36; correlation was higher between SF-36 MCS and the SNOT-22 emotional domain.
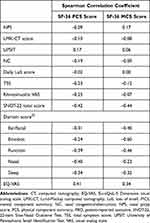 |
Table 3 Association Between SF-36 PCS and MCS and Clinical and PRO Measures at Baseline in SINUS-24 (Overall Study Population) |
Discussion
SF-36 is the most common tool for assessing impact on general HRQoL, which provides insights into the overall health burden of patients and allows comparisons with other diseases. This analysis found that patients with severe CRSwNP had worse physical and mental HRQoL than the general population. General health status (EQ-VAS) in SINUS-24 was worse than the population norms and other chronic diseases perceived to be of higher burden. The assessment based on SF-36 showed that the burden was as bad as, if not worse than, rheumatoid arthritis, type 2 diabetes, and asthma. The subgroups of patients with previous NP surgery (with/without prior SCS use), or with coexisting asthma/NSAID-ERD, or with anosmia, had similar scores to the overall study population, indicating that the reduced HRQoL observed was not limited to those with comorbidities or prior surgery, often considered as markers of disease severity. These findings add to those of previous studies showing that patients with CRSwNP have impaired physical and mental HRQoL, and may be more generalizable as the study population analyzed here was global, not limited to one institution or region as in previous studies,2,12,34 and was assessed using the updated SF-36 scale (SF-36 V2).
General health status (EQ-VAS) score correlated with the scores for the SF-36 PCS (moderate correlation) and MCS (weak correlation), suggesting that a simple measure of health should signal the impact on both mental and physical aspects of the patient’s HRQoL burden. The generally very weak correlations observed between subjective assessment of burden and objective clinical measures of CRSwNP have been reported previously,35–37 and highlighting the importance of a holistic assessment of a patient’s burden and that a disease considered “mild” based on clinical parameters may not belie the huge impact on patient emotional health. The strongest correlations were observed between SF-36 and the CRSwNP-specific HRQoL instrument SNOT-22. These correlations were observed between both the Physical (PCS) and Mental (MCS) Component Summary scores with the SNOT-22 total score and all five domains, indicating that use of a disease-specific HRQoL measure, such as SNOT-22, would provide a good assessment of general HRQoL.
Reduced HRQoL may result from the myriad of symptoms associated with severe disease and its common comorbidities as well as from under-recognized humanistic and economic impacts of living with such disease on a daily basis.2 Separate assessment of a patient’s HRQoL is vital because clinical measures do not show HRQoL impact. The present findings suggest that SNOT-22 and EQ-VAS may be sufficient to elucidate disease-specific and general HRQoL impact, respectively. Finally, it is clear from these results that the negative impact on HRQoL in patients with severe CRSwNP is of a similar magnitude to that seen in other common chronic diseases such as rheumatoid arthritis, diabetes, and asthma.
Abbreviations
CRSwNP, Chronic rhinosinusitis with nasal polyps; EQ-VAS, EuroQoL-5 Dimension visual analog scale; HRQoL, health-related quality of life; ITT, intention-to-treat; LMK-CT, Lund-Mackay computed tomography; LoS, daily loss of smell; MCS, mental component summary; NC, nasal congestion; NP, nasal polyps; NPS, nasal polyp score; NSAID-ERD, non-steroidal anti-inflammatory drug-exacerbated respiratory disease; PCS, physical component summary; SCS, systemic corticosteroid; SF-36 V2, 36-item Short Form questionnaire version 2; SNOT-22, 22-item Sino-Nasal Outcome Test; UPSIT, University of Pennsylvania Smell Identification Test.
Data Sharing Statement
Qualified researchers may request access to data and related study documents. Patient level data will be anonymized, and study documents will be redacted to protect the privacy of trial patients. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at: https://www.vivli.org/.
Ethics Approval and Informed Consent
The SINUS-24 study was conducted in accordance with the Declaration of Helsinki, approved by the local institutional review board or ethics committee at each study site (see Table S1), and all patients provided written informed consent.
Acknowledgments
Research sponsored by Sanofi and Regeneron Pharmaceuticals Inc. ClinicalTrials.gov Identifier: NCT02912468 (SINUS-24). Medical writing/editorial assistance provided by Dorothy Keine, PhD and Adelphi Group, funded by Sanofi and Regeneron Pharmaceuticals Inc. The authors thank Christine Taniou and Carole Mercier, analysts from Aixial, Boulogne-Billancourt, France, funded by Sanofi and Regeneron Pharmaceuticals Inc. The abstract of this paper was presented at the American Academy of Allergy Asthma & Immunology (AAAAI) 2021 Annual Meeting as a poster presentation with interim findings. The poster’s abstract was published in “Poster Abstracts” in J Allergy Clin Immunol 2021;147(2):AB133. Abstract 425. doi: https://doi.org/10.1016/j.jaci.2020.12.485
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Research sponsored by Sanofi and Regeneron Pharmaceuticals Inc. ClinicalTrials.gov Identifier: NCT02912468 (SINUS-24).
Disclosure
JFM reports research grants, speaker fees, and advisory board membership from AstraZeneca, Novartis, and Sanofi, speaker fees and advisory board membership from Boehringer Ingelheim, research grants and advisory board membership from GlaxoSmithKline, speaker fees from Menarini and Uriach, and advisory board membership from Merck Sharpe & Dohme. AHK, JM, C-CC, PJR, and JAJ-N are employees and may hold stock and/or stock options in Sanofi. CP reports advisory board membership and/or consultancy fees from GlaxoSmithKline, Guidepoint, M3 Global Research, Olympus, Sanofi, and Stryker, receiving grants from the Bernice Bibby Research Trust, NIHR, Rosetrees Trust, Royal College of Surgeons, and Sir Jules Thorn Trust, and is a trustee of Fifth Sense. PWH reports advisory board membership of Regeneron Pharmaceuticals Inc. and Sanofi. CH reports advisory board membership from GlaxoSmithKline, OptiNose, Sanofi, and Smith & Nephew. MW is a member of national and international scientific advisory board (consulting), has received fees for lectures, grants for research projects from ALK-Abelló A/S, Allakos, AstraZeneca, GlaxoSmithKline, HAL, Meda Pharmaceuticals, Novartis, Otonomy, Inc., Roche, Sanofi-Aventis, Stallergenes Greer, Strekin AG, and Teva Pharmaceuticals. SS is a former employee and may hold stock and/or stock options in Regeneron Pharmaceuticals Inc. SN, SK, and YD are employees and may hold stock and/or stock options in Regeneron Pharmaceuticals Inc. The authors report no other conflicts of interest in this work.
References
1. Hopkins C. Chronic rhinosinusitis with nasal polyps. N Engl J Med. 2019;381(1):55–63. doi:10.1056/NEJMcp1800215
2. Bachert C, Bhattacharyya N, Desrosiers M, Khan AH. Burden of disease in chronic rhinosinusitis with nasal polyps. J Asthma Allergy. 2021;14:127–134. doi:10.2147/JAA.S290424
3. Stevens WW, Schleimer RP, Kern RC. Chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol Pract. 2016;4(4):565–572. doi:10.1016/j.jaip.2016.04.012
4. Abdalla S, Alreefy H, Hopkins C. Prevalence of sinonasal outcome test (SNOT-22) symptoms in patients undergoing surgery for chronic rhinosinusitis in the England and Wales National prospective audit. Clin Otolaryngol. 2012;37(4):276–282. doi:10.1111/j.1749-4486.2012.02527.x
5. Seys SF, De Bont S, Fokkens WJ, et al. Real-life assessment of chronic rhinosinusitis patients using mobile technology: the mySinusitisCoach project by EUFOREA. Allergy. 2020;75(11):2867–2878. doi:10.1111/all.14408
6. Hall R, Trennery C, Chan R, et al. Understanding the patient experience of severe, recurrent, bilateral nasal polyps: a qualitative interview study in the United States and Germany. Value Health. 2020;23(5):632–641. doi:10.1016/j.jval.2019.11.005
7. Vennik J, Eyles C, Thomas M, et al. Chronic rhinosinusitis: a qualitative study of patient views and experiences of current management in primary and secondary care. BMJ Open. 2019;9(4):e022644. doi:10.1136/bmjopen-2018-022644
8. Khan AH, Abbe A, Falissard B, et al. Data mining of free-text responses: an innovative approach to analyzing patient perspectives on treatment for chronic rhinosinusitis with nasal polyps in a phase IIa proof-of-concept study for dupilumab. Patient Prefer Adherence. 2021;15:2577–2586. doi:10.2147/PPA.S320242
9. Bhattacharyya N, Villeneuve S, Joish VN, et al. Cost burden and resource utilization in patients with chronic rhinosinusitis and nasal polyps. Laryngoscope. 2019;129(9):1969–1975. doi:10.1002/lary.27852
10. Khan A, Vandeplas G, Huynh TMT, et al. The Global Allergy and Asthma European Network (GALEN) rhinosinusitis cohort: a large European cross-sectional study of chronic rhinosinusitis patients with and without nasal polyps. Rhinology. 2019;57(1):32–42. doi:10.4193/Rhin17.255
11. Tomassen P, Vandeplas G, Van Zele T, et al. Inflammatory endotypes of chronic rhinosinusitis based on cluster analysis of biomarkers. J Allergy Clin Immunol. 2016;137(5):1449–1456.e1444. doi:10.1016/j.jaci.2015.12.1324
12. Khan A, Huynh TMT, Vandeplas G, et al. The GALEN rhinosinusitis cohort: chronic rhinosinusitis with nasal polyps affects health-related quality of life. Rhinology. 2019;57(5):343–351. doi:10.4193/Rhin19.158
13. Murphy AJ, Macdonald LE, Stevens S, et al. Mice with megabase humanization of their immunoglobulin genes generate antibodies as efficiently as normal mice. Proc Natl Acad Sci USA. 2014;111(14):5153–5158. doi:10.1073/pnas.1324022111
14. Gandhi NA, Pirozzi G, Graham NMH. Commonality of the IL-4/IL-13 pathway in atopic diseases. Expert Rev Clin Immunol. 2017;13(5):425–437. doi:10.1080/1744666X.2017.1298443
15. Macdonald LE, Karow M, Stevens S, et al. Precise and in situ genetic humanization of 6 Mb of mouse immunoglobulin genes. Proc Natl Acad Sci USA. 2014;111(14):5147–5152. doi:10.1073/pnas.1323896111
16. Bachert C, Han JK, Desrosiers M, et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet. 2019;394(10209):1638–1650. doi:10.1016/S0140-6736(19)31881-1
17. Lee SE, Hopkins C, Mullol J, et al. Dupilumab improves health related quality of life: results from the phase 3 SINUS studies. Allergy. 2022;77(7):2211–2221. doi:10.1111/all.15222.
18. Maspero JF, Bachert C, Martinez FJ, et al. Clinical efficacy among patients with chronic rhinosinusitis with nasal polyps and clinical features of obstructive lung disease: post hoc analysis of the Phase III SINUS-24 and SINUS-52 studies. J Asthma Allergy. 2023;: In Press.
19. Bachert C, Han JK, Wagenmann M, et al. EUFOREA expert board meeting on uncontrolled severe chronic rhinosinusitis with nasal polyps (CRSwNP) and biologics: definitions and management. J Allergy Clin Immunol. 2021;147(1):29–36. doi:10.1016/j.jaci.2020.11.013
20. Hawthorne G, Osborne RH, Taylor A, Sansoni J. The SF36 version 2: critical analyses of population weights, scoring algorithms and population norms. Qual Life Res. 2007;16(4):661–673. doi:10.1007/s11136-006-9154-4
21. Bachert C, Mannent L, Naclerio RM, et al. Effect of subcutaneous dupilumab on nasal polyp burden in patients with chronic sinusitis and nasal polyposis: a randomized clinical trial. JAMA. 2016;315(5):469–479. doi:10.1001/jama.2015.19330
22. Rand Corporation. 36-item short form survey instrument; 2022. Available from: https://www.rand.org/health-care/surveys_tools/mos/36-item-short-form/survey-instrument.html.
23. Ware JE
24. Altinkesen E, Gelecek N. Functional status and quality of life in patients with early and late stage rheumatoid arthritis. Fiz Rehabil. 2011;22(2):93–99.
25. Siroux V, Boudier A, Anto JM, et al. Quality-of-life and asthma-severity in general population asthmatics: results of the ECRHS II study. Allergy. 2008;63(5):547–554. doi:10.1111/j.1398-9995.2008.01638.x
26. Svedbo Engstrom M, Leksell J, Johansson UB, et al. Health-related quality of life and glycaemic control among adults with type 1 and type 2 diabetes - a nationwide cross-sectional study. Health Qual Life Outcomes. 2019;17(1):141. doi:10.1186/s12955-019-1212-z
27. EuroQol G. EuroQol--a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208.
28. EuroQol Research Foundation. EQ-5D-Y User Guide; 2020. Available from: https://euroqol.org/publications/user-guides.
29. Maglinte GA, Hays RD, Kaplan RM. US general population norms for telephone administration of the SF-36v2. J Clin Epidemiol. 2012;65(5):497–502. doi:10.1016/j.jclinepi.2011.09.008
30. Janssen MF, Szende A, Cabases J, Ramos-Goni JM, Vilagut G, Konig HH. Population norms for the EQ-5D-3L: a cross-country analysis of population surveys for 20 countries. Eur J Health Econ. 2019;20(2):205–216. doi:10.1007/s10198-018-0955-5
31. Scott M, Chuang C-C, Khan A, Small M, Kamat SA. Clinical characteristics and patient-reported outcomes (PROs) among chronic rhinosinusitis with nasal polyps (CRSwNP) patients with a history of sinus surgery.
32. Khan AH, Reaney M, Guillemin I, et al. Development of sinonasal outcome test (SNOT-22) domains in chronic rhinosinusitis with nasal polyps. Laryngoscope. 2022;132(5):933–941. doi:10.1002/lary.29766
33. Swinscow TDV. Correlation and regression. In statistics at square one 9th edition; 2021. Available from: https://www.bmj.com/about-bmj/resources-readers/publications/statistics-square-one/11-correlation-and-regression.
34. Ference EH, Reddy SR, Tieu R, Gokhale S, Park S, Lecocq J. Burden of nasal polyps in the United States. OTO Open. 2020;4(3):2473974X2095072. doi:10.1177/2473974X20950727
35. Basu S, Georgalas C, Kumar BN, Desai S. Correlation between symptoms and radiological findings in patients with chronic rhinosinusitis: an evaluation study using the sinonasal assessment questionnaire and Lund-Mackay grading system. Eur Arch Otorhinolaryngol. 2005;262(9):751–754. doi:10.1007/s00405-004-0891-0
36. Ryan WR, Ramachandra T, Hwang PH. Correlations between symptoms, nasal endoscopy, and in-office computed tomography in post-surgical chronic rhinosinusitis patients. Laryngoscope. 2011;121(3):674–678. doi:10.1002/lary.21394
37. Bhattacharyya N. Relationship between mucosal inflammation, computed tomography, and symptomatology in chronic rhinosinusitis without polyposis. Ann Otol Rhinol Laryngol. 2008;117(7):517–522. doi:10.1177/000348940811700709
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
