Back to Journals » Drug Design, Development and Therapy » Volume 16
Guanmaitong Granule Attenuates Atherosclerosis by Inhibiting Inflammatory Immune Response in ApoE−/− Mice Fed High-Fat Diet
Authors Yang M , Jiao H, Li Y, Zhang L, Zhang J , Zhong X , Xue Y
Received 3 May 2022
Accepted for publication 5 September 2022
Published 16 September 2022 Volume 2022:16 Pages 3145—3168
DOI https://doi.org/10.2147/DDDT.S372143
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Georgios Panos
Mengqi Yang,1 Huachen Jiao,2 Yan Li,2 Lei Zhang,1 Juan Zhang,2 Xia Zhong,1 Yitao Xue2
1First College for Clinical Medicine, Shandong University of Traditional Chinese Medicine, Jinan, 250014, People’s Republic of China; 2Cardiology Department, Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan, 250014, People’s Republic of China
Correspondence: Yitao Xue, Cardiology Department, Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jing Shi Road, Lixia District, Jinan, 250014, People’s Republic of China, Tel +8613505313455, Email [email protected]
Background: Atherosclerosis (AS) is the leading cause of cardiovascular diseases, such as myocardial infarction and stroke. Guanmaitong granule (GMTG) is a TCM (Traditional Chinese medicine) prescribed to treat AS. However, its mechanism remains unclear.
Methods: We obtained reliable ingredients and targets of GMTG using the HERB database. AS-related targets were obtained from HERB and GeneCards databases. The target database was constructed by intersecting the ingredients of GMTG with the AS-related targets. STRING and Cytoscape were used to create protein-protein interaction (PPI) network and screen core targets. GO enrichment analysis and KEGG pathway analyses were performed using R. Finally, the ApoE−/− mice AS model was induced by a high-fat diet (HFD) for in vivo validation of core pathways and targets.
Results: A total of 124 ingredients and 418 potential targets of GMTG for treating AS were obtained. Numerous ingredients and targets were related to Panax notoginseng, Salvia miltiorrhiza, and Astragalus. Most core targets and pathways were involved in the inflammatory immune response. GMTG could decrease serum triglycerides, total cholesterol, low-density lipoprotein-cholesterol, and oxidized low-density lipoprotein level and increase the serum high-density lipoprotein-cholesterol level. Furthermore, GMTG reduced the plaque burden and promoted plaque remodeling by reducing plaque area, lipid deposition, foam cell content, and collagen fiber content in the plaque in the aortic root of ApoE−/− mice. GMTG inhibited systemic and plaque inflammatory immune response and increased plaque stability by inhibiting the excessive release of the TLR4/MyD88/NF-κB pathway-induced inflammatory cytokines, tumor necrosis factor, interleukin-6, and interleukin-1 beta.
Conclusion: Radix notoginseng, Radix salviae liguliobae, and Radix astragali are the main ingredients of GMTG for treating AS. Further, GMTG could regulate the level of serum lipids and inhibit inflammatory immune response, which resulted in anti-AS effects such as plaque stabilization, reduction of plaque burden, and plaque remodeling. GMTG is a promising multi-target treatment for AS.
Keywords: Guanmaitong granule, atherosclerosis, inflammatory immune response, plaque burden, plaque remodeling
Introduction
Cardiovascular diseases, especially acute myocardial infarction (AMI), are the leading cause of death, morbidity, and disability worldwide.1 Atherosclerosis (AS) is a chronic and progressive inflammatory arterial wall disease characterized by intimal lipid accumulation, thickening of the arterial wall, and narrowing of the vascular lumen.2 Unstable atherosclerotic plaque rupture, platelet aggregation, and thrombosis cause vascular stenosis or occlusion, leading to acute cardiovascular disease,3,4 a major global health threat that poses a heavy societal burden.5 Interventional therapy and Western medicine therapies are the main methods of AS treatment.6 Although revascularization corrects severe stenosis, it does not alter the biological process of AS, and plaque instability is preserved. Statins, lipidaidemic, and anti-inflammatory drugs can be used to treat AS, protect endothelial blood vessels, and even reverse plaques.7–9 However, long-term, high-dose statin treatment could lead to myopathy/myolysis, diabetes mellitus, liver damage, and other adverse effects.10–12 The intestinal cholesterol absorption inhibitor ezetimibe only reduces low-density lipoprotein-cholesterol (LDL-C) level and can thus only be used as an adjunct to statins.13 The proprotein convertase subtilisin/kexin type 9 (PCSK9) monoclonal antibody significantly reduces serum LDL-C. However, this is a high-cost alternative for the few patients with poor response or intolerance to statins.14,15 Canakinumab can block the interaction with the interleukin-1 (IL-1) receptor, neutralize the biological activity of human IL-1, and avoid IL-1-induced gene activation and the generation of inflammatory mediators. However, it is expensive, has numerous side effects, and can lead to fatal infections.16 Therefore, finding safe, effective, and economical anti-AS drugs is imperative.
It is generally believed that AS results from lipid accumulation in the vascular wall. However, the pathogenesis of AS is more complex. Immune cells, inflammation associated with hyperlipidemia, and elevated oxidized low-density lipoprotein (ox-LDL) levels play a crucial role.17 Extensive experimental and clinical evidence shows that AS is a chronic inflammatory disease,18 and the inflammatory response plays an essential role in the entire process of atherosclerotic plaque formation. In early AS, vascular endothelial injury, abnormal lipid metabolism, and hemodynamic damage are the leading causes. AS is thought to be accompanied by inflammatory changes in blood flow-mediated endothelial cells (ECs).19 After the ECs are activated, they expressed monocyte chemotaxis protein 1 (MCP-1), intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion protein 1 (VCAM-1), and other cytokines, attracting lymphocytes and monocytes, which combine with endothelial biomolecules and infiltrate artery walls, leading to the initiation of inflammation. A large amount of LDL-C is modified to ox-LDL and accumulates in the inner wall of the blood vessels, which promotes the formation of AS plaque.20 In advanced AS lesions, many inflammatory cells, such as macrophages infiltrate the vascular wall and secrete matrix metalloproteinases to degrade collagen fibers in the extracellular matrix of the plaque, leading to plaque rupture, bleeding, and thrombosis.21,22 Anti-inflammation therapies provide a new perspective for the developing therapeutic strategies for AS.
Guanmaitong granule (GMTG) is a commonly used prescription of Professor Yitao Xue for treating AS and has had remarkable clinical effects.23,24 GMTG is composed of the root of Membranous Milkvetch (Radix Astragali), root of Ligulilobe sage (Radix Salviae liguliobae), Trichosanthes kirilowii Maxim (Trichosanthis Fructus), rhizome of Chinese Goldthread (Rhizoma Coptidis), Sanchi (Radix Notoginseng), Figwort Root (Radix Scrophulariae), bulb of Thunberg Fritillary (Bulbus Fritillariae thunbergii), rhizome of Gaint Knotweed (Rhizoma Polygoni cuspidati), Bigflower Cape Jasmine (Gardenia jasminoides var.grandiflora), and Oyster (Crassostrea gigas). Previous studies have shown that various ingredients of Traditional Chinese medicine (TCM) herbs contained in GMTG have anti-AS effects. Astragaloside IV can prevent ox-LDL-induced ECs injury by reducing oxidative stress and inflammation.25 The combination of Astragalus polysaccharide and hirudin can reduce the apoptosis rate of macrophages induced by ox-LDL, which may be used to treat AS because of the regulation of mitochondrial membrane potential and the expression of related proapoptotic proteins Caspase-3 (CASP3), Bax and anti-apoptotic protein Bcl-2.26 Tanshinone IIA plays a role by inhibiting LDL oxidation,27 smooth muscle cell migration and development, arterial intimal monocyte adhesion, pro-inflammatory cytokine expression, platelet aggregation,28 and macrophage-mediated cholesterol accumulation in AS.29 Berberine can effectively improve the levels of blood lipids, reduce the area of atherosclerotic plaque in mice, and exert an anti-inflammatory effect by lowering blood CRP and interleukin-6 (IL-6) levels.30 Panax notoginseng saponins can inhibit the formation of foam cells in ApoE−/− mice by regulating the TLR4/SYK signaling pathway.31 However, the specific mechanism of GMTG in treating AS is unclear because of the diverse ingredients of TCM and their complex interaction with the human body. Network pharmacology has become a powerful strategy for studying TCM prescriptions and is a breakthrough method in applying bioinformatics and systems biology in TCM.32
The goal of this study was to explore the potential mechanism of action of GMTG in the treatment of AS based on network pharmacology. The core mechanism was validated through ApoE−/− mice. The flowchart of the process is shown in Figure 1.
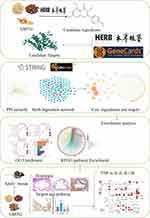 |
Figure 1 Flow Chart. |
Materials and Methods
Database Building and Prediction of Potential Targets
HERB (http://herb.ac.cn/), a high-throughput experiment and reference-guided database of TCM, integrates multiple TCM databases containing the most comprehensive list of TCMs and ingredients, providing high-quality and evidence-based links between TCM and modern medicines. Ten TCM herbs in GMTG were screened using the HERB database, with FDR < 0.05 as the screening standard, to obtain the ingredients and targets of GMTG.
The HERB database was searched with the keywords of “atherosclerosis” to obtain AS-related ingredients. Taking the intersection of GMTG and AS ingredients as potential ingredients. The GeneCards database was searched with the keywords of “atherosclerosis” to obtain AS-related targets. The AS-related targets and targets of GMTG were intersected to identify the potential targets.
Construction of the TCM Herb-Potential Target Network
The TCM herb-potential target network was constructed using Cytoscape software,33 and the topology of the network was analyzed. The core ingredients of GMTG used to treat AS were obtained by screening the degree values (≥2.62 (mean degree value)).
Protein-Protein Interaction (PPI) Network
The PPI network of potential targets was constructed using STRING34 and Cytoscape, and the network topology was analyzed. The degree value was screened to identify the core targets of GMTG in the treatment of AS.
GO and KEGG Enrichment Analyses
Enrichment analysis was performed using Cluster Profiler and Pathview packages in R. P < 0.05 indicated that the enrichment was statistically significant. Pathways with low P-values were selected for experimental verification.
Drug Preparation
GMTG was composed of 10 single TCM granules: Radix Astragali (HUANG QI), Radix Salviae liguliobae (DAN SHEN), Trichosanthis Fructus (GUA LOU), Rhizoma Coptidis (HAUNG LIAN), Radix Notoginseng (SAN QI), Radix Scrophulariae (XUAN SHEN), Bulbus Fritillariae thunbergii (ZHE BEI MU), Rhizoma Polygoni cuspidati (HU ZHANG), Gardenia jasminoides var.grandiflora (SHUI ZHI), Crassostrea gigas (MU LI), which was manufactured by Jiangyin Tianjiang Pharmaceutical Co., Ltd. (Wuxi, Jiangsu, China) and Beijing Kangrentang Pharmaceutical Co., Ltd (Beijing, China). All the above granules were authenticated by the outpatient granule pharmacy of Affiliated Hospital of Shandong University of Traditional Chinese Medicine (Jinan, Shandong, China). The details of GMTG are listed in Tables 1 and 2.
 |
Table 1 Composition of GMTG |
 |
Table 2 Properties and Meridians of GMTG |
Establishment of as Model
Fifteen SPF male C57BL/6J mice (8 weeks, 20±2g) and 48 SPF male ApoE−/−All mice were purchased (Vital River Laboratory Animal Technology Co., Ltd., Beijing, China) and raised in a barrier environment. All experimental procedures were in accordance with the standards specified in the Animal Experimental Guidelines of the Chinese Medical Ethics Committee and approved by the Animal Experimental Ethics Committee of Shandong University of Traditional Chinese Medicine (2021–35). We strictly abided by relevant provisions on animal handling.
After 1 week of adaptive feeding with common feed, feeding of common feed was continued in C57BL/6J mice, while ApoE−/− mice were fed an HFD (0.15% cholesterol, 21% lard, and 78.85% standard feed; Keao Co., Ltd., Beijing, China) to establish an AS model. The drug dose (based on crude drug content) for mice was 9.1 times that for humans. In this study, the equivalent dose in mice was obtained from the clinical dose in humans (clinical dose of GMTG: 198 g/d, adult human weight 70 kg, mouse dose of GMTG: 198g/70kg×9.1≈25.74g/kg).35 After 16 weeks of continuous feeding, three ApoE−/− mice were randomly selected for oil red and hematoxylin and eosin (HE) staining of the aorta to verify the model. After successful molding, ApoE−/− mice were randomly divided into three groups (n=15 per group): the AS model group (MOD), conventional dose GMTG-treatment group (GMTG), and atorvastatin-treatment group (ATO). C57BL/6J mice were regarded as a control group (NC). NC and MOD groups were administered saline 0.2 mL/d via gavage, the GMTG group was administered GMTG 25.74g/kg/d (0.2mL/20g), and the ATO group was administered atorvastatin calcium (Pfizer Pharmaceuticals, Co., Ltd., New York, USA) 5mg/kg/d (0.2mL) via gavage. All mice were given an intraperitoneal injection of pentobarbital for euthanasia after 8-weeks of intervention.
Sample Collection
After 8 weeks of treatment, blood was collected from the eyeball after anesthesia, allowed to stand at room temperature for 2 h, and then centrifuged at 3500 rpm for 30 min at 4°C. The supernatant was collected for an enzyme-linked immunosorbent assay (ELISA). After exposing the thoracic and abdominal cavity, 0.9% saline was perfused into the apex of the heart until the effluent was clear, the heart and aorta were quickly removed, and the surrounding adipose tissue was discarded. Part of the adipose tissue was fixed with 4% paraformaldehyde for slicing and staining, and the other part was immediately put in liquid nitrogen and transferred to −80 °C for RT-qPCR.
Measurement of Level of Serum Lipids
The levels of serum total cholesterol (TC) (A111-1-1), serum triglyceride (TG) (A110-1-1), LDL-C (A113-1-1), and serum high-density lipoprotein-cholesterol (HDL-C) (A112-1-1) were measured using kits purchased from Nanjing JianCheng Bioengineering Institute (Jiangsu, China), according to the manufacturer’s instructions, and the absorbance was measured by enzyme calibration. The level of serum ox-LDL was detected using an ELISA kit (JL10819, Jianglaibio Co., Ltd., Shanghai, China), and the absorbance at A562 was measured using an enzyme-labeled instrument.
Enzyme-Linked Immunosorbent Assay (ELISA)
The levels of serum tumor necrosis factor (TNF-α, E-EL-M0049c, Elabscience Biotech, Wuhan, China), IL-6 (JL20268, Jianglaibio Co., Ltd., Shanghai, China), and IL-1β (MM-0040M1, Mmbio, Jiangsu, China) were measured according to the manufacturer’s instructions, and the absorbance at A562 nm was measured by enzyme calibration.
Histological Analysis
Samples were fixed with 4% paraformaldehyde, dehydrated using an alcohol concentration gradient, removed in xylene, embedded in paraffin, and cut into 4 μm slices. The sections were stained with HE, Oil red, and Movat, and imaged using a Pannoramic MIDI digital scanner (3DHISTECH Ltd., Hungary). Image-Pro Plus 6.0 (Media Cybernetics, Inc., Rockville, MD, USA) was used to analyze the atherosclerotic plaque area, lipid deposition, foam cell content, and collagen fiber content. The formulae are as follows: lipid deposition=red area of oil red staining/plaque area, foam cell content =foam cell area of Movat staining/plaque area, and collagen fiber content=collagen area of Movat staining/plaque area.
Reverse Transcription-Quantitative Polymerase Chain Reaction (RT-qPCR)
Total RNA was extracted from aortic samples using a modified tissue/cell RNA rapid extraction kit (AC0202, Sparkjade, Shandong, China), followed by RNA concentration and purity measurement. Total RNA was then reverse transcribed into cDNA on PCR Thermal Cycler DiceTM (Takara Bio Inc., Kusatsu, Japan) and a SPARK script II-RT Plus Kit (R223-01, Vazyme, Nanjing, China). The cDNA was amplified using 2×SYBR Green qPCR Mix (AH0104-B, Sparkjade, Shandong, China), and the threshold cycle was recorded using the Light Cycler® 96 system (Roche Applied Sciences, Penzberg, Germany) using the following reaction conditions: 95.0 °C, 5 min 1 cycle; 95.0 °C for 10s, 56°C for 30s, 72°C for 30s for 40 cycles, 95°C for 15s, 65°C for 60s, 95°C continuous 1 cycles, and 40°C for 30s. The fold-change in mRNA expression was determined using the 2−ΔΔCT method. Primers for TLR4, MyD88, NF-κB p65, and actin were designed and synthesized by Biosune Biotech (Shanghai, China). (Table 3)
 |
Table 3 Primer Sequences for RT-qPCR Amplification |
Immunohistochemistry (IHC)
Immunohistochemical analysis was performed using anti-TLR4 (1:1000, GB11519, Servicebio), anti-MyD88 (1:200, GB11269, Servicebio), and anti-NF-κB p65 (1:400, bs-0465R, Bioss) antibodies. Fixed tissue gradient alcohol dehydration, paraffin embedding, and slicing to a thickness of 4 μm were performed. After deparaffinization and rehydration, the tissue sections were antigen-repaired with citric acid antigen-repair buffer (pH 6.0) and heated for the specified time. After natural cooling, the slides were placed in PBS (pH 7.4) and washed by shaking on a decolorization shaker three times for 5 min each. After treatment with 3% H2O2 and incubation at room temperature in the dark for 25 min, the slides were placed in PBS (pH 7.4) and washed by shaking on a decolorization shaker 3 times for 5 min each. Subsequently, 3% BSA was added to the circle to cover the tissue evenly, and the tissues were sealed for 30 min at room temperature. After gently removing the sealing solution, the sections were placed flat in a wet box with primary antibodies and incubated overnight at 4°C, followed by incubation with secondary antibodies for 30 min. The sections were then stained with diaminobenzidine (DAB) (G1211, Servicebio) and hematoxylin and mounted with neutral balsam. Brownish-yellow granules indicated positive signals. The slides were scanned and evaluated using Image-Pro Plus software.
Statistical Analysis
We used QQ plots and Shapiro–Wilk test to assess data distribution. Graphpad Prism 9.0.0 software (San Diego, California, USA) was used to analyze and visualize the data. The measurement data are expressed as the mean ± SD. Analysis of variance (ANOVA) and Dunnett’s T3 tests were carried out to determine statistical significance for multiple comparisons. The least significant difference (LSD) test was performed under the assumption of equal variances. The indigenous α level was set to 0.05 (bilateral), and P<0.05 indicated a statistically significant difference.
Results
Screening results of Ingredients and Targets
A total of 124 ingredients and 418 targets of GMTG for AS treatment were screened from the HERB database. Radix Astragali contained 24, Radix Salviae liguliobae contained 40, Trichosanthis Fructus contained 14, Rhizoma Coptidis contained 16, Radix Notoginseng contained 32, Radix Scrophulariae contained 17, Bulbus Fritillariae thunbergii contained 12, Rhizoma Polygoni cuspidati contained 20, Gardenia jasminoides var. grandiflora contained 6 ingredients. Eleven ingredients were contained in more than two TCM herbs, eight of which were in Radix Notoginseng (Table 4).
 |
Table 4 Core Ingredients of GMTG |
A total of 4710 AS-related targets were identified from the GeneCards database. The intersection of GMTG-related and AS-related targets yielded 418 targets for GMTG treatment of AS.
Network Construction
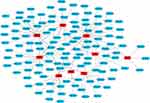
The TCM herb-ingredient network of GMTG used to treat AS (Figure 2) was constructed using Cytoscape software 3.7.0. It contained 133 nodes, including nine TCM herbs and 124 related ingredients. (Appendix 1) The PPI network of potential targets was constructed using STRING and Cytoscape with a confidence of 0.700, and 418 targets were screened and analyzed (Figure 3).
The top 11 ingredients in the network are listed in Table 5. Figure 4A showed the relationship between the TCM herbs and 11 core ingredients. Radix Notoginseng, Radix Salviae liguliobae, and Radix Astragali contained most of the ingredients and were the core TCM herbs in the network. The top 100 targets in the PPI network were selected as core targets according to the degree (Figure 4B). The top 15 core targets based on the degree in the PPI network are listed in Table 6. TNF, IL-6, and IL 1β were the core inflammatory cytokine genes. The targets encoded by AKT1, TP53, EGFR, STAT3, and CASP3 were closely associated with the inflammatory response. MAPK1 and MAPK3 were the MAPK family proteins involved in inflammation.
 |  |  |  |
Table 5 Information of the Top 11 Ingredients in TCM Herb-Ingredient Network |
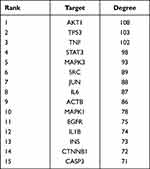 |
Table 6 Top 15 Targets in PPI Network |
GO and KEGG Pathway Analysis
Biological process enrichment analysis indicated that the potential targets were mainly enriched in response to lipopolysaccharides, molecules of bacterial origin, nutrient levels, and cellular response to chemical stress and biotic stimulus. (Figure 5A) The enriched cell components were the external side of the plasma membrane and a membrane raft (Figure 5B), and the enriched molecular function terms were primarily G protein-coupled receptor binding, signaling receptor activator activity, and receptor-ligand activity. (Figure 5C) GO enrichment results for the first 10 items are shown in Figure 5D.
 |
Figure 5 GO Enrichment Analysis. (A) Biological Process, (B) Cellular Component, (C) Molecular Function, (D) Results of three Ontologies. |
KEGG pathway enrichment analysis identified 191 pathways with significant enrichment (P<0.05). The AGE-RAGE signaling pathway in diabetic complications, Lipid and atherosclerosis, TNF signaling pathway, IL-17 signaling pathway, Apoptosis, PI3K-Akt signaling pathway, Toll-like receptor signaling pathway, NF-κB signaling pathway, and many other pathways related to the inflammatory response were among the top 50 pathways ranked by P-value. (Appendix 2) The results of the top 10 KEGG pathways were shown in Figure 6. In addition, the Lipid and atherosclerosis pathway was the most significant and was closely related to core targets TNF, IL-6, and IL-1β.
 |
Figure 6 KEGG pathway Enrichment Analysis. The red node represents target, the yellow node represents pathway, the line represents the relationship between pathway and target. |
Model Verification
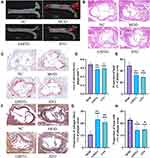
Oil red staining from the aortic arch to the thoracic aorta showed atherosclerotic plaque formation in the MOD group, while no apparent plaque occurred in the NC group, which was consistent with the HE staining results. These results indicated the successful construction of the AS model (Figure 7).
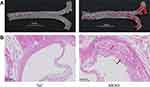 |
Figure 7 (A) Oil red staining of thoracic aorta, (B) HE staining. The black arrow represents plaque, the red arrow represents cholesterol crystals, the green arrow represents lymphocyte infiltration. |
GMTG Reduced the Serum Lipid Level in ApoE−/− Mice
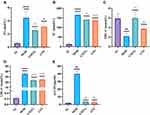
Results showed that GMTG has a lipid-lowering effect. Compared with those in the NC group, the serum TG, TC, LDL-C, and ox-LDL levels in the MOD group were significantly higher, and the HDL-C level was lower, conforming to hyperlipidemia characteristics in the AS model of ApoE−/− mice. Compared with the MOD group, the serum TG, TC, LDL-C, and ox-LDL levels in the GMTG and ATO groups were lower. (Figure 8)
GMTG Inhibited the Level of Serum Inflammatory Cytokines in ApoE−/− Mice
Based on the results of PPI and Cytoscape analyses, we selected inflammatory cytokines, including TNF-α, IL-6, and IL-1β, as the core targets for experimental verification. Compared with those in the NC group, the levels of serum TNF-α, IL-6, and IL-1β in the MOD group were increased. Compared with those in the MOD group, the levels of the above inflammatory cytokines in the GMTG and ATO groups were significantly decreased. (Figure 9)
GMTG Ameliorated Atherosclerotic Plaque Lesions and Pathological Damage in ApoE−/− Mice
Oil red staining showed that atherosclerotic plaques in the thoracic aorta of the MOD group were significantly formed, and after GMTG and ATO treatment, the formation of aortic plaque was reduced. (Figure 10A) HE staining showed obvious atherosclerotic plaques in the aortic root of the MOD group, and a lipid core and cholesterol crystals were also formed, with inflammatory cell infiltration. (Figure 10B) The results were consistent with the oil red staining results. Oil red staining analysis of aortic root showed that GMTG and ATO reduced the atherosclerotic plaque area and lipid deposition in aortic root compared to that in the MOD group. (Figure 10C, D and E) In addition, the results of Movat staining showed that the content of foam cells in the GMTG group was lower than that in the MOD group, and the content of collagen fibers was higher than that in the MOD group. (Figure 10F, G and H) In summary, GMTG could reduce the area of aortic atherosclerotic plaque, reduce plaque lipid deposition and plaque foam cell content, and increase plaque collagen fiber content, thereby reducing the burden of plaque and promoting plaque remodeling to play an anti-AS role.
GMTG Regulated the TLR4/MyD88/NF-κB Signaling Pathways in ApoE−/− Mice
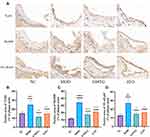
Based on the results of KEGG pathway enrichment and PPI analyses, the TLR4/MyD88/NF-κB signaling pathway in AS was selected as the core inflammation-related pathway for experimental verification. The mRNA levels of TLR4, MyD88, and NF-κB in the MOD group were higher than those in the NC group (Figure 11). Compared with those in the MOD group, TLR4, MyD88, and NF-κB mRNA levels were significantly decreased after GMTG or ATO treatment.
The IHC results showed that the positive areas of TLR4, MyD88, and NF-κB in the MOD group were higher than those in the NC group, and the positive areas of TLR4, MyD88, and NF-κB in the GMTG and ATO groups were lower than those in the MOD group (Figure 12). It was consistent with RT-qPCR results. The above results showed that GMTG could inhibit the inflammatory immune response of atherosclerotic plaque through the TLR4/MyD88/NF-κB pathway in ApoE−/− mice and has the anti-AS effect of stabilizing the plaque.
Discussion
In this study, the main ingredients and mechanisms of GMTG in treating AS were comprehensively and reliably analyzed using a high-quality database of TCM. The key targets of the core signaling pathway and downstream inflammatory cytokines were verified in vivo by constructing an ApoE−/− mice AS model, which provided objective data supporting the anti-AS effect of TCM, that is the inhibition of inflammatory and immune response in AS.
We screened ingredients and targets related to GMTG through high-throughput experiments of TCM and reference database HERB to ensure the data obtained were comprehensive and reliable. The HERB database, also known as BENCAO ZUJIAN, was jointly developed by the Peking University of TCM, Institute of Computing Technology of the Chinese Academy of Sciences, and Institute of Kidney Research of West China Hospital of Sichuan University. It is advantageous because of the ability to cross-reference multiple TCM database, including the most comprehensive list of TCM herbs and ingredients compiled to date, which makes it convenient to use. In addition, it provides high-quality, evidence-based links between TCM herbs and modern drugs by linking targets, diseases, and TCM herbs/ingredients by integrating the results of high-throughput experimental results, literature search, and statistical inference results of TCM herbs/ingredients.36 Therefore, considering the high-quality of retrieval results from the HERB database, we directly search for ingredients and targets related to TCM herbs to reduce the false positive rate of indirect searching, providing reliable data supporting further experimental research.
ApoE−/− mice develop atherosclerotic lesions naturally, however, they mostly form stable plaques and fail to form a vulnerable plaque model.37 Our goal was to simulate the complex pathological changes in the late stage of AS, especially the mechanism of vulnerable plaque and plaque rupture in the late stage. Based on the existing research,38 we constructed an HFD-induced atherosclerotic vulnerable plaque model using ApoE−/− mice. First, 8-weeks-old ApoE−/− mice were fed an HFD for 16 weeks. After 8 weeks of continuous administration, pathological changes in plaque and various indicators were detected. In the MOD group, large lipid cores, foam cells and cholesterol crystals, infiltration of inflammatory cells, and the rupture of some plaques were observed in the typical aortic root plaque.37
The inflammatory response is involved in all stages of AS development.39 CANTOS experiment confirmed the correctness of AS inflammation theory and opened the second era of AS prevention and treatment. It focused on anti-inflammatory benefits.16 TCM herbs have therapeutic potential in preventing and treating AS inflammation and immunity because of their multi-target effects and overall regulation. Analysis of the composition of GMTG showed that Radix Notoginseng, Radix Salviae liguliobae, and Radix Astragali play a more important role in the formula, which contains many ingredients and targets for the treatment of AS by GMTG. These three TCM herbs are commonly used for the clinical treatment of AS.40 Existing studies have shown that various main ingredients of GMTG have anti-AS effects, including β-sitosterol, palmitic acid, quercetin, etc. β-sitosterol can reduce cholesterol synthesis by inhibiting cholesterol synthase expression and regulate inflammation by regulating the hypothalamus-pituitary-adrenal axis.41 Palmitic acid, a saturated fatty acid, is the leading free fatty acid in plasma lipids. It may lead to an inflammatory reaction,42–45 cell dysfunction, and even cell death46,47 by inducing oxidative stress and continuous endoplasmic reticulum stress in cardiomyocytes. Kaempferol is a flavonoid antioxidant, which has anti-inflammatory and anti-atherosclerotic effects.48,49 It can increase the expression of Bcl-2 by silencing the mitochondrial pathway mediated by regulatory factor 1 and play a protective role in hypoxic myocardial cells. Kaempferol can effectively reduce vascular inflammation and prevent AS development.50 Rutin is an anti-inflammatory, anti-oxidant, anti-allergic, and antiviral flavonoid molecule, known to have anti-atherosclerotic and autophagy-inducing properties, which could inhibit ox-LDL-mediated macrophage inflammation and foam cell formation by inducing autophagy and modulating PI3K/ATK signaling, showing potential in treating atherosclerosis.51 Oleic acid is a monounsaturated fatty acid, which can stimulate vascular smooth muscle cells proliferation and migration.52 It also has an anti-inflammatory effect, reducing LDL oxidation and lowering cholesterol.53–55 Higenamine has anti-thrombotic, anti-apoptotic, antioxidant, anti-inflammatory, and immunomodulatory effects.56 Oleanolic acid and ursolic acid belong to pentacyclic triterpenoid acids, and they are isomers, so their pharmacological effects are almost the same. They have been proved to have pharmacological effects such as anti-inflammatory, anti-atherosclerosis, lowering lipid, and inhibiting smooth muscle cell proliferation.57 Adenosine, an extracellular signal molecule, has the effects of anti-inflammatory and anti-thrombotic, reducing blood pressure and heart rate, and may delay the occurrence of early atherosclerotic events.58,59 Obaculatone has anti-inflammatory, analgesic, and antioxidant effects, which can regulate blood lipids and prevent atherosclerosis.60,61
Dyslipidemia is one of the most critical risk factors for AS. It is well known that hypercholesterolemia is the primary risk factor for atherosclerosis cardiovascular disease (ASCVD), and treatment for reducing LDL-C has become the cornerstone of primary and secondary prevention of ASCVD.62 However, there is still a residual cardiovascular risk after effectively lowering LDL-C levels using statins or combined lipid-lowering strategies.63,64 Recent studies have shown that inflammation and cholesterol are both pathogenic factors of AS. AS develop only when these two factors coexist.65 CANTOS showed that the cardiovascular benefits of canakinumab were directly related to the reduction in IL-6.16 Furthermore, evidence shows that hypertriglyceridemia has a causal relationship with an increased risk of AS,66,67 TG-rich lipoprotein and its residues are also independent risk factors for AS.65 Ox-LDL is a causal factor of AS and plays an important role in the formation by promoting inflammation and lipid deposition in the arterial wall. LDL-C is oxidized and modified to ox-LDL in the intimal region of the blood vessel, which induces the injury and adhesion of ECs to monocytes and chemotaxis to the subcutaneous tissue, allowing the formation of macrophage-derived foam cells, which leads to the dissolution of fibrous caps and eventually affects plaques stability. It plays an important role in all aspects of AS.68 Overall, reducing circulating TC, TG, LDL-C, and ox-LDL levels is beneficial to AS, which is consistent with the results of this study. GMTG can regulate lipid levels by reducing the TG, TC, LDL-C, and ox-LDL levels, and increasing HDL-C level. In addition, GMTG reduced the levels of circulating TNF-α, IL-6, and IL-1β in ApoE−/− mice, indicating that it has anti-inflammatory and lipid-lowering effects.
We identified a large number of pathways that might be involved in the treatment of AS by GMTG through enrichment analysis. The TLR4 pathway not only exists in the top one pathway set, but its downstream cytokines, including TNF-α, IL-6, and IL-1β, are also the core targets in the PPI network. Therefore, we selected the TLR4 pathway and its downstream inflammatory cytokines for further experimental verification. Studies have shown that the TLR4 pathway might be involved in the inflammatory and immune responses in AS.69,70 The activation of Toll-like receptors and MyD88 is an important part of the immune and inflammatory mechanism of AS and plaque formation.71 NF-κB is a downstream transcription factor of the TLR4 pathway. Activated NF-κB translocates into the nucleus and binds to its related DNA motif to induce the transcription of target genes, including pro-inflammatory cytokines, such as TNF, IL-1, and mitogen.72 The TLR4/MyD88/NF-κB pathway is an important inflammatory signal transduction pathway in the body and is closely related to the development of AS. Upregulation of TLR4 protein expression in vascular ECs can activate NF-κB through MyD88 and induce the production and release of pro-inflammatory cytokines such as TNF-α, IL-6, and IL-1β, to promote the formation of AS plaques.73–75 TNF-α is a pro-inflammatory cytokine closely related to AS, thrombosis, and plaque rupture. It can promote the endocytosis and transport of LDL-C in vascular ECs,76 inhibit the formation of nitric oxide synthase, stimulate ECs to express adhesion molecules, and lead to vascular endothelial dysfunction.77,78 As an important pro-inflammatory cytokine, IL-6 is involved in the inflammatory response and aggravates the development of AS.79 IL-1β, another important pro-inflammatory cytokine, is involved in various autoimmune inflammatory reactions and cell activities, including cell proliferation, differentiation, and apoptosis.80–83 IL-1β also synergistically induced VEGF production with TNF and IL-6, and plays a role in angiogenesis.80 Our study showed that GMTG could inhibit the TLR4/MyD88/NF-κB pathway, reduce the release of downstream inflammatory cytokines TNF-α, IL-6, and IL-1β, and play an anti-AS role by inhibiting plaque inflammation and increasing plaque stability.
Although GMGT seems a little better than statin in lipid regulation and anti-inflammation, most of the results were not statistically significant. Thus, further research is necessary.
Conclusion
Overall, the effective mechanism of GMTG in treating AS involves the regulation of targets and pathways in various biological processes, especially inflammation and immune response. The results showed that GMTG could exert anti-inflammatory and immune-regulating effects on AS partially via the TLR4/MyD88/NF-κB signaling pathway. GMTG could also regulate the levels of serum lipids, reduce plaque burden, promote plaque remodeling and increase plaque stability. In addition, GMTG can potentially reduce the risk of residual cardiovascular inflammation. Therefore, GMTGs could be a potential therapeutic drug with multiple anti-AS effects, which warrants further study.
Abbreviations
AMI, Acute myocardial infarction; AS, Atherosclerosis; ASCVD, Atherosclerosis cardiovascular disease; DAB, Diaminobenzidine; ECs, Endothelial cells; ELISA, Enzyme-Linked Immunosorbent Assay; GMTG, Guanmaitong granule; LDL-C, Low-density lipoprotein-cholesterol; HDL-C, High-density lipoprotein-cholesterol; HFD, High-fat diet; ICAM-1, Intercellular adhesion molecule 1; IHC, Immunohistochemistry; IL-1β, Interleukin-1 beta; IL-6, Interleukin-6 LDL-C, Low density lipoprotein-cholesterol; MCP-1, Monocyte chemoattractant protein 1; MyD88, Myeloid differentiation primary response protein MyD88; NF-κB, Transcription factor p65; ox-LDL, oxidized low density lipoprotein; PCSK9, Proprotein convertase subtilisin/kexin type 9; RT-qPCR, Reverse transcription quantitative polymerase chain reaction; SYK, Tyrosine-protein kinase SYK; TC, Total cholesterol; TCM, Traditional chinese medicine; TG, Triglycerids; TLR4, Toll-like receptor 4; TNF-α, Tumor necrosis factor; VCAM-1, Vascular cell adhesion protein 1.
Acknowledgments
This work was supported by the “Combination of sports and medicine” demonstration project of precise prevention and treatment of traditional Chinese medicine for people with normal and high blood pressure.(2021SFGC0503)
Disclosure
The authors declare that there are no conflicts of interest in this work.
References
1. Du SL, Jia ZQ, Zhong JC, et al. TRPC5 in cardiovascular diseases. Rev Cardiovasc Med. 2021;22(1):127–135. doi:10.31083/j.rcm.2021.01.212
2. Rosamond W, Flegal K, Furie K, et al. Heart disease and stroke statistics–2008 update: a report from the American Heart Association statistics committee and stroke statistics subcommittee. Circulation. 2008;117(4):e125–146. doi:10.1161/CIRCULATIONAHA.107.187998
3. Kanter JE, Kramer F, Barnhart S, et al. Diabetes promotes an inflammatory macrophage phenotype and atherosclerosis through acyl-CoA synthetase 1. Proc Natl Acad Sci USA. 2012;109(12):E715–E724. doi:10.1073/pnas.1111600109
4. Li JJ, Chen JL. Inflammation may be a bridge connecting hypertension and atherosclerosis. Med Hypotheses. 2005;64(5):925–929. doi:10.1016/j.mehy.2004.10.016
5. Xu HL, Jiang JX, Chen WZ, et al. Vascular macrophages in atherosclerosis. J Immunol Res. 2019;2019:4354786. doi:10.1155/2019/4354786
6. Ge JB, Xu YJ, Wang C. Medicine.
7. Arsenault BJ, Kritikou EA, Tardif JC. Regression of atherosclerosis. Curr Cardiol Rep. 2012;14(4):443–449. doi:10.1007/s11886-012-0285-7
8. Cuchel M, Rader DJ. Macrophage reverse cholesterol transport: keyto the regression of atherosclerosis? Circulation. 2006;113(21):2548–2555. doi:10.1161/CIRCULATIONAHA.104.475715
9. Fukumoto Y, Libby P, Rabkin E, et al. Statins alter smoothmuscle cell accumulation and collagen content in establishedatheroma of watanabe heritable hyperlipidemic rabbits. Circulation. 2001;103(7):993–999. doi:10.1161/01.cir.103.7.993
10. Armitage J, Bowman L, Wallendszus K, et al.; Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH) Collaborative Group. Intensive lowering of LDL cholesterol with 80 mg versus 20 mg simvastatin daily in 12,064 survivors of myocardial infarction: a double-blind randomised trial. Lancet. 2010;376(9753):1658–1669. doi:10.1016/S0140-6736(10)60310-8
11. Preiss D, Seshasai SR, Welsh P, et al. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a meta-analysis. JAMA. 2011;305(24):2556–2564. doi:10.1001/jama.2011.860
12. Newman CB, Preiss D, Tobert JA, et al. Statin safety and associated adverse events: a scientific statement from the American Heart Association. Arterioscler Thromb Vasc Biol. 2019;39(2):e38–e81. doi:10.1161/ATV.0000000000000073
13. Kastelein JJ, Akdim F, Stroes ES, et al. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N Engl J Med. 2008;358(14):1431–1443. doi:10.1056/NEJMoa0800742
14. Qiu Y, Huang XQ, Huang ZG. Current status and prospect of PCSK9 inhibitors for atherosclerotic cardiovascular diseases. Central South Pharm. 2021;19(07):1353–1357.
15. Arrieta A, Hong JC, Khera R, et al. Updated cost-effectiveness assessments of PCSK9 inhibitors from the perspectives of the health system and private payers: insights derived from the Fourier trial. JAMA Cardiol. 2017;2(12):1369–1374. doi:10.1001/jamacardio.2017.3655
16. Ridker PM, Everett BM, Thuren T, et al.; CANTOS Trial Group. Antiinflammatory therapy with Canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119–1131. doi:10.1056/NEJMoa1707914.
17. Schaftenaar F, Frodermann V, Kuiper J, et al. Atherosclerosis: the interplay between lipids and immune cells. Curr Opin Lipidol. 2016;27(3):209–215. doi:10.1097/MOL.0000000000000302
18. Wolf D, Ley K. Immunity and Inflammation in Atherosclerosis. Circ Res. 2019;124(2):315–327. doi:10.1161/CIRCRESAHA.118.313591
19. Tabas I, García-Cardeña G, Owens GK. Recent insights into the cellular biology of atherosclerosis. J Cell Biol. 2015;209(1):1–22. doi:10.1083/jcb.201412052
20. Chistiakov DA, Melnichenko AA, Grechko AV, et al. Potential of anti-inflammatory agents for treatment of atherosclerosis. Exp Mol Pathol. 2018;104(2):114–124. doi:10.1016/j.yexmp.2018.01.008
21. Liu YF, Yu HM, Zhang Y, et al. TLRs are important inflammatory factors in atherosclerosis and may be a therapeutic target. Med Hypotheses. 2008;70(2):314–316. doi:10.1016/j.mehy.2007.05.030
22. Libby P. Current concepts of the pathogenesis of the acute coronary syndromes. Circulation. 2001;104(3):365–372. doi:10.1161/01.cir.104.3.365
23. Zheng XY. Guiding Principles for Clinical Research on New Drugs of Traditional Chinese Medicine.
24. Di PY, Kang L, Meng YJ, et al. Medication regularity and characteristics analysis of chinese medicine in the treatment of atherosclerosis based on data mining and network pharmacology. Pharmacol Clin Chin Mater Med. 2022:1–22. doi:10.13412/j.cnki.zyyl.20211015.004.
25. Zhu ZS, Li JY, Zhang XR. Astragaloside IV protects against oxidized Low-Density Lipoprotein (ox-LDL)-induced endothelial cell injury by reducing oxidative stress and inflammation. Med Sci Monit. 2019;25:2132–2140. doi:10.12659/MSM.912894
26. Pan Y. Study on the effect and mechanism of the effective components of Astragalus and leech on lipid accumulation in macrophages. Changchun Univ Tradit Chin Med. 2021. doi:10.26980/d.cnki.gcczc.2021.000105
27. Hartley A, Haskard D, Khamis R. Oxidized LDL and antioxidized LDL antibodies in atherosclerosis-novel insights and future directions in diagnosis and therapy. Trends Cardiovasc Med. 2019;29(1):22–26. doi:10.1016/j.tcm.2018.05.010
28. Ahmadsei M, Lievens D, Weber C, et al. Immune-mediated and lipid-mediated platelet function in atherosclerosis. Curr Opin Lipidol. 2015;26(5):438–448. doi:10.1097/MOL.0000000000000212
29. Tabas I, Bornfeldt KE. Macrophage phenotype and function in different stages of atherosclerosis. Circ Res. 2016;118(4):653–667. doi:10.1161/CIRCRESAHA.115.306256
30. Ren XN, Zhang XL, Song C. Effects and action mechanism of anti-atherosclerosis of berberine in rat models with atherosclerosis. Hebei Med J. 2021;43(01):115–118.
31. Zhao P, Li YH, Gao W, et al. Panax notoginseng saponins inhibited the formation of atherosclerotic foam cells in Apo E knockout mice by regulating TLR4/SYK signaling. Nat Product Res Dev. 2021;33(08):1267–1273. doi:10.16333/j.1001-6880.2021.8.001
32. Zhang GB, Li QY, Chen QL, et al. Network pharmacology: a new approach for Chinese herbal medicine research. Evid Based Complement Alternat Med. 2013;2013:621423. doi:10.1155/2013/621423
33. Shannon P, Markiel A, Ozier O, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498–2504. doi:10.1101/gr.1239303
34. Szklarczyk D, Gable AL, Lyon D, et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47(D1):D607–13. doi:10.1093/nar/gky1131
35. Chen Q. Methodology of Pharmacological Research of Traditional Chinese Medicine.
36. Fang SS, Dong L, Liu L, et al. HERB: a high-throughput experiment- and reference-guided database of traditional Chinese medicine. Nucleic Acids Res. 2021;49(D1):D1197–D206. doi:10.1093/nar/gkaa1063
37. Ni M, Chen WQ, Zhang Y. Animal models and potential mechanisms of plaque destabilisation and disruption. Heart. 2009;95(17):1393–1398. doi:10.1136/hrt.2008.143461
38. Chen Z. Anti-inflammatory and immunoregulatory mechanisms of tanshinone IIA on vulnerable atherosclerotic plaques through TLR4/MyD88/NF-κB pathway. Beijing Univ Tradit Chin Med. 2016. doi:10.3389/fphar.2019.00850
39. Gerhardt T, Haghikia A, Stapmanns P, et al. Immune mechanisms of plaque instability. Front Cardiovasc Med. 2022;8:797046. doi:10.3389/fcvm.2021.797046
40. Mo Q, Wu Y, Wei D, et al. Research on the prescription rules for the treatment of atherosclerosis based on data mining. World Chin Med. 2021;16(16):
41. Liu WL, Ji Y, Huang AX. Research and development progress of β-sitosterol. Farm Products Process. 2019;1:77–79,82. doi:10.16693/j.cnki.1671-9646(X).2019.01.022
42. Xu Q, Chen SY, Deng LD, et al. Antioxidant effect of mogrosides against oxidative stress induced by palmitic acid in mouse insulinoma NIT-1 cells. Braz J Med Biol Res. 2013;46(11):949–955. doi:10.1590/1414-431X20133163
43. Akoumi A, Haffar T, Mousterji M, et al. Palmitate mediated diacylglycerol accumulation causes endoplasmic reticulum stress Plin2 degradation and cell death in H9C2 cardiomyoblasts. Exp Cell Res. 2017;354(2):85–94. doi:10.1016/j.yexcr.2017.03.032
44. Rizzi F, Naponelli V, Silva A, et al. Polyphenon E (R), a standardized green tea extract, induces endoplasmic reticulum stress, leading to death of immortalized PNT1a cells by anoikis and tumorigenic PC3 by necroptosis. Carcinogenesis. 2014;35(4):828–839. doi:10.1093/carcin/bgt481
45. Wen SY, Velmurugan BK, Day CH, et al. High density lipoprotein (HDL) reverses palmitic acid induced energy metabolism imbalance by switching CD36 and GLUT4 signaling pathways in cardiomyocyte. J Cell Physiol. 2017;232(11):3020–3029. doi:10.1002/jcp.26007
46. Park EJ, Lee AY, Park S, et al. Multiple pathways are involved in palmitic acid-induced toxicity. Food Chem Toxicol. 2014;67(17):26–34. doi:10.1016/j.fct.2014.01.027
47. Cetrullo S, Tantini B, Flamigni F, et al. Antiapoptotic and antiautophagic effects of eicosapentaenoic acid in cardiac myoblasts exposed to palmitic acid. Nutrients. 2012;4(2):78–90. doi:10.3390/nu4020078
48. Silva Dos Santos J, Gonçalves Cirino JP, de Oliveira Carvalho P, et al. The pharmacological action of kaempferol in central nervous system diseases: a review. Front Pharmacol. 2021;11:565700. doi:10.3389/fphar.2020.565700
49. Tu YC, Lian TW, Yen JH, et al. Antiatherogenic effects of kaempferol and rhamnocitrin. J Agric Food Chem. 2007;55(24):9969–9976. doi:10.1021/jf0717788
50. Zhang YW, Shao DY, Shi JL, et al. A review on biological activities of kaempferol. Chin Bull Life Sci. 2017;29(4):400–405. doi:10.13376/j.cbls/2017053
51. Li B, Ji Y, Yi C, et al. Rutin Inhibits Ox-LDL-mediated macrophage inflammation and foam cell formation by inducing autophagy and modulating PI3K/ATK signaling. Molecules. 2022;27(13):4201. doi:10.3390/molecules27134201
52. Zhang Y, Liu C, Zhu L, et al. PGC-1alpha inhibits oleic acid induced proliferation and migration of rat vascular smooth muscle cells. PLoS One. 2007;2(11):e1137. doi:10.1371/journal.pone.0001137
53. Basu A, Devaraj S, Jialal I. Dietary factors that promote or retard inflammation. Arterioscler Thromb Vasc Biol. 2006;26(5):995–1001. doi:10.1161/01.ATV.0000214295.86079.d1
54. Parthasarathy S, Khoo JC, Miller E, et al. Low density lipoprotein rich in oleic acid is protected against oxidative modification: implications for dietary prevention of atherosclerosis. Proc Natl Acad Sci USA. 1990;87(10):3894–3898. doi:10.1073/pnas.87.10.3894
55. Tang CK, Yang JH, Yi GH, et al. Effects of oleate on ATP binding cassette transporter A1 expression and cholesterol efflux in THP-1 macrophage-derived foam cells. Acta Biochim Biophys Sin (Shanghai). 2003;12:1077–1082.
56. Zhang N, Lian Z, Peng X, et al. Applications of Higenamine in pharmacology and medicine. J Ethnopharmacol. 2017;196:242–252. doi:10.1016/j.jep.2016.12.033
57. Zhang MF, Shen YQ. Research progress on vascular pharmacologic effects of oleanolic and ursolic acids. Drug Eval Res. 2017;40(10):1510–1519.
58. Koupenova M, Johnston-Cox H, Ravid K. Regulation of atherosclerosis and associated risk factors by adenosine and adenosine receptors. Curr Atheroscler Rep. 2012;14(5):460–468. doi:10.1007/s11883-012-0263-y
59. Layland J, Carrick D, Lee M, et al. Adenosine: physiology, pharmacology, and clinical applications. JACC Cardiovasc Interv. 2014;7(6):581–591. doi:10.1016/j.jcin.2014.02.009
60. Yan M, Zhou Y, He XH, et al. Research progress on the bioactivity of limonin and its analogues in citrus. Food Ferment Indus. 2018;44(02):290–296. doi:10.13995/j.cnki.11-1802/ts.014827
61. Sah AN, Joshi A, Juyal V, et al. Antidiabetic and hypolipidemic activity of Citrus medica Linn.seed extract in streptozotocin induced diabetic rats. Pharmacogn J. 2011;3(23):80–84. doi:10.5530/pj.2011.23.12
62. Peng J, Luo F, Ruan GY, et al. Hypertriglyceridemia and atherosclerosis. Lipids Health Dis. 2017;16(1):233. doi:10.1186/s12944-017-0625-0
63. Chapman MJ, Ginsberg HN, Amarenco P, et al. Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: evidence and guidance for management. Eur Heart J. 2011;32(11):1345–1361. doi:10.1093/eurheartj/ehr112
64. Sampson UK, Fazio S, Linton MF. Residual cardiovascular risk despite optimal LDL cholesterol reduction with statins: the evidence, etiology, and therapeutic challenges. Curr Atheroscler Rep. 2012;14(1):1–10. doi:10.1007/s11883-011-0219-7
65. Libby P. The changing landscape of atherosclerosis. Nature. 2021;592(7855):524–533. doi:10.1038/s41586-021-03392-8
66. Thomsen M, Varbo A, Tybjaerg-Hansen A, et al. Low nonfasting triglycerides and reduced all-cause mortality: a mendelian randomization study. Clin Chem. 2014;60(5):737–746. doi:10.1373/clinchem.2013.219881
67. Do R, Willer CJ, Schmidt EM, et al. Common variants associated with plasma triglycerides and risk for coronary artery disease. Nat Genet. 2013;45(11):1345–1352. doi:10.1038/ng.2795
68. Wang CY. Research Progress of oxidized low density lipoprotein and atherosclerosis. Cardiovasc Des Electron J Integr Tradit Chin West Med. 2017;5(29):34. doi:10.16282/j.cnki.cn11-9336/r.2017.29.023
69. Li Y, Zhang L, Ren P, et al. Qing-Xue-Xiao-Zhi formula attenuates atherosclerosis by inhibiting macrophage lipid accumulation and inflammatory response via TLR4/MyD88/NF-κB pathway regulation. Phytomedicine. 2021;93:153812. doi:10.1016/j.phymed.2021.153812
70. Zhang XS, Xue CY, Xu Q, et al. Caprylic acid suppresses inflammation via TLR4/NF-κB signaling and improves atherosclerosis in ApoE-deficient mice. Nutr Metab (Lond). 2019;16(1):40. doi:10.1186/s12986-019-0359-2
71. Björkbacka H, Kunjathoor VV, Moore KJ, et al. Reduced atherosclerosis in MyD88-null mice links elevated serum cholesterol levels to activation of innate immunity signaling pathways. Nat Med. 2004;10(4):416–421. doi:10.1038/nm1008
72. Xu YJ, Chen XS. Cholesterol metabolism of macrophage foam cells and atherosclerosis. Basic Clin Med. 2013;33(2):235–238. doi:10.16352/j.issn.1001-6325.2013.02.014
73. Yin YW, Liao SQ, Zhang MJ, et al. TLR4-mediated inflammation promotes foam cell formation of vascular smooth muscle cell by upregulating ACA-T1 expression. Cell Death Dis. 2014;5(12):e1574. doi:10.1038/cddis.2014.535
74. Chen TW, Luo W, Wu GJ, et al. A novel MyD88 inhibitor LM9 prevents atherosclerosis by regulating inflammatory responses and oxidative stress in macrophages. Toxicol Appl Pharmacol. 2019;370:44–55. doi:10.1016/j.taap.2019.03.012
75. Zhu LB, Gong XY, Gong JP, et al. Notoginsenoside R1upregulates miR-221-3p expression to alleviate ox-LDL-induced apoptosis, inflammation, and oxidative stress by inhibiting the TLR4/NF-κB pathway in HUVECs. Braz J Med Biol Res. 2020;53(6):e9346. doi:10.1590/1414-431x20209346
76. Zhang YZ, Yang XY, Bian F, et al. TNF-α promotes early atherosclerosis by increasing transcytosis of LDL across endothelial cells: crosstalk between NF-κB and PPAR-γ. J Mol Cell Cardiol. 2014;72:85–94. doi:10.1016/j.yjmcc.2014.02.012
77. Kleemann R, Zadelaar S, Kooistra T. Cytokines and atherosclerosis: a comprehensive review of studies in mice. Cardiovasc Res. 2008;79(3):360–376. doi:10.1093/cvr/cvn120
78. Zhang HR, Park Y, Wu JX, et al. Role of TNF-alpha in vascular dysfunction. Clin Sci (Lond). 2009;116(3):219–230. doi:10.1042/CS20080196
79. Tao M, Zheng KC, Xiao MM, et al. Research progress of correlation between IL-6 and coronary heart disease. Acta Med Univ Sci Technol Huazhong. 2016;45(5):585–587.
80. Nakahara H, Song J, Sugimoto M, et al. Anti-interleukin-6 receptor antibody therapy reduces vascular endothelial growth factor production in rheumatoid arthritis. Arthritis Rheum. 2003;48(6):1521–1529. doi:10.1002/art.11143
81. Van Damme J, De Ley M, Opdenakker G, et al. Homogeneous interferon-inducing 22K factor is related to endogenous pyrogen and interleukin-1. Nature. 1985;314(6008):266–268. doi:10.1038/314266a0
82. Tominaga K, Yoshimoto T, Torigoe K, et al. IL-12 synergizes with IL-18 or IL-1beta for IFN-gamma production from human T cells. Int Immunol. 2000;12(2):151–160. doi:10.1093/intimm/12.2.151
83. Xia SY, Zhang ZB, Magupalli VG, et al. Gasdermin D pore structure reveals preferential release of mature interleukin-1. Nature. 2021;593(7860):607–611. doi:10.1038/s41586-021-03478-3
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.