Back to Journals » OncoTargets and Therapy » Volume 12
Harmine suppresses the proliferation of pancreatic cancer cells and sensitizes pancreatic cancer to gemcitabine treatment
Authors Wu LW, Zhang JK , Rao M, Zhang ZY, Zhu HJ, Zhang C
Received 12 February 2019
Accepted for publication 21 May 2019
Published 12 June 2019 Volume 2019:12 Pages 4585—4593
DOI https://doi.org/10.2147/OTT.S205097
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Yao Dai
Lin-Wen Wu,1,2,* Jian-Kang Zhang,1,2,* Mingjun Rao,3 Zuo-Yan Zhang,1,2 Hua-Jian Zhu,1,2 Chong Zhang1,2
1School of Medicine, Zhejiang University City College, Hangzhou, Zhejiang 310015, People’s Republic of China; 2College of Pharmaceutical Sciences, Zhejiang University, Hangzhou, Zhejiang 310058, People’s Republic of China; 3Department of Clinical Pharmacology, Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang 310006, People’s Republic of China
*These authors contributed equally to this work
Purpose: Pancreatic carcinoma is one of the most deadliest types of cancer, and relatively insensitive to the currently available chemotherapy. Thus, the discovery of novel therapeutic agents to prolong the survival times of patients with pancreatic cancer is urgently required.
Methods: Cell proliferation was assessed using the sulforhodamine B and cell clone formation assay, apoptosis was analyzed through Annexin V/PI staining, analysis of cell cycle distribution was determined by PI staining, and the expression of proteins was detected via Western blotting.
Results: Our data showed that harmine exerted an anti-proliferative effect and cell cycle arrest at G2/M in pancreatic cancer cells. Meanwhile, harmine plus gemcitabine showed strong synergy in inhibiting the proliferation of pancreatic cancer cells. Furthermore, harmine induced apoptosis and enhanced the gemcitabine-induced apoptosis in pancreatic cancer cells. The AKT/mTOR pathway is involved in mechanisms of gemcitabine resistance in pancreatic cancer cells, our data demonstrated that harmine plus gemcitabine significantly suppressed the AKT/mTOR signaling pathway.
Conclusion: Harmine may be a potential candidate for the treatment of pancreatic cancer. Morever, the combination of harmine with gemcitabine appears to be an attractive option for the treatment of patients with pancreatic cancer.
Keywords: harmine, gemcitabine, pancreatic cancer, AKT/mTOR, combination
Introduction
Pancreatic carcinoma is one of the most deadliest types of cancer worldwide. In addition, it is estimated that pancreatic carcinoma will become the second leading cause of cancer-related death by 2030.1 During the previous several decades, there has not been significant improvement in the clinical outcome of pancreatic cancer, with the average survival rate being 5%.2 The anatomical location of the pancreatic gland is close to many important organs, main arteries and veins. Consequently, a large proportion of patients with pancreatic carcinoma (80–85%) present with an extension to adjacent organs or distant metastases, rendering surgical resection.3 Currently, gemcitabine is recommended as the first-line drug for the treatment of advanced pancreatic cancer.4 However, pancreatic cancer is relatively insensitive to chemotherapy, and the poor outcomes associated with chemotherapy is largely related to acquired chemoresistance of pancreatic cancer cells.5 Thus, the discovery of novel agents or gemcitabine-based combination regimens are urgently required to overcome chemoresistance and prolong the survival time of patients with pancreatic cancer.
Harmine is a tricyclic β-carboline alkaloid isolated from the seeds of the medical plant Peganum harmala L.6 The plant is the only salt-tolerant perennial herb in the Peganum genus of the family Zygophyllaceae. Its seeds are traditionally used to relieve pain, to promote blood circulation, and to treat rheumatism and symptoms (ie, cough and asthma).7 Furthermore, harmine can also inhibit all members of the DYRK family (ie, DYRK1A, DYRK1B, DYRK2, and DYRK4), with the highest affinity demonstrated for DYRK1A.8 In addition, several molecules, such as AChE, BChE, MAO-A and GABA receptor, were also considered to be targets of harmine for other pharmacological effects.9 Harmine possesses multiple types of pharmacological activity such as anti-microbial, anti-fungal, anti-tumor, anti-plasmodial, anti-oxidant, anti-mutagenic, and anti-genotoxic properties.10 Harmine suppresses the proliferation of various types of cancer cells (eg, ovarian and gastric cancer).11,12 It has been shown to exhibit anti-cancer properties by inhibiting tumor growth, metastasis, and inducing apoptosis.13 Our data demonstrated for the first time that harmine may exhibit anti-cancer activity in pancreatic cancer cells. In addition, harmine may sensitize pancreatic cancer cells to treatment with gemcitabine via AKT/mTOR pathway. Our study has revealed that harmine may be a potential candidate for the treatment of pancreatic cancer treatment, and the combination of harmine with gemcitabine appears to be an attractive option for the treatment of patients with pancreatic cancer.
Materials and methods
Materials
Harmine (cat. no. HY-N0737A) and gemcitabine hydrochloride (cat. no. HY-B0003) were supplied by Med Chem Express (Monmouth Junction, NJ, USA).
Cell culture
PANC-1 (cat. no. TCHu98), CFPAC-1 (cat. no. TCHu112), SW-1990 (cat. no. TCHu201) and BxPC-3 (cat. no. TCHu12) were obtained from Shanghai Institute of Biochemistry and Cell Biology (Shanghai, China). BxPC-3, CFPAC-1 and SW1990 cells were maintained under humidified conditions (37 °C, 5% CO2) in RPMI-1640 (Life Technologies) plus 5% FBS. PANC-1 cells were cultured in DMEM medium.
Sulforhodamine B (SRB) assay
Cell survival was determined by SRB assay as described previously.14 Firstly, pancreatic cancer cells were seeded into 96-well plates at a density of 4×103 per well and treated with the compounds at the indicated concentrations for 72 h, Then, the cells were fixed by 10% trichloroacetic acid solution at 4 °C for 1 h, washed with tap water, dried and stained with 0.4% SRB solution for 30 min. Then, the excess dye was removed by washing repeatedly with 1% acetic acid. Following this, wells were dried and SRB dye was dissolved in 10 mM Tris base solution for OD determination at 515 nm using a multi-scan spectrum.
Cell clone formation assay
Pancreatic cancer cells were seeded onto 6-well plates at a density of 1,000 cells per well and treated with harmine at the indicated concentrations, plated in 35 mm dishes and incubated at 37 °C for 14 days. The compound-containing medium was replaced every 2–3 days during the whole experiment.
Apoptosis detection by annexin v/propidium iodide (PI) staining
Apoptosis was detected by Annexin V - FITC apoptosis detection kit (cat. no. 556547, BD bioscience, San Jose, CA) according to the manufacturer’s recommendations. Briefly, cells were collected and incubated in 200 μL binding buffer containing 10 μL Annexin V at room temperature for 10 mins. Prior to flow cytometric analysis, 5 μL propidium iodide solution was added.
Analysis cell cycle distribution by PI staining
Cell cycle distribution was detected by PI staining as described previously.15 Samples were analyzed by a FACSCalibur cytometer.
Western blotting
Whole-cell extracts for SDS-PAGE were prepared and Western blotting analysis was performed as previously described.14 The antibodies used for Western blotting were obtained from different resources: anti-c-myc antibody (cat. no. 13987), anti-cleaved PARP antibody (cat. no. 9541), anti-cleaved-caspase-3 antibody (cat. no. 9661), anti-caspase-3 antibody (cat. no. 9662), anti-AKT antibody (cat. no. 9272), anti-phospho-AKT (Ser-473) antibody (cat. no. 4051), anti-phospho-p70S6 Kinase (Thr-389) antibody (cat. no. 9234), anti-mTOR antibody (cat. no. 2983), anti-p70S6 Kinase antibody (cat. no. 2708) and anti-phospho-mTOR (Ser-2448) antibody (cat. no. 5536) were purchased from Cell Signaling Technology (Danvers, MA, USA); anti-p21 antibody (cat. no. sc-6246) was purchased from Santa Cruz Biotechnology (Dallas, Texas, USA); anti-GAPDH antibody (cat. no. db106) was acquired from Diagbio Biosciences (Hangzhou, China); anti-cyclin B1 antibody (cat. no. 55004-1-AP) was purchased from Proteintech (Rosemont, IL, USA).
Statistical analyses
The data were presented as means ± SD from at least three independent experiments. Differences between the groups were determined with Student’s t-test analysis and considered significant when P<0.05. Combination index (CI) values were calculated using Calcusyn (Biosoft, Great Shelford, Cambridge, UK) and it indicated synergism when CI value <0.9.
Results
Harmine suppresses the proliferation of pancreatic cancer cells
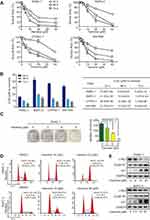
Harmine shows anti-cancer activity in multiple types of cancer, such as neuroblastoma, ovarian, and gastric cancer. However, thus far, its activity against pancreatic cancer has not been evaluated.11,12,16 Firstly, we determined the anti-cancer effect of harmine on the growth of pancreatic cancer cell lines, including PANC-1, CFPAC-1, SW-1990, and BxPC-3. Pancreatic cells were incubated with indicated concentrations of harmine for 24 h, 48 h, and 72 h. As shown in Figure 1A, the growth of pancreatic cancer cells was significantly suppressed by harmine in a dose- and time-dependent manner. The half-maximal inhibitory concentrations (IC50) of harmine were shown in Figure 1B. In addition, harmine significantly suppressed the colon formation of pancreatic cells in a dose-dependent manner (Figure 1C). Moreover, we observed that harmine induced cell cycle arrest at G2/M in PANC-1 and bxPC-3 cells (Figure 1D). Meanwhile, harmine-induced G2/M arrest was accompanied by the activation of p21 and cyclin B1, as well as inhibition of c-Myc in pancreatic cancer cells (Figure 1E). Thus, our data demonstrated that harmine may exert an anti-proliferative effect and cell cycle arrest at G2/M in pancreatic cancer cells.
Harmine enhanced the cytotoxicity of gemcitabine in pancreatic cancer cells
Pancreatic cancer is typically diagnosed at an advanced stage and gemcitabine-based treatment has been used as first-line therapy for metastatic pancreatic cancer.17 Thus, we are encouraged to investigate whether harmine may enhance the cytotoxicity of gemcitabine in pancreatic cancer cells. As shown in Figure 2A, we determined its anti-cancer activity by combining harmine with gemcitabine at clinically achievable concentrations in four human pancreatic cancer cell lines.18 The combination of harmine plus gemcitabine showed strong synergy in these pancreatic cancer cell lines (mean CI values: <0.9, Figure 2B).
Harmine enhanced the gemcitabine induced apoptosis in pancreatic cancer cells
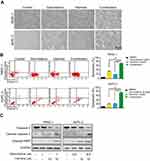
Initially, we investigated the morphological changes induced by the combination of harmine and gemcitabine in BxPC-3 and PANC-1 cells. Figure 3A showed that a large proportion of pancreatic cancer cells treated with harmine plus gemcitabine were detached from the culture dishes, whereas the remaining adherent cells showed loss of adhesion, shrinkage, and rounding, which are typical morphological changes associated with apoptosis. Subsequently, we investigated the apoptotic effect of harmine, gemcitabine, and harmine plus gemcitabine in PANC-1 and BxPC-3 cells. As shown in Figure 3B, according to Annexin V positivity, the percentages of apoptotic PANC-1 cells were as follows: vehicle control, 7.33%±0.18%; gemcitabine, 35.60%±1.56% (harmine vs harmine plus gemcitabine, P=0.0032); harmine, 19.75%±1.34% (gemcitabine vs harmine plus gemcitabine, P=0.0015); and harmine plus gemcitabine, 74.63%±2.72%. Harmine enhanced gemcitabine induced apoptosis by in both PANC-1 and BxPC-3 cells (Figure 3B). We also found that the cleavage of PARP and activation of caspase-3 were enhanced following treatment with harmine plus gemcitabine versus either single-agent treatment in pancreatic cancer cells (Figure 3C). Thus, we concluded that harmine induces apoptosis and enhances gemcitabine induced apoptosis in pancreatic cancer cells.
Harmine plus gemcitabine inhibited the AKT/mTOR signaling pathway
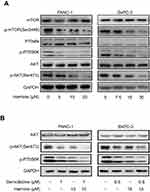
The PI3K/AKT/mTOR pathway is involved in the development of resistance to gemcitabine. Our data indicated that harmine suppressed the AKT/mTOR pathway in PANC-1 and BxPC-3 cells (Figure 4A). In addition, we subsequently evaluated the effect of harmine plus gemcitabine on the AKT/mTOR pathway in pancreatic cancer cells. As shown in Figure 4B, in PANC-1 and BxPC-3 cells, treatment with harmine plus gemcitabine significantly inhibited the expression of the members of the AKT/mTOR signaling pathway, including p-AKT (ser473), p-mTOR (Ser2448) and p-P70S6K.
Discussion
Pancreatic cancer is associated with a very poor prognosis, highlighted by the close parallel between the incidence and mortality associated with this disease. Gemcitabine has been a standard of chemotherapy for the treatment of metastatic pancreatic cancer.19 An array of different agents (eg, anti-metabolites, nucleoside analogs, and DNA intercalating compounds), have been used against pancreatic cancer, alone or in combination, with limited improvement observed in patient survival.20 Thus, the evolution of chemotherapy for pancreatic cancer is urgently warranted. In our study, we evaluated the anti-proliferative activity of harmine in four pancreatic cancer cell lines, including PANC-1, CFPAC-1, SW1990 and BxPC-3. Our data indicated that harmine inhibited the proliferation of pancreatic cancer cells, with the IC50 ranging between 5.40 μM and 13.67 μM. Furthermore, harmine enhanced the anti-proliferative and apoptotic induction activity of gemcitabine in pancreatic cancer cells. Our findings provide a novel strategy for the use of harmine in the treatment of pancreatic cancer. Moreover, the combination of harmine plus gemcitabine may be an effective combination regimen against this disease.
Patients with advanced pancreatic cancer are linked to a poor prognosis. Furthermore, there has been no improvement in patient survival since the introduction of gemcitabine in 1996.21 Pancreatic cancer remains one of the most deadliest types of cancer, with extremely low survival rates. This is attributed to delayed diagnosis and the development of resistance to standard therapies.22 The AKT/mTOR pathway is involved in mechanisms of resistance to gemcitabine in pancreatic cancer cells.23 Furthermore, the development of resistance to gemcitabine in breast cancer cells is mainly mediated by activation of the AKT/mTOR signaling pathway.24 In the present study, we showed that harmine suppressed the AKT/mTOR pathway, and harmine plus gemcitabine also significantly suppressed the AKT/mTOR signaling pathway. Thus, harmine may enhance the anti-cancer activity of gemcitabine via suppressing the AKT/mTOR pathway. Therefore, our data indicated that the combination of gemcitabine with harmine may be an effective therapeutic regimens for the treatment of patients with gemcitabine-resistant pancreatic tumors. Although gemcitabine exhibits a good toxicity profile, myelosuppression remains the most common adverse effect associated with this agent.25 Recently, it was reported that harmine may enhance the trabecular bone mass and osteogenic responses, and increased the number of preosteoclasts.26 Hence, we suggest that harmine plus gemcitabine may be an attractive option for sensitization of cells to anti-pancreatic cancer activity. Moreover, this regimen may reverse the myelosuppression induced by gemcitabine during the treatment of pancreatic cancer. However, further in-vivo studies are warranted to test the hypothesis.
Conclusion
In conclusion, harmine may be a promising drug for the treatment of pancreatic cancer. The combination of harmine with gemcitabine may be an effective therapeutic strategy, offering synergistic efficacy and low toxicity in patients with pancreatic cancer. Furthermore, gemcitabine plus harmine may be an efficacious therapeutic regimen for patients with gemcitabine-resistant pancreatic tumors.
Acknowledgments
This work was supported by the National Natural Science Foundation of China [81702887, 2017]; Hangzhou Major Science and Technology Project [20172016A01, 2017]; Fund of Hangzhou Medical Key Discipline Construction [2017-51-07, 2017]; Zhejiang Province Medical Key Discipline Construction [2018-2-03, 2018]; Teachers Research Fund of Zhejiang University City College [J-19006, 2019] and Scientific and Technological Developing Scheme of Hangzhou City [20191203B49 and 20191203B50, 2019].
Disclosure
The authors report no conflicts of interest in this work.
References
1. Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913–2921. doi:10.1158/0008-5472.CAN-14-0155
2. Deshmukh SK, Tyagi N, Khan MA, et al. Gemcitabine treatment promotes immunosuppressive microenvironment in pancreatic tumors by supporting the infiltration, growth, and polarization of macrophages. Sci Rep. 2018;8(1):12000. doi:10.1038/s41598-018-30437-2
3. Lauffer DC, Kuhn PA, Kueng M, et al. Pancreatic cancer: feasibility and outcome after radiochemotherapy with high dose external radiotherapy for non-resected and R1 resected patients. Cureus. 2018;10(5):e2713.
4. Lankadasari MB, Aparna JS, Mohammed S, et al. Targeting S1PR1/STAT3 loop abrogates desmoplasia and chemosensitizes pancreatic cancer to gemcitabine. Theranostics. 2018;8(14):3824–3840. doi:10.7150/thno.25308
5. Mao Y, Xi L, Li Q, et al. Combination of PI3K/Akt Pathway Inhibition and Plk1 Depletion Can Enhance Chemosensitivity to Gemcitabine in Pancreatic Carcinoma. Transl Oncol. 2018;11(4):852–863. doi:10.1016/j.tranon.2018.04.011
6. Herraiz T, Gonzalez D, Ancin-Azpilicueta C, Aran VJ, Guillen H. beta-Carboline alkaloids in Peganum harmala and inhibition of human monoamine oxidase (MAO). Food Chem Toxicol. 2010;48(3):839–845. doi:10.1016/j.fct.2009.12.019
7. Li Y, He Q, Du S, Guo S, Geng Z, Deng Z. Study of methanol extracts from different parts of peganum harmala L. Using (1)H-NMR plant metabolomics. J Anal Methods Chem. 2018;2018:6532789. doi:10.1155/2018/6532789
8. Ogawa Y, Nonaka Y, Goto T, et al. Development of a novel selective inhibitor of the down syndrome-related kinase Dyrk1A. Nat Commun. 2010;1:86. doi:10.1038/ncomms1090
9. Shu B, Zhang J, Jiang Z, Cui G, Veeran S, Zhong G. Harmine induced apoptosis in Spodoptera frugiperda Sf9 cells by activating the endogenous apoptotic pathways and inhibiting DNA topoisomerase I activity. Pestic Biochem Physiol. 2019;155:26–35. doi:10.1016/j.pestbp.2019.01.002
10. Patel K, Gadewar M, Tripathi R, Prasad SK, Patel DK. A review on medicinal importance, pharmacological activity and bioanalytical aspects of beta-carboline alkaloid “Harmine”. Asian Pac J Trop Biomed. 2012;2(8):660–664. doi:10.1016/S2221-1691(12)60116-6
11. Gao J, Zhu H, Wan H, Zou X, Ma X, Gao G. Harmine suppresses the proliferation and migration of human ovarian cancer cells through inhibiting ERK/CREB pathway. Oncol Rep. 2017;38(5):2927–2934. doi:10.3892/or.2017.5952
12. Li C, Wang Y, Wang C, Yi X, Li M, He X. Anticancer activities of harmine by inducing a pro-death autophagy and apoptosis in human gastric cancer cells. Phytomedicine. 2017;28:10–18. doi:10.1016/j.phymed.2017.02.008
13. Zhang H, Sun K, Ding J, et al. Harmine induces apoptosis and inhibits tumor cell proliferation, migration and invasion through down-regulation of cyclooxygenase-2 expression in gastric cancer. Phytomedicine. 2014;21(3):348–355. doi:10.1016/j.phymed.2013.09.007
14. Hu X, Wu LW, Zhang ZY, Chen ML, Li YL, Zhang C. The anti-tumor effect of regorafenib in lung squamous cell carcinoma in vitro. Biochem Biophys Res Commun. 2018;503:1123–1129. doi:10.1016/j.bbrc.2018.06.129
15. Zhang C, Shi J, Mao SY, et al. Role of p38 MAPK in enhanced human cancer cells killing by the combination of aspirin and ABT-737. J Cell Mol Med. 2015;19(2):408–417. doi:10.1111/jcmm.12461
16. Uhl KL, Schultz CR, Geerts D, Bachmann AS. Harmine, a dual-specificity tyrosine phosphorylation-regulated kinase (DYRK) inhibitor induces caspase-mediated apoptosis in neuroblastoma. Cancer Cell Int. 2018;18:82. doi:10.1186/s12935-018-0574-3
17. Fuchs CS, Azevedo S, Okusaka T, et al. A phase 3 randomized, double-blind, placebo-controlled trial of ganitumab or placebo in combination with gemcitabine as first-line therapy for metastatic adenocarcinoma of the pancreas: the GAMMA trial. Ann Oncol. 2015;26(5):921–927. doi:10.1093/annonc/mdv027
18. Zhang C, Cai TY, Zhu H, et al. Synergistic antitumor activity of gemcitabine and ABT-737 in vitro and in vivo through disrupting the interaction of USP9X and MCL-1. Mol Cancer Ther. 2011;10(7):1264–1275. doi:10.1158/1535-7163.MCT-10-1091
19. Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet. 2016;388(10039):73–85. doi:10.1016/S0140-6736(16)00141-0
20. Saluja AK, Dudeja V, Banerjee S. Evolution of novel therapeutic options for pancreatic cancer. Curr Opin Gastroenterol. 2016;32(5):401–407. doi:10.1097/MOG.0000000000000298
21. Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25(15):1960–1966. doi:10.1200/JCO.2006.07.9525
22. Orozco CA, Martinez-Bosch N, Guerrero PE, et al. Targeting galectin-1 inhibits pancreatic cancer progression by modulating tumor-stroma crosstalk. Proc Natl Acad Sci U S A. 2018;115(16):E3769–E3778. doi:10.1073/pnas.1722434115
23. Kagawa S, Takano S, Yoshitomi H, et al. Akt/mTOR signaling pathway is crucial for gemcitabine resistance induced by Annexin II in pancreatic cancer cells. J Surg Res. 2012;178(2):758–767. doi:10.1016/j.jss.2012.05.065
24. Yang XL, Lin FJ, Guo YJ, Shao ZM, Ou ZL. Gemcitabine resistance in breast cancer cells regulated by PI3K/AKT-mediated cellular proliferation exerts negative feedback via the MEK/MAPK and mTOR pathways. Onco Targets Ther. 2014;7:1033–1042. doi:10.2147/OTT.S63145
25. Toschi L, Finocchiaro G, Bartolini S, Gioia V, Cappuzzo F. Role of gemcitabine in cancer therapy. Future Oncol. 2005;1(1):7–17. doi:10.1517/14796694.1.1.7
26. Huang J, Yin H, Rao SS, et al. Harmine enhances type H vessel formation and prevents bone loss in ovariectomized mice. Theranostics. 2018;8(9):2435–2446. doi:10.7150/thno.22144
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.