Back to Journals » Blood and Lymphatic Cancer: Targets and Therapy » Volume 7
Harnessing the immune system through programmed death-1 blockade in the management of Hodgkin lymphoma
Authors Oncale MB , Maymani H, Nastoupil LJ
Received 9 November 2016
Accepted for publication 21 January 2017
Published 16 February 2017 Volume 2017:7 Pages 1—7
DOI https://doi.org/10.2147/BLCTT.S110665
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor David Dingli
Melody B Oncale, Hossein Maymani, Loretta J Nastoupil
Department of Lymphoma and Myeloma, Division of Cancer Medicine, The University of Texas MD Anderson Cancer Center, Houston, TX, USA
Abstract: Immunotherapy is a rapidly evolving therapeutic option in the treatment of lymphoma. Neoplastic cells evade immune recognition through the programmed death (PD)-1/PD-ligand immune checkpoint pathway. Several novel agents have been developed to restore the immune system’s ability to recognize and destroy cancer cells. Nivolumab and pembrolizumab are two anti-PD-1 antibodies that have demonstrated success in the treatment of refractory Hodgkin lymphoma. Harnessing the immune system’s ability to target neoplastic cells, ideally without the use of cytotoxic chemotherapeutic agents, is one way in which these novel agents are changing the therapeutic landscape in the treatment of lymphomas. Here, we review the emerging data regarding checkpoint inhibitors in the management of Hodgkin lymphoma, the unique adverse effects encountered with the use of these agents, and a practical approach to the management of these adverse effects. Additionally, we discuss upcoming trials that will further assess the promising future developments of checkpoint inhibition in the treatment of not only Hodgkin lymphoma but also other B cell lymphomas and myeloma. These agents offer immense promise of a future where many lymphomas can be treated without the toxic effects of chemotherapeutic agents.
Keywords: Hodgkin lymphoma, programmed death-1, nivolumab, pembrolizumab, lymphoma
Introduction
Eradication of cancer cells is a pivotal function of the immune system in its ability to maintain a healthy host and is a very attractive approach to cancer if the immune system can be harnessed as a therapeutic intervention. One of the most successful applications of immune therapy in the treatment of hematologic malignancies is the use of allogeneic hematopoietic stem cell transplant (allo-SCT). Allo-SCT harnesses a donor’s immune system to identify the host’s cancer antigens creating a graft-versus-cancer effect. The unfortunate outcome of such therapy is the morbidity of graft-versus-host disease and the limited application impacted by several variables, including available donors and optimal candidates. Therefore, more widely available and less toxic means of harnessing the immune system as a therapeutic intervention for cancer have been highly sought after.
Cell-mediated surveillance and elimination of cancer cells are critical to the immune response, but the endogenous immune system can be highly ineffective at eliminating established cancer. Cancer cells have evolved various mechanisms to escape immune recognition. The programmed death (PD)-1 pathway is an important pathway by which cancer cells escape immune-mediated antitumor response.1 The use of monoclonal antibodies to block PD-1 has been shown to restore the immune system’s ability to identity and eradicate cancer cells.2 Durable clinical responses using anti-PD-1 antibodies have been achieved in both solid tumors and hematologic malignancies.3–6 Here, we review the development and role of PD-1 blockade with the two leading monoclonal antibodies, nivolumab and pembrolizumab, in the management of refractory Hodgkin lymphoma, the unique adverse effects encountered with these agents, and future developments.
PD-1/PD-ligand pathway
Alteration in the PD-1 and PD-ligand PD-L1 axis is a well-known mechanism by which cancer cells thwart immune response. PD-1 is located on the surface of immune cells including activated CD4+ and CD8+ T cells, T regulatory cells (Tregs), B cells, natural killer (NK) cells, and myeloid cells.7 PD-L1 and PD-L2 can be expressed on a variety of cell types, including antigen-presenting cells (APCs), cancer cells, and stromal cells.7,8 Aberrant expression of PD-1 and the receptor ligands results in impaired T cell function, T cell exhaustion, and immune escape.9–11 The interaction of PD-L1 and PD-1 allows for the transmission of intracellular inhibitory signals that ultimately lead to cycle arrest and T cell apoptosis, while PD-L1 expression by tumor cells leads to suppression of Treg function, which allows tumor evasion of the immune system.12 The interaction of PD-L2 and PD-1 inhibits cytokine generation and proliferation of the T cells.9 Therapeutic blocking of PD-1 with a monoclonal antibody allows for the enhancement of B cells and NK cells, while therapeutic blockade of PD-L1 can lead to the enhancement of T cells by dendritic cells. Taken together, these augmentations of the immune system assist with and restore the function of T cells against autologous tumor cells.7,12–14
Hodgkin lymphoma and the PD-L1 pathway
Hodgkin lymphoma is unique in that the malignant Hodgkin Reed–Sternberg cells represent a minority of cells (<10% of tumor mass) as compared to the microenvironment, which is rich in host immune cell infiltrate. In classical Hodgkin lymphoma (cHL), alterations in PD-L1 and PD-L2 are nearly universal.15 Additionally, genetic analyses have shown that Reed–Sternberg cells in cHL frequently exhibit amplification of 9p24.1 leading to increased activity of the JAK/STAT pathway, and overexpression of PD-L1 and PD-L2.15 Some studies suggest that PD-L1 is overexpressed in nearly every case of Hodgkin lymphoma studied. Fluorescence in situ hybridization assays have recently been used to determine the incidence and prognostic significance of 9p24.1, PD-L1, and PD-L2 alterations, in 108 patients with uniformly treated cHL.15 9p24.1 amplification was commonly associated with advanced-stage disease and inferior progression-free survival (PFS), suggesting that PD-1-mediated immune evasion may foster tumor spread in Hodgkin lymphoma. In addition, the high rate of alteration in the PD-L1/PD-L2 loci may explain the robust clinical activity of PD-1 blockade in refractory Hodgkin lymphoma as compared to other lymphomas. In addition, Epstein–Barr virus infection, also common in Hodgkin lymphoma, is another mechanism of PD-L1 overexpression consistent with the known ability of the virus to usurp the PD-1 pathway to allow viral persistence in the host.16 Taken together, Hodgkin lymphoma tumor cells may rely heavily on the PD-1/PD-L pathway to evade immune surveillance and confer a survival advantage. These biologic features of Hodgkin lymphoma indicate that the PD-1/PD-L axis is an attractive therapeutic target.
Therapeutic targeting of the PD-1/PD-L pathway
Multiple monoclonal antibodies have been developed to block the PD-1/PD-L pathway. Nivolumab (fully human monoclonal IgG4 antibody) was the first anti-PD-1 antibody to be approved by the US Food and Drug Administration (FDA) for metastatic melanoma in 2014. Since then, it has gained FDA approval for metastatic non-small-cell lung cancer and renal cell carcinoma, and recently has received breakthrough designation for patients with relapsed/refractory Hodgkin lymphoma who have failed high-dose therapy and autologous stem cell transplant (ASCT) and brentuximab vedotin (May 2016). The breakthrough designation followed the published reports of the phase I dose escalation and expansion study of nivolumab in patients with relapsed/refractory Hodgkin lymphoma.3
Twenty-three patients were treated with nivolumab (escalated from 1 to 3 mg/kg every 2 weeks) until disease progression or until a complete response (CR) of 2-year duration.3 Median age was 35 years (range 20–54), 52% were male, all had an Eastern Cooperative Oncology Group performance status of 0 (26%) or 1 (74%), and 17% had extranodal involvement. Patients represented a heavily pretreated population with 87% having received three or more prior treatment regimens (87% had received Adriamycin [doxorubicin], bleomycin, vinblastine, dacarbazine; 78% had prior brentuximab vedotin; 78% had undergone ASCT). Grade 3 or 4 adverse events (AEs) occurred in 52% (N=12) and included myelodysplastic syndrome (MDS), pancreatitis, pneumonitis, stomatitis, colitis, gastrointestinal inflammation, thrombocytopenia, elevated lipase level, lymphopenia, and leukopenia. The most common drug-related AEs were rash (22%) and thrombocytopenia (17%). AEs were reversible in all except two patients (MDS; pancreatitis) who discontinued treatment. There were no treatment-related deaths. The median number of nivolumab doses was 16 (range 6–37), with 65% receiving 90% or higher of the intended dose.
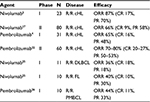
At the time of publication, overall response rate (ORR) was 87% in relapsed/refractory Hodgkin lymphoma patients, with a CR rate of 17% (N=4), partial response in 70% (N=16), and stable disease in 13% (N=3).3 Six patients who were previously ineligible for transplant chose to undergo SCT at the time of best overall response. Extended follow-up presented at the American Society of Hematology (ASH) 2015 Annual Meeting suggested the meaningful clinical response observed early on was durable. Median duration of response and median PFS were still not reached with an overall survival (OS) >90%. The CR rate at extended follow-up had increased to 22% (N=5) indicating ongoing responses.
This phase I study represents the first published clinical study using nivolumab in patients with relapsed/refractory Hodgkin. In this heavily pretreated population, nivolumab was associated with a very high response rate, durable responses, and a manageable side effect profile. The clinical implications and justification for a phase I study with a small number of subjects resulting in a New England Journal of Medicine publication are based on the finding that this well-tolerated immune therapy approach is revolutionary for this patient population who were chemorefractory and facing a dismal outcome prior to this breakthrough.
The phase II multicenter, single-arm study investigating the safety and efficacy defined by ORR of nivolumab in relapsed/refractory cHL patients who had failed ASCT and subsequent brentuximab vedotin has been reported.17 Patients received nivolumab (3 mg/kg) every 2 weeks until disease progression, unacceptable toxicity, or study end. Median age was 37 years. Median number of prior lines of therapy was four. Median time from the most recent brentuximab vedotin exposure was 0.7 years, and median time from ASCT was 3.4 years. The most common drug-related AEs were fatigue (25%), infusion reaction (20%), rash (16%), arthralgia (14%), pyrexia (14%), nausea (13%), diarrhea (10%), and pruritus (10%). The majority of these were grades 1–2 with the exception of one case of grade 3 rash.
The ORR of 80 subjects as determined by an independent review committee was 66% (slightly higher ORR of 73% by investigator assessment).17 The 6-month estimated PFS and OS were 77% and 99%, respectively. In this registrational study, nivolumab resulted in durable responses and an acceptable safety profile in heavily pretreated patients with relapsed/refractory cHL who had failed ASCT and brentuximab vedotin and were without an alternative treatment option. This study continues to enroll with additional cohorts including brentuximab vedotin-naïve patients and patients who received brentuximab vedotin prior to ASCT.
The phase II study provides information on a larger number of subjects, though this is still a relatively small study population. Similar to the phase I study, the majority of subjects had a response, with a small subset achieving a CR. What is more meaningful than response rates are the robust PFS and OS suggesting this is an effective, well-tolerated therapeutic option that can result in meaningful outcomes for young patients with limited to no options.
Additional PD-1 inhibitors have subsequently been developed, including pembrolizumab, a humanized IgG4 antibody to PD-1 that has been associated with promising efficacy for solid tumors including non-small-cell lung cancer, melanoma, and head and neck cancer.18–21 The efficacy in and safety of patients with relapsed/refractory Hodgkin lymphoma who failed brentuximab vedotin and were not candidates for SCT enrolled in the phase I study, KEYNOTE-013, have been reported.4 Patients received 10 mg/kg of pembrolizumab every 2 weeks up to 2 years or until confirmed disease progression or unacceptable toxicity. Similar to the nivolumab studies in relapsed Hodgkin lymphoma, this was a young patient population (median age 32 years) with good performance status. All patients had received prior brentuximab vedotin therapy, and 71% had failed ASCT. The most common treatment-related AEs included hypothyroidism (16%), diarrhea (16%), nausea (13%), and pneumonitis (10%). There were no grade 4 treatment-related AEs and no deaths due to study treatment. Of the 31 patients with relapsed/refractory Hodgkin lymphoma, the ORR was 65%, and 6-month PFS and OS estimates were 69% and 100%, respectively.
Correlative studies examined demonstrated that pembrolizumab increased circulating immune cell subsets (expansion of T cells and NK cells) and upregulated interferon gamma (IFN-γ) pathways; however, gene signatures did not appear to predict response in this small cohort. This phase I study demonstrated that pembrolizumab is also an effective PD-1 antibody in relapsed/refractory Hodgkin lymphoma and was associated with an acceptable safety profile. Further clinical development of pembrolizumab in Hodgkin lymphoma is warranted.
Preliminary results of a multi-cohort phase II study of pembrolizumab (200 mg every 3 weeks) in heavily pretreated Hodgkin lymphoma patients suggest a favorable safety profile and high ORR (70–80%) with pembrolizumab in chemoresistant, transplant-ineligible patients who have had prior brentuximab vedotin and in patients who have failed ASCT and subsequent brentuximab vedotin therapy.22 The phase II study of pembrolizumab in relapsed/refractory cHL demonstrated similarly favorable efficacy and safety as the phase I study. This study is ongoing, and future reports are eagerly awaited. Though there are subtle differences in the study populations, it appears that nivolumab and pembrolizumab are equally effective and well tolerated and offer hope for many patients with Hodgkin lymphoma facing poor outcomes. The unmet need currently are those patients who have failed chemotherapy and brentuximab vedotin and have not been candidates for ASCT; it is expected that these patients would benefit from PD-1 antibody therapy and are in need of novel, effective therapy.
Response of PD-1 blockade in other lymphomas (non-Hodgkin lymphomas)
Given the impressive results in Hodgkin lymphoma, the use of PD-1 inhibitors has been investigated in other hematologic malignancies. PD-1 and its ligands PD-L1 and PD-L2 are also expressed in non-Hodgkin lymphoma (NHL) and/or the neoplastic microenvironment; however, the expression of PD-L1 varies significantly among lymphoma subtypes.23,24 Early phase studies suggest that response rates with PD-1 antibodies are most promising in Hodgkin lymphoma and have been disappointing as single agents in relapsed diffuse large B cell lymphoma (DLBCL) and follicular lymphoma. Eighty-one patients with relapsed NHL or multiple myeloma were treated in a phase I study with 1 or 3 mg/kg of nivolumab every 2 weeks up to 2 years.25 The ORR in 11 patients with relapsed DLBCL was 36% (18% CR) with a median PFS of 7 weeks. The ORR was also modest in relapsed follicular lymphoma (N=10) (40%; CR 10%); however, the responses appeared more durable in follicular lymphoma with median PFS not yet reached in this small subgroup. Genetic alterations of PD-L1 and PD-L2 were rare among the NHL subgroups in this study, a very different observation compared to the near universal 9p24.1 alterations seen in Hodgkin lymphoma.
Primary mediastinal large B cell lymphoma (PMBCL) commonly harbors 9p24.1 amplification or rearrangement, and concomitant overexpression of both PD-L1 and PD-L2. Ten patients with relapsed/refractory PMBCL were included in the phase I study of pembrolizumab.26 The ORR was 44% in patients with relapsed/refractory PMBCL, and with very short median follow-up (144 days), the median duration of response had not been reached. The results of this phase I study subgroup analysis are very promising particularly given this is a very poor-risk population. Ongoing and future studies examining the efficacy of PD-1/PD-L blockade in PMCBL are eagerly awaited.
Monotherapy with PD-1 antibodies holds the most promise in relapsed/refractory cHL. There are promising early results in relapsed/refractory PMBCL and possible durable responses in follicular lymphoma. Table 1 summarizes the efficacy of PD-1 antibodies across lymphoma subtypes. What is lacking is a predictive biomarker to identify patients that will likely do well with PD-1 blockade in lymphoma. Amplification of 9p24.1 more than PD-L1 expression may predict response to PD-1 blockade. However, we need tools that utilize clinically available information to help guide therapy for the numerous patients with lymphoma who are failing chemotherapy and are in need of novel treatment.
Immune-related AEs
The mechanisms used to block the checkpoints in T cell function that enhance antitumor activity are present against both tumor and normal cells, which disrupts the normal immunologic milieu leading to immune-related AEs (irAEs) such as dermatologic, gastrointestinal, hepatic, endocrine, and pulmonary AEs.27 Given the direct relationship to immunologic enhancement, irAEs can often be managed with temporary dose interruption and immunosuppression with corticosteroids or infliximab. As there is currently limited data to guide effective management, clinical experience and prompt identification and intervention are important in moderating severity. It should be noted that the management of irAE in the literature is largely based on experience in solid tumors rather than hematologic malignancies. PD-1/PD-L1 inhibition may be associated with a more favorable safety profile compared to CTLA-4 inhibitors; however, similar side effects are observed across immune checkpoint inhibitors. The most common irAE reported is rash, with an incidence of approximately 40% with PD-1 antibodies. Reactions can range from mild papular eruptions to severe bullous pemphigoid reactions. For mild symptoms (grades 1–2), management is generally supportive with topical steroids. In addition, system antihistamines may be utilized to relieve pruritus. Systemic corticosteroids (1 mg/kg daily) are needed for moderate-to-severe (grade 3 or higher) rash. Fortunately, grade 3 or 4 rash is uncommon (<10%). Treatment with PD-1 inhibitors should be delayed until symptoms improve to baseline or grade 1. Treatment with immune checkpoint inhibitors should be discontinued if the rash does not improve after 12 weeks of supportive therapy and should be permanently avoided if associated with Stevens–Johnson syndrome.27
Diarrhea or colitis can be seen in patients treated with PD-1 inhibitors, though the incidence of severe colitis (grade 3 or higher) is higher with CTLA-4 blocking antibodies (7% versus 2%).28 Onset of diarrhea or colitis can vary, but is often seen within 8 weeks of initiation of treatment. During the evaluation of these symptoms, infectious etiologies such as Clostridium difficile or viral pathogens must first be excluded. Discussion of the number of bowel movements per day is important in discerning the severity of diarrhea. Mild symptoms can be treated with antidiarrheal agents such as loperamide hydrochloride. If symptoms persist (>3 days) or worsen in intensity, systemic corticosteroid (budesonide or prednisone 1–2 mg/kg daily equivalent) treatment should be considered. Infliximab (5 mg/kg once every 2 weeks) has been used in those who fail to respond to corticosteroids.29 Treatment with immune checkpoint inhibitors should be held until symptoms resolve to grade 1 or less. A prolonged course of corticosteroids (>3 weeks) may also be necessary for colitis to fully resolve.
Hepatitis or transaminitis associated with PD-1 inhibition generally begins within the first 12 weeks of therapy, though similar to other irAEs, it can occur at any time. Liver enzymes and bilirubin should be monitored closely, and if elevated, viral and other drug-induced causes should be excluded. The immune checkpoint inhibitor should be held until recovery. Prompt corticosteroid initiation for severe cases is recommended. For steroid-refractory cases, mycophenolate or tacrolimus may be useful. Infliximab is contraindicated in this setting due to increased risk of hepatotoxicity.
Pneumonitis is also uncommon (<10%), but unlike most other irAEs, pneumonitis is more common with PD-1 inhibitors compared to other classes of checkpoint inhibitors.28 Patients presenting with any new pulmonary complaint (shortness of breath, cough) or hypoxia require urgent assessment including chest imaging. Radiologic findings can be subtle and generally reveal bilateral, peripheral infiltrates. Mild cases can be managed with systemic corticosteroids, but patients with more advanced symptoms should undergo bronchoscopy prior to immunosuppression to evaluate for superinfection. Importantly, patients who experience moderate-to-severe symptoms should not be retreated with checkpoint inhibitors.
Neurologic symptoms are rare (<5%) and can range from mild neuropathy to meningitis, vasculitis, and Guillain–Barré syndrome. Treatment with systemic corticosteroids may not be effective in severe disease, and intravenous immunoglobulin may be helpful. No further checkpoint inhibitors should be administered for cases of grade 3 or higher neurologic irAEs.
Other irAEs such as various endocinopathies (thyroid disease, panhypopituitarism secondary to hypophysitis), nephritis, and pancreatitis have also been reported with PD-1/PD-L1 inhibitors. Given the more widely available use of immune checkpoint inhibitors, identification and intervention are critical to avoid serious morbidity and mortality associated with immune checkpoint inhibitors. Continued vigilance is critical as the onset of irAEs can be variable. Fortunately, most irAEs are mild. With combination therapy appearing promising, the additive toxicity with combination therapy also warrants close monitoring.
Future directions
The promising results seen with immune therapy in lymphoma suggest the application of these agents will continue. In addition, there is room for improvement with many refractory lymphoma patients failing to respond to monotherapy with PD-1 antibodies. Similar to solid tumors, the favorable toxicity profile associated with PD-1 antibodies suggests combination therapies may be very promising in hematologic malignancies, particularly in lymphoma subtypes in which single-agent efficacy is modest. The combination of brentuximab vedotin and nivolumab is currently being explored in several settings including relapsed/refractory Hodgkin lymphoma (NCT02572167) and in patients who are deemed poor candidates for frontline combination chemotherapy (NCT02758717). In addition, combination ipilimumab, nivolumab, and brentuximab vedotin (NCT01896999) is being explored in relapsed/refractory Hodgkin lymphoma. Combination approaches will be practice changing if they indeed improve the efficacy without excess toxicity.
Furthermore, additional trials are ongoing with pembrolizumab combinations. The KEYNOTE-204 trial, which compares pembrolizumab to brentuximab vedotin in relapsed Hodgkin lymphoma, is currently ongoing and may help clinicians decide the optimal sequencing of therapy now that there are more options for patients with relapsed Hodgkin lymphoma. Pembrolizumab in combination with rituximab is currently being explored in relapsed follicular lymphoma (NCT02446457) and may be a promising synergistic combination particularly in follicular lymphoma. A phase I trial of CA-170, an oral, small molecule that targets the PD-L1 and PD-L2 as well as the V-domain Ig suppressor of T cell activation immune checkpoints, is currently ongoing in patients with relapsed Hodgkin lymphoma and solid tumors. This study is exciting as this is the first oral medication in this class.
The natural evolution of trial development suggests that these agents will be moved earlier in the treatment of patients with lymphoma, and perhaps become frontline therapy. Nivolumab, in combination with doxorubicin, vinblastine, and dacarbazine, is currently being evaluated in Hodgkin lymphoma as frontline therapy. Additionally, pembrolizumab in combination with chemotherapeutic agents is being evaluated as frontline therapy in DLBCL (NCT02541565).30 The current unanswered question is whether chemotherapy can be abrogated with optimal immune therapy combination, which is a very enticing concept. The results of such studies evaluating this, such as those combining bretuximab with either nivolumab or CTLA-4 blockade in cHL, are highly anticipated.30 Additionally, several studies will soon be recruiting participants to evaluate PD-1 inhibitors in combination with immunomodulatory agents in myeloma, chronic lymphocytic leukemia, and low-grade B cell lymphomas. Even with the ongoing studies and those that are actively recruiting, many unanswered questions regarding treatment with PD-1 and PD-L1 inhibitors remain unanswered. Specifically, further investigation is needed in specific underrepresented populations, such as minority patients and those with preexisting autoimmune diseases, viral hepatitis, or HIV. These unanswered questions will hopefully lead to the development of further trials to assess these promising agents in this specific subpopulation of patients.
Choosing between the PD-1 inhibitors
Currently, very little data are available to guide clinicians in the selection of a particular PD-1 inhibitor. With respect to the two most commonly used agents, nivolumab and pembrolizumab, their efficiency and safety profile appear to be very similar. Some data suggest that pembrolizumab has a higher degree of PD-1 blockade, but this observation has not translated into higher clinical efficacy. In the absence of head-to-head comparison studies, the choice of agent will depend on the provider’s experience or comfort level with a particular agent. In addition, subtle differences in the treatment schedule (every 2 weeks versus every 3 weeks) may lead to a patient preference in selection of antibody (nivolumab is dosed every 2 weeks, while pembrolizumab is dosed every 3 weeks).
Conclusion
Immunotherapeutic agents targeting the PD-1/PD-L1 pathway offer a promising therapeutic approach for patients with a variety of lymphomas, particularly for those with refractory Hodgkin lymphoma facing dismal outcomes. Optimal duration and sequencing of therapy are currently unknown. Randomized studies are needed to better understand the efficacy and safety profile of checkpoint inhibitors in lymphoma. Predictive biomarkers are desired to identify the patients likely to respond to PD-1 blockade across the lymphoma subtype spectrum. Optimism surrounds the combination therapies currently under investigation that may overcome resistance to monotherapy with PD-1 antibodies that may extend the clinical benefits to more patients who are chemorefractory and in need of novel therapy. Ultimately, many questions remain, but the early success of these agents has sparked interest in further development of immune modulation in the management of lymphoma. The promising outcomes noted thus far with checkpoint inhibitors currently offer hope for the treatment of patients with chemorefractory disease, and may ultimately lead to less toxic treatment options in challenging conditions where chemotherapy has previously been the standard approach.
Disclosure
Nastoupil receives honorarium from Abbvie, Celgene, Genentech, Gilead, Pharmacyclics, and TG Therapeutics. The other authors report no conflicts of interest in this work.
References
Okazaki T, Honjo T. The PD-1-PD-L pathway in immunological tolerance. Trends Immunol. 2006;27(4):195–201. | ||
Okazaki T, Chikuma S, Iwai Y, Fagarasan S, Honjo T. A rheostat for immune responses: the unique properties of PD-1 and their advantages for clinical application. Nat Immunol. 2013;14(12):1212–1218. | ||
Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372(4):311–319. | ||
Armand P, Shipp MA, Ribrag V, et al. Programmed death-1 blockade with pembrolizumab in patients with classical Hodgkin lymphoma after brentuximab vedotin failure. J Clin Oncol. Epub 2016 Jun 27. | ||
Reck M, Rodríguez-Abreu D, Robinson AG, et al; KEYNOTE-024 Investigators. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. | ||
Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369(2):122–133. | ||
Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. | ||
Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8(6):467–477. | ||
Carreras J, Lopez-Guillermo A, Roncador G, et al. High numbers of tumor-infiltrating programmed cell death 1-positive regulatory lymphocytes are associated with improved overall survival in follicular lymphoma. J Clin Oncol. 2009;27(9):1470–1476. | ||
Tzankov A, Meier C, Hirschmann P, Went P, Pileri SA, Dirnhofer S. Correlation of high numbers of intratumoral FOXP3+ regulatory T cells with improved survival in germinal center-like diffuse large B-cell lymphoma, follicular lymphoma and classical Hodgkin’s lymphoma. Haematologica. 2008;93(2):193–200. | ||
Muenst S, Hoeller S, Dirnhofer S, Tzankov A. Increased programmed death-1+ tumor-infiltrating lymphocytes in classical Hodgkin lymphoma substantiate reduced overall survival. Hum Pathol. 2009;40(12):1715–1722. | ||
Intlekofer AM, Thompson CB. At the bench: preclinical rationale for CTLA-4 and PD-1 blockade as cancer immunotherapy. J Leukoc Biol. 2013;94(1):25–39. | ||
Yang ZZ, Novak AJ, Stenson MJ, Witzig TE, Ansell SM. Intratumoral CD4+CD25+ regulatory T-cell-mediated suppression of infiltrating CD4+ T cells in B-cell non-Hodgkin lymphoma. Blood. 2006;107(9):3639–3646. | ||
Myklebust JH, Irish JM, Brody J, et al. High PD-1 expression and suppressed cytokine signaling distinguish T cells infiltrating follicular lymphoma tumors from peripheral T cells. Blood. 2013;121(8):1367–1376. | ||
Roemer MG, Advani RH, Ligon AH, et al. PD-L1 and PD-L2 genetic alterations define classical Hodgkin lymphoma and predict outcome. J Clin Oncol. 2016;34(23):2690–2697. | ||
Green MR, Monti S, Rodig SJ, et al. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood. 2010;116(17):3268–3277. | ||
Younes A, Santoro A, Shipp M, et al. Nivolumab for classical Hodgkin’s lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol. 2016;17(9):1283–1294. | ||
Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–1550. | ||
Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16(8):908–918. | ||
Seiwert TY, Burtness B, Mehra R, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol. 2016;17(7):956–965. | ||
Chow LQ, Haddad R, Gupta S, et al. Antitumor activity of pembrolizumab in biomarker-unselected patients with recurrent and/or metastatic head and neck squamous cell carcinoma: results from the phase Ib KEYNOTE-012 expansion cohort. J Clin Oncol. Epub 2016 Sep 19. | ||
Moskowitz C, Zinzani PL, Fanale MA, et al. Multicohort phase 2 study of pembrolizumab for relapsed/refractory classical Hodgkin lymphoma (R/R Chl): KEYNOTE-087. Haematologica. 2016;101:319. | ||
Chen BJ, Chapuy B, Ouyang J, et al. PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin Cancer Res. 2013;19(13):3462–3473. | ||
Andorsky DJ, Yamada RE, Said J, Pinkus GS, Betting DJ, Timmerman JM. Programmed death ligand 1 is expressed by non-Hodgkin lymphomas and inhibits the activity of tumor-associated T cells. Clin Cancer Res. 2011;17(13):4232–4244. | ||
Lesokhin AM, Ansell SM, Armand P, et al. Nivolumab in patients with relapsed or refractory hematologic malignancy: preliminary results of a phase Ib study. J Clin Oncol. 2016;34(23):2698–2704. | ||
Zinzani PL, Ribrag V, Moskowitz CH, et al. Phase 1b study of PD-1 blockade with pembrolizumab in patients with relapsed/refractory primary mediastinal large B-cell lymphoma (PMBCL). Blood. 2015;126(23):3986. | ||
Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the immune-related adverse effects of immune checkpoint inhibitors: a review. JAMA Oncol. 2016;2(10):1346–1353. | ||
Robert C, Schachter J, Long GV, et al; KEYNOTE-006 investigators. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372(26):2521–2532. | ||
Pagès C, Gornet JM, Monsel G, et al. Ipilimumab-induced acute severe colitis treated by infliximab. Melanoma Res. 2013;23(3):227–230. | ||
Goodman A, Patel SP, Kurzrock R. PD-1-PD-L1 immune-checkpoint blockade in B-cell lymphomas. Nat Rev Clin Oncol. Epub 2016 Nov 2. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.