Back to Journals » International Journal of General Medicine » Volume 14
Habitual Hyperthermia: An Interpretive Paradigm of the 20th Century? Not Really
Authors Ginier-Gillet M , Esparcieux A
Received 19 February 2021
Accepted for publication 21 April 2021
Published 25 May 2021 Volume 2021:14 Pages 2063—2068
DOI https://doi.org/10.2147/IJGM.S306423
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Mathieu Ginier-Gillet,1 Aurelie Esparcieux2
1Grenoble Faculty of Medicine, Grenoble Alpes University, La Tronche, France; 2Department of Internal Medicine and Infectious Diseases, Clinique de l’Infirmerie Protestante de Lyon, Caluire-et-Cuire, France
Correspondence: Mathieu Ginier-Gillet 55 Rue Ney, Lyon, 69006, France
Tel +33680854615
Email [email protected]
Abstract: Prolonged and unexplained fevers in young adults are uncommon, especially when access to diagnostic tests is simplified. Therefore, the definition of unexplained fever depends on the volume of tests performed. However, low-grade fever has not been a priority in research. Management of low-grade fever [eg, an oral temperature of ≥ 37.8°C (100°F) and < 38.3°C (101°F) at any time of the day] is not codified. The presented case of a 37-year-old nurse with an intermittent fever for three months, with no clear diagnostic evidence and no elevated markers of inflammation, illustrates “habitual hyperthermia” (HH)—retained after ordering tests sequentially in town and at the hospital. HH was made known by Prof. H.A. Reimann (1897– 1986) an American virologist, although the diagnostic criteria are fallible. The article reviews the criteria and then discusses how to select diagnostic tests in family practice for prolonged fever in young adults without clinical signs of orientation. Given the polymorphism of febrile illnesses, the principle of parsimony must be transgressed, and in the event of an early suspicion of HH, surveillance is a rule to be further amended.
Keywords: habitual hyperthermia, low-grade fever, patient-centered care, primary health care, pyrexia of unknown origin, undifferentiated febrile illness
More is not less, less is a bore.
—Robert C. Venturi, 1991 Pritzker Architecture Prize Laureate
Introduction
In Europe and the United States, up to a third of fevers can go undiagnosed after extensive examination at a tertiary referral center.1 A debate continues as to whether the choice of a minimal diagnostic workup (as the volume of standardized examinations) is a factor influencing the epidemiology of fevers or inflammations of unknown origin (FUO/IUO). In the only identified systematic review, Fusco et al2 did not consider that these types of check-ups changed their occurrences, but only four series out of the 18 selected (three European, one Asian) described in a precise manner the tests to be performed in the case of a prolonged fever.
Consequently, the definitions of FUO/IUO beyond the hospital, by the concentration of expertise and technical means, are heterogeneous as well as the diagnostic tactics which result among teams.
Chronologically, it was in 1991 that Durack and Street3 paved the way for outpatient management of persistent fevers, but it was in the 2000s that de Kleijn et al4 revised the definition of a FUO according to investigations in line with the existence of potential diagnostic clues. However, it was in 2003 that Knockaert et al5 encouraged abandoning these quantitative criteria and, in 2009, that Vanderschueren et al6 incorporated common biomarkers of inflammation into these definitions. The four definitions of FUO/IUO are shown in Table 1.
 |
Table 1 Definition of a Case of Fever of Unknown Origin or Inflammation of Unknown Origin |
Case History
A 37-year-old Caucasian female hospital nurse presented with a prolonged fever in a private practice in June. The fever was intermittent, present for 11 weeks after an episode of a resolved cough in March, and regularly registered between 37.5–38.5°C (99.5–101.3°F) on a tympanic thermometer—in the context of screening for access to units.
The patient was not taking any treatments and has no significant medical history.
In March, a diagnosis of SARS-CoV-2 was ruled out. In May, her gynecologist removed her copper IUD. The first blood test did not show an inflammatory response, the SARS-CoV-2 serological test was negative, and the cultures of the device were sterile.
Upon questioning, no stay abroad was reported in the last 24 months. However, a year earlier, the patient reported close contact with a bacilliferous tuberculosis (TB) patient. The complaints were fatigue and neck pain without any morning stiffness. Coughing was absent, there was no weight loss, warm feeling was moderate, and chills were absent.
The extent of the physical examination was nonrevealing. The temperature was 37.2°C (99°F) using a forehead infrared thermometer, the pulse was 65 beats per minute, the arterial pressure was at 125/75 mmHg and some mobile latero-cervical ganglia were present.
The first intuition was toward a hospital-acquired or community-acquired infection.
Tests were ordered on a large scale: white blood cell count differential within normal limits (WNL), C-reactive protein (CRP) 0.7 mg/L, basic metabolic panel WNL, hepatic profile WNL, ferritinemia 85 µg/L, s-TSH 2.3 mIU/L, serological tests CMV − (IgM and IgG), EBV + (anti-VCA IgM −, anti-VCA IgG +, anti-EBNA IgG +), HCB + (vaccine-induced immunity), HCV −, HIV-1/-2 −, syphilis −, urine test strip LEU − NIT −, rheumatoid factor 10 IU/mL, antinuclear antibodies (ANA) + at 1:160th (on HEp-2 cells) with a nuclear speckled pattern and gamma-interferon release assay − 14 months after exposure to TB.
A second intuition was an imprecise measurement of body temperature. However, rectal temperature was measured at 8 a.m. as between 37.7–37.9°C (99.9–100.2°F) for 3 days in a row with a fever peak of 38.4°C (101.1°F) at 6 p.m. Therefore, as a result, the patient was referred for an outpatient consultation at an internal medical clinic, in which no origin other than “habitual hyperthermia” was mentioned, particularly after normal blood cultures, echocardiography and CT-TAP scan.
Research
Should more diagnostic tests be carried out in a young adult with a prolonged fever (especially low-grade) in the absence of an inflammatory (biological) response?
Articles were collected through systematic search of the electronic MEDLINE database. The document search strategy focused primarily on European and North American literature reviews, published in English and/or French. The keywords have been combined in the following order: “fever of unknown origin” OR “pyrexia of unknown origin” OR “neurogenic fever” OR “habitual hyperthermia” OR “low-grade fever” OR “factitious fever” NOT “children” AND “review”.
The bibliography obtained by automated means was also supplemented by a manual search of cohorts or series of cases which provided a definition of persistent fever around a minimal diagnostic assessment. Research on the diagnostic contribution of PET imaging with 18F-FDG was excluded. This also applies to studies which concerned mixed cases of fever, ie cases of fever in immunocompromised subjects.
Discussion
20th Century Terminology
In the Petersdorf and Beeson7 cohort of 1952–1957, fevers lasting over three weeks less than or equal to 38.3°C (101°F) were ruled out based on data from Reimann8—who, in 1931, observed a 23-year-old female student, whose rectal temperature could rise to 38.2°C (100.8°F) after an exercise activity (climbing five flights of stairs). “Habitual hyperthermia” (HH) came to be characterized as a paraphysiological state, ie constitutional. Kintner and Rowntree9 were more cautious. In their inventory of 100 cases of prolonged idiopathic fever, the term HH never appears. The authors simply noticed that one in four cases had emotional lability. The choice not to include cases of low-grade fever therefore did not allow Petersdorf and Beeson to untie the Gordian knot of their trigger factors, but instead blocked their investigations. Consequently, Petersdorf and Bennett’s10 message in 1957 was more moderate—a prolonged febrile state below 38.3°C (101°F) with few physical signs is not necessarily an HH, but one probability among others. Table 2 summarizes the misconceptions about HH.
 |
Table 2 Misconceptions and Facts Concerning “Habitual Hyperthermia” (HH) |
Fraudulent Fevers
These belong to history, much like mercury thermometers. However, they pose the generic (unresolved) questions of how to correctly measure body temperature in ambulatory care? Which instrument to use? And how to clinically justify a cascade of tests without documentation of the fever? If there is no universal (ie, quantitative) definition of fever and the measurement artifacts are well known.11 It was Rumans and Vosti12 in 1978, who proposed potential clues in favor of a fraudulent fever. One of their criteria was “Normal laboratory studies, especially complete blood count and erythrocyte sedimentation rate, in a patient with a true organic fever is unexpected.”12 In 1979, Aduan et al13 added a supplement through a series of 32 cases, in which one in three falsities was observed among caregivers. Among the leading mechanisms, the manipulation of the thermometer and the injection or oral intake of pyrogenic substances.
Concerning “Shotgun Testing”
Failure to act exposes legal sanctions. Doing too much can lead to cascade effects. The probability of having an abnormal result after 12 tests is almost 50%.14 In this case, the screening of antinuclear antibodies (ANA) was positive, but no specific subserologies were detected. Weak positive (or equivocal) results are common in healthy subjects. Between 2011–2012, ~41million Americans had ANA titers of 1:80.15
A negative, weak positive (or equivocal) and positive titer usually refers to dilutions of <1:40, 1:40 to 1:80, and ≥1:160 under fluorescence microscopy.16 However, the following tests [anti-double stranded DNA antibodies and antibodies against extractable nuclear antigens (eg, in routine work-ups, SS-A/Ro 60 kDa and 52 kDa, SS-B/La, Sm, U1-RNP, CENP-B, Topoisomerase I, Pol III and Jo-1)] should not be performed here and now, since, for one, positive serologies are not always required in non-organ-specific autoimmune diseases and, secondly, multiplex technology is not (yet) the gold standard. In case of suspected connective tissue disorders, a decision algorithm, the ANA-reflex test, has been proposed by the Italian Society of Clinical Pathology and Laboratory Medicine to limit expenses outside the hospital sector.17
“Plurality Should Not Be Posited without Necessity”
It is known that a nonspecific sign has multiple meanings. However, the goal is not to recreate the medieval battle between Ockham and Chatton, well synthesized by Maurer.18 Anyway this principle, which has become universal, has no scientific, realistic, or argumentative value. It provides no information on events and allows no differentiation of two equiprobable explanations. So, should we generate only hypotheses that are easy to test? No.
Semiologist Peirce (1839–1914) identified three processes for reaching an explanation: “Deduction proves that something must be; Induction shows that something actually is operative; Abduction merely suggests that something may be.”19 Unfortunately, the criteria for a good abduction, generating plausible hypotheses, are no longer clinical but strictly economical. It’s obvious that the clinical decision threshold responds to institutional factors, rather than pure science values. Yet, is it possible to have a correct scope of practice analysis without asking the following questions? What diagnostic tools are available? What is the economic environment? No again.
The epistemological obstacle to defining a febrile illness as idiopathic or cryptogenic20 relates only to equipment. Hence, the following ad hoc comments apply: (1) the apparent rate of FOI/IOI should not be confused with the actual rate of unexplained fevers evolving for 7, 14, or 21 days (classic hospital threshold) without diagnostic examinations being carried out; (2) the time that elapses between nonspecific symptoms and a diagnosis depends on experience in reconsidering the observed phenomena (meta-abduction); (3) it depends on the capacity to perform tests, not no more but no less than necessary.
Diagnostic Method
Medicine is an art. That is why healthcare must be patient-centered and nothing can replace a good clinical examination and multidisciplinary dialogue for diagnostic procedures. However, perhaps the patient should be referred once all the nearby available diagnostic resources have been exhausted. From experience, fevers in adults encountered in family practice (FP) generally have less than 5–7 days of progression at the time of the first consultation, with an intermittent pattern, particularly in view of early self-medication. If certain viral respiratory tract infections (ie, common cold) are self-limiting diseases—an ambiguous word attributed to infections that do not justify any etiological confirmation—their auto-regressive process cannot be predicted.21
The study by Affronti et al22 is the most complete on the diagnostic procedure in prolonged low-grade fevers [ie, according to their inclusion criteria, an axillary temp. between 37.5–38.3°C (99.5–101°F)]. The authors argue that, in the event of a very early suspicion of HH, only a complete blood count, an erythrocyte sedimentation rate (ESR), CRP and urinalysis should be performed with more or less up to two years of monitoring. The team also considered complaints. In Group A, in which organicity was confirmed, weight loss was significantly more frequent (P < 0.05). In Group B, in which a paraphysiologic origin was suspected, dizziness (P < 0.02) and poorly defined discomfort (P < 0.0001) were common. What is most remarkable is the number of diagnoses involved in the group suspected of an organic febrile disease (adults with an average age of 34 ± 14 y): brucellosis, mononucleosis, autoimmune thyroiditis, toxoplasmosis, Crohn disease, endocarditis, etc.
Anyway, the low prevalence of inflammatory diseases in the sense of Vanderschueren and Knockaert23 (“Big 3” for infections, neoplasia and noninfectious inflammatory disorders) in FP, calls for gaining information via exclusion. The corollary is that the (extrinsic) performance of biomarkers of inflammation (eg, CRP, ESR, plasma viscosity) to fit into one of the three major nosological frameworks is limited. To be convinced of this, Watson et al24 measured the area under the curve (AUC) of biomarkers using data from a population of British general practitioners. The AUC of CRP was 0.65 (95% CI, 0.64–0.66; P < 0.001), whereas the combination of CRP + ESR assays does not improve the index.
Therefore, CRP is not always a good rule to rule out inflammatory diseases’ ex tempore. However, the testing time may be a little less. This message is very well defended by Irving and Holden.25 Time is an efficient variable for reducing uncertainty. Theoretically, for each small increase in the pre-test probability of the disease, an excellent test will detect more appropriately the sickness status of an individual (ie, each time the total net gain in certainty formalized by the Predictive Summary Index [Ψ = PPV + NPV − 1] tends towards 1, the overall clinical utility of a test is optimal). Figure 1 provides a decision tree for managing prolonged fever in young adults in FP.4,11,22–24,26,27 Finally, the decision to consider HH very early on, or even psychological stress-induced hyperthermia according to the findings of Oka et al,28 justifies patient monitoring.
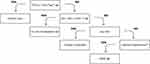 |
Figure 1 An example of decision tree for managing prolonged febrile illness in young adults in family practice. Abbreviations: BMP, basic metabolic panel; CBC, complete blood count w/diff; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; PDCs, potentially diagnostic clues; UAI, uncertainty avoidance index; WAW, watch and wait. Notes: Fever, sublingual temp. of ≥37.8°C (100°F) at any time during the day.11 “Red flag” symptoms, involuntary weight loss, toxic-appearing. In Zenone’s study27 (ie, in a French community hospital with a mean age of 59.5 y) 13 entities accounted for ~ two-thirds of community acquired fever: giant cell arteritis, habitual hyperthermia, EBV infections, sinusitis and occult dental infections, factitious fever, CMV infections, Q fever, lymphoma, colorectal carcinoma, adult-onset Still disease, rheumatoid arthritis, systemic lupus erythematosus, polyarteritis nodosa. *HH is pyrexia with no signs, normal inflammation blood tests and an erratic circadian rhythm of body temperature, usually not exceeding 38.3°C (100.4°F). Nevertheless, check errors in body temperature measurement and eliminate a drug-induced fever. (a) Any localizing signs or “clues that could lead to certain specific diagnoses”4 after a comprehensive history and general multi-system exam. (b) Be careful, a low CRP level is not considered proof of a mild and harmlessillness.24 (c) The geographical history and knowledge of ubiquitous pathogens is essential. Suggested outpatient testing (adapted from Carmoi et al):26 BMP, liver function panel, s-TSH, protein electrophoresis, LDH, ferritin, ANA titer and pattern, serological profile EBV, CMV, HIV-1/-2, blood cultures, urine test strip, chest radiography, abdominal and pelvic ultrasonography, and pantomography. (d) No specific rules are available for patient monitoring. Affronti et al22 recommends re-examination every 2 months w/CBC + ESR + CRP + urinalysis at the 6th month of follow-up. |
Conclusion
The diagnostic spectrum of recurrent fevers has not been detailed; however, it remains challenging for the clinician. In this sense, Fabry disease is a good example of a rare disease, sometimes expressed by episodes of low-grade fever. Ultimately, in a young adult with a prolonged fever and no biological inflammatory response, monitoring is more important than increasing the number of tests. Therefore, nothing can replace the clinician’s intuition, eyes and touch and a multidisciplinary dialogue to discuss the diagnostic strategy. The paradigm “habitual hyperthermia” should be updated to avoid defensive practices. Future research should focus on improving the continuity of care in unexplained fevers.
Ethics Approval and Informed Consent for Publication
Written informed consent has been provided by the patient to have the case details published. Ethical approval from a research ethics committee was not required, as this is a case report. Institutional approval was not needed to publish the case.
Acknowledgments
We would like to thank Dr. Stéphane Vinzio (Head of Internal Medicine at the Groupe Hospitalier Mutualiste de Grenoble, France) for his knowledgeable comments while writing the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Mulders-Manders CM, Pietersz G, Simon A, Bleeker-Rovers CP. Referral of patients with fever of unknown origin to an expertise center has high diagnostic and therapeutic value. QJM. 2017;110(12):793–801. doi:10.1093/qjmed/hcx158
2. Fusco FM, Pisapia R, Nardiello S, Cicala SD, Gaeta GB, Brancaccio G. Fever of unknown origin (FUO): which are the factors influencing the final diagnosis? A 2005–2015 systematic review. BMC Infect Dis. 2019;19(653). doi:10.1186/s12879-019-4285-8
3. Durack DT, Street AC. Fever of unknown origin: re-examined and redefined. In: Remington JS, Swartz MN, editors. Current Clinical Topics in Infectious Diseases. Boston, MA: Blackwell Science; 1991:35–51.
4. de Kleijn EMHA, Knockaert DC, van der Meer JWM. Fever of unknown origin: a new definition and proposal for diagnostic work-up. Eur J Intern Med. 2000;11(1):1–3. doi:10.1016/S0953-6205(99)00073-4
5. Knockaert DC, Vanderschueren S, Blockmans D. Fever of unknown origin in adults: 40 years on. J Intern Med. 2003;253(3):263–275. doi:10.1046/j.1365-2796.2003.01120.x
6. Vanderschueren S, Del Biondo E, Ruttens D, Van Boxelaer I, Wauters E, KnockaertDC. Inflammation of unknown origin versus fever of unknown origin: two of a kind. Eur J Intern Med. 2009;20(4):415–418. doi:10.1016/j.ejim.2009.01.002
7. Petersdorf RG, Beeson PB. Fever of unexplained origin: report on 100 cases. Medicine (Baltimore). 1961;40(1):1–30. doi:10.1097/00005792-196102000-00001
8. Reimann HA. Habitual hyperthermia: a clinical study of four cases with long continued low grade fever. Arch Intern Med (Chic). 1935;55(5):792–808. doi:10.1001/archinte.1935.00160230085006
9. Kintner AR, Rowntree LG. Long continued, low grade, idiopathic fever: analysis of one hundred cases. JAMA. 1934;102(12):889–892. doi:10.1001/jama.1934.02750120001001
10. Petersdorf RG, Bennett IL Jr. Factitious fever. Ann Intern Med. 1957;46(6):1039–1062. doi:10.7326/0003-4819-46-6-1039
11. Mackowiak PA, Wasserman SS, Levine MM. A critical appraisal of 98.6 degrees F, the upper limit of the normal body temperature, and other legacies of Carl Reinhold August Wunderlich. JAMA. 1992;268(12):1578–1580. doi:10.1001/jama.1992.03490120092034
12. Rumans LW, Vosti KL. Factitious and fraudulent fever. Am J Med. 1978;65(5):745–755. doi:10.1016/0002-9343(78)90792-1
13. Aduan RP, Fauci AS, Dale DC, Herzberg JH, Wolff SM. Factitious fever and self-induced infection: a report of 32 cases and review of the literature. Ann Intern Med. 1979;90(2):230–242. doi:10.7326/0003-4819-90-2-230
14. Deyo RA. Cascade effects of medical technology. Annu Rev Public Health. 2002;23:23–44. doi:10.1146/annurev.publhealth.23.092101.134534
15. Dinse GE, Parks CG, Weinberg CR, et al. Increasing prevalence of antinuclear antibodies in the United States. Arthritis Rheumatol. 2020;72(6):1026–1035. doi:10.1002/art.41214
16. Tozzoli R, Bizzaro N, Tonutti E, et al. Guidelines for the laboratory use of autoantibody tests in the diagnosis and monitoring of autoimmune rheumatic diseases. Am J Clin Pathol. 2002;117(2):316–324. doi:10.1309/Y5VF-C3DM-L8XV-U053
17. Tonutti E, Bizzaro N, Morozzi G, et al. The ANA-reflex test as a model for improving clinical appropriateness in autoimmune diagnostics. Auto Immun Highlights. 2016;7(1):9. doi:10.1007/s13317-016-0080-3
18. Maurer A. Ockham’s razor and Chatton’s anti-razor. Mediaev Stud. 1984;46:463–475. doi:10.1484/J.MS.2.306670
19. Hartshorne C, Weiss P, eds. Collected Papers of Charles Sanders Peirce. Vol. V: Pragmatism and Pragmaticism. Cambridge, MA: Harvard University Press; 1935. [Book I, Lecture VI, par. 171].
20. Sloan LH. Cryptogenic fever. Postgrad Med. 1951;9(2):118–122. doi:10.1080/00325481.1951.11694080
21. van Vugt SF, Butler CC, Hood K, et al. Predicting benign course and prolonged illness in lower respiratory tract infections: a 13 European country study. Fam Pract. 2012;29(2):131–138. doi:10.1093/fampra/cmr081
22. Affronti M, Mansueto P, Soresi M, et al. Low-grade fever: how to distinguish organic from non-organic forms. Int J Clin Pract. 2010;64(3):316–321. doi:10.1111/j.1742-1241.2009.02256.x
23. Vanderschueren S, Knockaert DC. Tackling fever and inflammation of unknown origin: the do’s and don’ts. Acta Clin Belg. 2014;69(6):412–417. doi:10.1179/2295333714Y.0000000070
24. Watson J, Salisbury C, Whiting P, Banks J, Pyne Y, Hamilton W. Added value and cascade effects of inflammatory marker tests in UK primary care: a cohort study from the clinical practice research datalink. Br J Gen Pract. 2019;69(684):e470–e478. doi:10.3399/bjgp19X704321
25. Irving G, Holden J. The time-efficiency principle: time as the key diagnostic strategy in primary care. Fam Pract. 2013;30(4):386–389. doi:10.1093/fampra/cmt007
26. Carmoi T, Grateau B, Billhot M, et al. [Prolonged fever: specific issues in the young adult population]. Rev Med Interne. 2010;31(12):838–845. French. doi:10.1016/j.revmed.2009.10.437
27. Zenone T. Fever of unknown origin in adults: evaluation of 144 cases in a non-university hospital. Scand J Infect Dis. 2006;38(8):632–638. doi:10.1080/00365540600606564
28. Oka T, Kanemitsu Y, Sudo N, Hayashi H, Oka K. Psychological stress contributed to the development of low-grade fever in a patient with chronic fatigue syndrome: a case report. Bio Psycho Social Med. 2013;7(1):7. doi:10.1186/1751-0759-7-7
29. Carruthers BM, van de Sande MI, De Meirleir KL, et al. Myalgic encephalomyelitis: international consensus criteria. J Intern Med. 2011;270(4):327–338. doi:10.1111/j.1365-2796.2011.02428.x
30. Weinstein L. Clinically benign fever of unknown origin: a personal retrospective. Rev Infect Dis. 1985;7(5):692–699. doi:10.1093/clinids/7.5.692
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
