Back to Journals » Risk Management and Healthcare Policy » Volume 13
Guidelines and Current Assessment of Health Care Responsibility in Italy
Authors Zerbo S, Malta G, Argo A
Received 12 November 2019
Accepted for publication 29 January 2020
Published 10 March 2020 Volume 2020:13 Pages 183—189
DOI https://doi.org/10.2147/RMHP.S238353
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Marco Carotenuto
Stefania Zerbo, Ginevra Malta, Antonina Argo
Department of Health Promotion, Maternal and Child Care, “G. D’Alessandro”, Legal Medicine Section, University of Palermo, Palermo, Italy
Correspondence: Stefania Zerbo
Department of Health Promotion, Maternal and Child Care, “G. D’Alessandro”, Legal Medicine Section, University of Palermo, via del Vespro N. 129, Palermo 90127, Italy
Tel +39 33 3725 0209
Fax +39 09 1655 3203
Email [email protected]
Abstract: Clinical guidelines are a potential tool for improving the effectiveness and quality of healthcare, decreasing variability in clinical practice, and preventing adverse events. In the purview of Law no. 24/2017, adherence to national guidelines can lead to a reduction in medical malpractice claims and the practice of so-called “defensive medicine”. The law has assigned a central role to the guidelines, establishing the National Institute of Health through the new Italian National Center for Clinical Excellence, Quality, and Security (CNEC) as the methodological guarantor in the process of national guideline development. Here we discuss the issue of professional liability as recently outlined by the Gelli-Bianco Law (no. 24/2017), taking into account the clinical significance and medicolegal value of the guidelines.
Keywords: clinical guidelines, health care, liability, medical malpractice, Gelli-Bianco law
Introduction
In 1990, the Institute of Medicine (IOM) described the Clinical Practice Guidelines (CPG) as “systematically developed statements to assist practitioner and patient decisions about appropriate healthcare for specific clinical circumstances.”1 This definition was updated by IOM in 2011 as follows to highlight the rigorous methodology of the guideline development process:
Clinical practice guidelines are statements that include recommendations intended to optimize patient care that are informed by a systematic review of evidence and an assessment of the benefits and harms of alternative care options.2
According to the more recent definition, CPG summarize the best clinical evidence to support any clinical decision-making based on benefits and harms of alternative care options, with the aims of reducing unacceptable variability in practice, enhancing the translation of research into practice, and improving healthcare quality and safety.3 They are also an important tool for promoting operational integration between different areas of expertise and reducing preventable mistakes and adverse events.4,5 The standardization provided by guidelines may counteract unjustified discretionary decisions made by healthcare professionals that could result in inappropriate treatments. Thus, guidelines can prevent the application of arbitrary or obsolete clinical practices while ensuring uniformity of procedures and proper management of the healthcare system. It should be noted that clinical guidelines are not prescriptive, and do not prevent doctors from making assessments or restrict the freedom of patients to choose their healthcare provider.6,7
For guidelines to be considered acceptable and effective, they must be implemented locally within individual states based on input provided by the specialty physician, who will then apply them in clinical practice.4,8 In Italy, guidelines are implemented by the National Agency for Regional Health Care Services (AGENAS), the institute responsible for developing the health service quality monitoring system.
To be fully implemented, guidelines formulated and accepted by the national or international scientific community must be accompanied by training and support mechanisms for practitioners; these include lectures and courses, practical application based on patient examinations, and regular updates on items that are rarely encountered in clinical practice. After introducing the guidelines, outcome and process measures must be monitored as performance indicators in healthcare, with provisions made to revise these as necessary.6 Law no. 24/2017 has instituted a National Observatory of Good Practices relative to safety in healthcare within AGENAS with the aims of collecting from the abovementioned regional centers data related to risks and adverse events as well as to causes, extent, frequency, and financial burden of controversies; establishing guidelines with the support of qualified scientific associations; and identifying measures for preventing and managing health risk and monitoring good practices.
When various guidelines exist regarding the diagnosis and treatment of the same pathology, physician decision-making should be based on the risk–benefit balance for the patient and cost-effectiveness of heathcare.9 Absolute compliance with guidelines does not guarantee successful treatment outcome, and the Gelli-Bianco law stipulates that deviation from national medical guidelines may be justifiable when guided by professional judgment and/or the specific needs of the patient.
Over the last 2 decades, medical liability in Italy has become a prominent issue in healthcare policy and a major concern for healthcare economics. The dramatic increase in medical malpractice litigation and its impact on medical insurance have encouraged the practice of defensive medicine, which consists of administering or withholding treatment or subjecting patients to unnecessary tests as concern for avoiding malpractice lawsuits prevails over the duty to provide optimal treatment.10 There is significant variability in the types of malpractice and size of indemnity payments across specialties: data from 2013 obtained from the Italian Parliament Commission of National Health Inquiry showed an overall increase of 24% in compensation requests for malpractice claims in the period between 2006 (11.376 claims) and 2011 (14.088 claims), with an average annual increase of 5%; specializations including first aid, emergency surgery, and obstetrics accounted for the bulk of compensations. The inquiry also revealed that 10 billion euros paid by the Health System was attributable to the widespread practice of defensive medicine.
A retrospective study of 296 claims carried out between January 1, 2014 and December 31, 2017 by the Quality and Clinical Risk Management unit at the Policlinic Hospital of the University of Palermo in order to promote evidence-based interventions and increase patient safety showed that the most frequent claims were for wrong surgical procedure (33.8%), incorrect diagnosis (18.6%), fall (17.6%), infection (6.4%), postoperative complications (5.4%), and wrong therapy (3.1%). By the end of the survey, the judicial process had not concluded in the majority of cases (82.8%); claims were closed by receipt payment in 12.2% of cases, with no payments made in 5.1% of cases.11
In light of the increasing number of healthcare liability claims in Italy, the Gelli law (no. 24) entitled “Provisions relating to the safety of care and the assisted person, as well as the professional liability of health professionals” was implemented on March 8, 2017. The new law aimed to improve patient safety, reduce the number of medical malpractice lawsuits, and counter the practice of defensive medicine by introducing a more favorable regime of professional liability for healthcare providers—both from the civil and criminal law perspectives—that emphasizes clinical practice guidelines validated by the Italian Health Institute. It is presumed that safety of care based on best practice evidence and guidelines can reduce the risk of adverse events associated with treatment and consequently, medical malpractice claims.12 The Balduzzi law (no.189/2012) was the earliest attempt at legislative provision for medical malpractice that first introduced the guidelines concept; however, the law was vague and did not define criteria for selecting and identifying the recommendations to be adopted, and was replaced by the Gelli law. Within this new legal framework, the law introduced Article 590-sexies into the Italian Criminal Code to provide room for sanctions for manslaughter or personal injury due to negligence as already set forth by articles 589 and 590 of the Italian Criminal Code, in cases where these crimes are caused during the provision of healthcare services. That is, the article introduces a stipulation for cases where the conduct of the professional was due to inexperience, provided that he/she acted in accordance with recommendations set forth by established guidelines and considering the specifics of the case.
Important new concepts have been introduced in the field of civil law that distinguish between 2 types of liability and their effects for substantive and procedural laws. Contractual liability is subject to Article 1218 of the Italian Civil Code, which states that:
The debtor who does not correctly perform according to the contract asset is liable for damages. He must provide compensation if he does not prove the absolute impossibility which determined the delayed performance or the breach of the obligation.
This type of liability arises from the infringement of specific obligations—ie failure to implement the contract asset. Extra-contractual liability is subject to Article 2043, which states that: “Whoever causes an unjustified injury in situations of intentional or negligent behavior must provide compensation.” This type of liability arises from the breach of a generic duty without considering a previous obligation. The abovementioned content was inspired by the Latin principle neminem laedere (“nobody must be wronged”), and is intended to prevent injury in the legal sphere.
Until the enactment of Law 24/2017, the medical liability of doctors and health facilities was deemed contractual in nature. Within the new legal framework, the liability of health facilities (either private or public) remains contractual; in such cases, the statute of limitations will be the normal term of 10 years. The claimant alleging breach of contractual duties must provide evidence of damages or worsening of his/her health condition in the course of treatment. It is then the responsibility of the defendant to prove that professional duties were duly performed, and that the negative outcome was caused by an unforeseeable event that was unavoidable with ordinary professional care. In contrast, the liability of healthcare staff, who are not under any contractual obligation with the patient, will be extracontractual, with a tort statute of limitations of 5 years. In this case, the burden of proof lies with the claimant, who must meet all requirements for establishing liability on torts including fault of the doctor and causal link between damages and faulty conduct, without application of any presumption. Given the advantages provided to patients by contractual liability, the aim of the reform is to shift claims against the hospital and reduce litigations against healthcare professionals.
Guidelines and Best Practice
The term evidence-based practice (EBP) refers to any form of proof, strong or weak, obtained through experience, observational research, or experimental trial.13 EBP thus standardizes healthcare procedures in accordance with well-tested hypotheses and based on cost vs benefit for the patient and increased efficiency and productivity of the healthcare system.8 Features of the methodology used to establish guidelines, levels of evidence, and the strength of recommendations upon which they are based influence practical application of the guidelines. Quality assurance must be founded on internationally accepted, objective, accessible, and easily reproducible methods so that the guidelines are of a sufficiently high standard.14
In the last decades, the number of guidelines developed by government and private organizations worldwide (eg, National Guidelines Clearinghouse, Guidelines International Network, Canadian Medical Association MA Infobase, and National Institute of Health and Care Excellence) has increased exponentially. Although many address the same clinical issues, they are not all used in equal measure because of their conflicting recommendations. For example, a systematic literature review of patient preference for the antithrombotic guidelines of the American College of Chest Physicians found heterogeneous and low-quality evidence;15 moreover, several studies have shown that most previous international guidelines lacked supportive scientific evidence.6,16–19
At present, many countries including Italy have national schemes for establishing guidelines. With input from skilled professionals, these programs are designed to ensure that guidelines are produced in a coordinated manner using methods that guarantee the scientific quality of the end product.20 Requisites for good guidelines are multidisciplinary work groups; explicit bibliographic research processes; grading of evidence and strength of recommendations; clarity and comprehensibility of objects and scope; and consideration of costs and organizational consequences of the implementation of good clinical practices and of the frequency of updates.6
Appropriate methodologies and rigorous strategies must be employed for guideline development to ensure successful implementation of the resultant recommendations. To this end, the International Appraisal of Guidelines Research and Evaluation (AGREE) tool was developed in 2003 to assess the methodological quality of clinical practice guidelines and the strength of recommendations,21 with a revision (AGREE II) published in 200922 that has become the internationally accepted standard. AGREE II comprises 6 domains with a total of 23 items, each scored on a scale of 1–7. A score >1 is required for a valid score, and a scoring rubric combines scores into a single composite score for each domain. AGREE II items and criteria were recently revised to create the AGREE Reporting Checklist, which aims to improve and promote comprehensiveness and completeness of reporting in clinical practice guidelines.
As outlined by Law no. 24/2017, adherence to national guidelines validated by the National Institute of Health can improve healthcare quality while reducing malpractice claims and the practice of defensive medicine. Article no. 5 states that
Healthcare professionals, while providing services for preventive, diagnostic, therapeutic palliative, rehabilitation, and forensic medical purposes, must abide by the recommendations laid out in guidelines, except for individual case specificities.
Article 5 of the Gelli-Bianco Law also states that national clinical guidelines must be elaborated by accredited public and private scientific societies and technical associations of healthcare professionals recognized by an Italian Ministry of Health decree from August 2, 2017. The elaboration process includes drafting clinical guidelines ex novo, adapting national guidelines to create an international version, and updating preexisting guidelines according to the health needs of the population.
Guidelines were published on the National Institute of Health website in the National Guidelines System (SNLG) following verification of the conformity of methodology to international quality standards recognized by the institute and validation of scientific evidence supporting the clinical recommendations (Figure 1). The National Institute of Health through the Italian National Center for Clinical Excellence, Quality, and Security (CNEC) acts as an independent guarantor of guideline methodology and quality. The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) method is recommended by CNEC for establishing new guidelines for the SNLG, whereas the GRADE-ADOLOPMENT method is used to adapt international guidelines to national standards.23 GRADE is used to assess the quality of evidence and grading strength of recommendations in systematic reviews and guideline development; it requires specification of the setting, population, intervention, and comparator for each management question, and of the relative importance of expected outcomes. Evidence quality is evaluated in 5 domains—namely, study limitations, inconsistency of results, indirectness of the evidence, imprecision, and publication bias. To date, only a few guidelines have been published in the SNLG.24 The Gelli-Bianco law states that in the absence of guidelines, health professionals fulfilling their medical duties must adhere to the good clinical practices outlined by the National Institute of Health based on the relevance of the topic, date of publication (at least 3 years), and opinion of a multidisciplinary panel of experts.
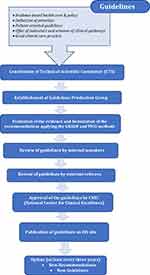 |
Figure 1 The Italian National Guidelines System. |
Guidelines for Assessing Liability of Italian Health Professionals
Clinical guidelines are developed to facilitate clinical decision-making regarding the treatment of specific diseases, and as a tool for evaluating the standard of care at a given time.25,26 Adherence to clinical practice guidelines in cases of professional negligence has aroused the interest of forensic medicine experts.27,28 The liability of medical professionals in Italy has recently been revisited as a result of Law 24/2017, which states that healthcare professionals fulfilling their medical duties must adhere to guidelines and good clinical practices recognized by the scientific community.25 Article 6 was designed to regulate criminal medical liability for healthcare professionals, and resulted in the introduction of a new article into the Italian Criminal Code (Article 590-sexies) with a special provision for healthcare professionals. The novelty of the new provision lies in the exemption of healthcare professionals from punishment for the cited offences when the event occurred as a result of inexperience, in cases where guideline recommendations were adhered to, or when best clinical practices were respected, and if these are relevant to the particularities of the specific case (second paragraph of Article 590-sexies). Exclusion from punishment as outlined by Article 590-sexies applies only to cases of incompetence and does not include those involving negligence and imprudence.
The abovementioned article absolves doctors of legal responsibility if they have followed the recommendations of published guidelines or good practices relevant to the case in question. Meanwhile, criminal liability can still be established for any negligent or imprudent treatment causing personal injury to or the death of the patient, which is considered as medical malpractice. The law safeguards doctors’ decision-making autonomy by stating that the guidelines are mandatory “except in specific cases.” In the absence of specific guidelines—or in particular cases determined by the patient’s needs—the health professional can deviate from guidelines but must adhere to the principles of good practice, which were defined in a statement by the Italian Supreme Court of Cassation (no. 8770) from February 22, 2018 as
Precautionary rules valid only if adequate with respect to the objective of the best care for the specific case of the patient and implying, in the contrary hypothesis, the duty on the part of the whole chain of health operators concretely implicated to depart from it.
As already explained, this departure requires professionals to follow so-called good clinical assistance practices, defined by Italian ministerial decree on July 15, 1997 as
An international standard of ethics and scientific quality to design, conduct, record, and report clinical studies involving human subjects that are valid and widely applicable.
Discussion and Conclusion
Clinical guidelines are an important tool for quality assurance because they facilitate medical practice and ensure that it evolves in line with research findings. They also reduce variability in actions, promote operational integration between different areas of expertise, and reduce clinical risk. However, guidelines do not represent absolute criteria and must always be considered in the context of individual cases; assessment of the compatibility between guidelines and specific cases is based on the expertise, prudence, and diligence of the medical professional acting in accordance with good clinical assistance practices.
Legal assessment of medical conduct should be carried out based on ex ante and not ex post criteria, in compliance with the principle of non-use of defensive medicine promulgated by the Gelli-Bianco law. Ex post evaluation of healthcare practices is a later element that can be used to justify the conduct in question in the event of a trial. The Gelli Law has assigned a central role to the guidelines, with the National Institute of Health through CNEC acting as methodological guarantor for the process of national guideline establishment. Although the law is intended to protect healthcare professionals from the surge in legal disputes while limiting defensive drift, it risks shifting emphasis from the optative and discretionary value of guidelines to a more slavishly prescriptive and thoughtless application by paradoxically encouraging the practice of defensive medicine. Thus, this approach does not guarantee a reduction in medicolegal disputes.
Although the new SNLG offers an opportunity to promote safe healthcare, there are several critical issues that hinder its development such as the requirement for updating the guidelines every 2 years according to the Gelli law, which is unrealistic given the time needed for new discoveries and practices to be adopted within the healthcare system and incorporated into existing guidelines.
A retrospective study carried out by the Italian National Institute of Health and Italian Association Clinical Evidence for Health in June 2017 on the quality and trustworthiness of clinical practice guidelines developed by Italian medical specialty societies from 2015 to 2016 (prior to enactment of the Gelli law) reported discouraging findings. Chief among these was a lack of cooperation between scientific societies to bring about necessary changes in the healthcare process and to define the benefits expected from adopting the guidelines. Additionally, poor evaluation of conflicts of interest by the guidelines’ architects hindered their reliability and credibility.
The Gelli law is still being implemented; therefore, it remains to be seen whether it can achieve the stated goals of protecting the safety of healthcare through monitoring of adverse events and relieving medical professionals of the burden of medical liability (including possible criminal proceedings) and associated insurance costs. However, the new law appears to offer healthcare professionals less protection than the previous Balduzzi Law, which removed culpability in all cases of gross negligence.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Institute of Medicine. Clinical Practice Guidelines: Directions for a New Program. Washington, DC: The National Academies Press; 1990. doi:10.17226/1626
2. Institute of Medicine (US). Committee on standards for developing trustworthy clinical practice guidelines. In: Graham R, Mancher M, Miller Wolman D, et al. editors. Clinical Practice Guidelines We Can Trust. Washington (DC): National Academies Press (US); 2011. doi:10.17226/13058
3. Woolf S, Schünemann HJ, Eccles MP, et al. Developing clinical practice guidelines: types of evidence and outcomes; values and economics, synthesis, grading, and presentation and deriving recommendations. Implement Sci. 2012;61:1–12.
4. Jena AB, Seabury S, Lakdawalla D, et al. Malpractice risk according to physician specialty. N Engl J Med. 2011;365:629–636. doi:10.1056/NEJMsa1012370
5. Gómez-durán EL, Martin-fumadó C, Arimany-manso J. Legal medicine contributions to patient safety. From ascertainment and evaluation to research in medical liability. Int J Legal Med. 2013;127:1051–1053. doi:10.1007/s00414-013-0885-9
6. Shaneyfelt TM, Mayo-smith MF, Rothwangl J. Are guidelines following guidelines? The methodological quality of clinical practice guidelines in the peer-reviewed medical literature. JAMA. 1999;281:1900–1905. doi:10.1001/jama.281.20.1900
7. Sackett DL, Rosemberg WMC, Gray J, et al. Evidence-based medicine: what it is and what it isn’t. BMJ. 1996;312:71–72. doi:10.1136/bmj.312.7023.71
8. Rosenberg W, Donald A. Evidence based medicine: an approach to clinical problem-solving. BMJ. 1995;310:1122–1126. doi:10.1136/bmj.310.6987.1122
9. Tilburt JC. Evidence-based medicine beyond the bedside: keeping an eye on context. J Eval Clin Pract. 2008;14:721–725.
10. US Congress. Office of technology assessment, defensive medicine and medical malpractice, OTA-H–6O2. Washington, DC: US Government Printing Office; July 1994. Available from: https://ota.fas.org/reports/9405.pdf.
11. Armetta F, Provenzano S, Raia DD, et al. Evolution of risk management in health care system: survey on the adverse events occurred in Palermo University Hospital Policlinico “Paolo Giaccone.”. Recenti Prog Med. 2019;110:244–250. doi:10.1701/3163.31447
12. Iannone P, Coclite D, Fauci AJ, et al. [Italian guidelines in accordance with the new National Guidelines System: critical issues and perspectives.]. Recenti Prog Med. 2017;108:360–362. doi:10.1701/2745.27986. Italian.
13. Dowie J. Evidence based medicine. Needs to be within framework of decision making based on decision analysis. BMJ. 1996;313:170. doi:10.1136/bmj.313.7050.170a
14. Kohn LT, Corrigan JM, Donaldson MS. To Err Is Human: Building a Safer Health System. Institute of Medicine (US) Committee on Quality of Health Care in America. Washington, DC, USA: National Academies Press; 1999.
15. Graham ID, Beardall S, Carter AO, et al. What is the quality of drug therapy clinical practice guidelines in Canada? Can Med Assoc J. 2001;165:157–163.
16. Steel N. Review of clinical practice guidelines found that they were often based on evidence of uncertain relevance to primary care patients. J Clin Epidemiol. 2014;67:1251–1257. doi:10.1016/j.jclinepi.2014.05.020
17. Grilli R, Magrini N, Penna A, et al. Quality of practice guidelines developed by specialty societies. The need for a critical appraisal. Lancet. 2000;355:103–106. doi:10.1016/S0140-6736(99)02171-6
18. Balducci L. Practical guidelines that may kill. J Med Pers. 2014;12:96–98. doi:10.1007/s12682-014-0169-2
19. Ferrara SD. Medical malpractice and legal medicine. Int J Legal Med. 2013;127:541–543. doi:10.1007/s00414-013-0839-2
20. AGREE Collaboration. Development and validation of an international appraisal instrument for assessing the quality of clinical practice guidelines: the AGREE project. Qual Saf Health Care. 2003;12:18–23. doi:10.1136/qhc.12.1.18
21. Brouwers MC, Kho ME, Browman GP, et al. AGREE Next Steps Consortium. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010;182:839–842. doi:10.1503/cmaj.090449
22. Schünemann HJ, Wiercioch W, Brozek J, et al. GRADE Evidence to Decision (EtD) frameworks for adoption, adaptation, and de novo development of trustworthy recommendations: GRADE-ADOLOPMENT. J Clin Epidemiol. 2017;81:101–110. doi:10.1016/j.jclinepi.2016.09.009
23. Iannone P, Coclite D, Napoletano A, et al. The new National Guidelines System in Italy: a first evaluation. G Ital Nefrol. 2019;36:1–10.
24. Rini MS, Argo A, Ventura Spagnolo E, et al. When is necessary not to apply guidelines? Pitfalls in dentistry practice. Dent Cadmos. 2018;86:686–695. doi:10.19256/d.cadmos.08.2018.07
25. Argo A, Seidita F, Zerbo S, et al. Orthodontic guidelines and assessment of medicolegal liability. Dent Cadmos. 2016;84:161–168. doi:10.1016/S0011-8524(16)30035-6
26. Davies J. Clinical guidelines as a tool for legal liability. An international perspective. Med Law. 2009;28:603–613.
27. Knaak JP, Parzeller M. Court decisions on medical malpractice. Int J Legal Med. 2014;128:1049–1057. doi:10.1007/s00414-014-0976-2
28. Montanari Vergallo G, Zaami S. Guidelines and best practices: remarks on the Gelli-Bianco law. Clin Ter. 2018;169:82–85.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
