Back to Journals » Biologics: Targets and Therapy » Volume 10
Guanine nucleotide exchange factor H1 can be a new biomarker of melanoma
Authors Shi J, Guo B, Zhang Y, Hui Q, Chang P, Tao Kai
Received 31 March 2016
Accepted for publication 26 May 2016
Published 5 July 2016 Volume 2016:10 Pages 89—98
DOI https://doi.org/10.2147/BTT.S109643
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Doris Benbrook
Jie Shi, Bingyu Guo, Yu Zhang, Qiang Hui, Peng Chang, Kai Tao
Reconstructive and Plastic Surgery, The General Hospital of Shenyang Military Region, Shenyang, People’s Republic of China
Abstract: Guanine nucleotide exchange factor H1 (GEF-H1), which couples microtubule dynamics to RhoA activation, is a microtubule-regulated exchange factor. Studies have shown that GEF-H1 can be involved in various cancer pathways; however, the clinical significance of GEF-H1 expression and functions in melanoma has not been established. In this study, we investigated the relationship between clinical outcomes and GEF-H1 functions in melanoma. A total of 60 cases of different grades of melanoma samples were used to detect the expression of GEF-H1. Results showed that both messenger RNA and protein levels of GEF-H1 were significantly higher in high-grade melanomas. Furthermore, patients with high GEF-H1 expression had a shorter overall survival (22 months) than patients with low level of GEF-H1 expression (33.38 months).We also found that GEF-H1 can promote the proliferation and metastasis of melanoma cells. In summary, these results suggested that GEF-H1 may be a valuable biomarker for assessing the degree and prognosis of melanoma following surgery.
Keywords: GEF-H1, melanoma, biomarker, proliferation, metastasis
Introduction
Melanoma has been known as one of the most aggressive and treatment-resistant human cancers.1 The 5-year survival rate of melanoma is <10% and it is the topmost cause of death from skin cancer.2,3 No especially effective therapeutic modality has yet been found, except for early surgical resection, which means melanoma has a very low overall survival.4 In recent years, genetic, epigenetic, and protein biomarkers appear in our field of vision with the advancements in molecular technologies,5,6 and new therapeutic strategies produce unquestionable clinical benefit in melanoma. However, these strategies still fail to prolong the survival of patients with melanoma. Therefore, it is necessary to explore sensitive and specific molecular markers associated with the diagnosis and progression of melanoma.4
As an upstream regulator of RhoA, guanine nucleotide exchange factor H1 (GEF-H1) is considered to regulate diverse biological functions in tumor cells.7 GEF-H1 induces the increase of GTP-bound form of RhoA to activate RhoA oncogene8–10 and then activates various signals into downstream signaling cascades, such as proliferation, metastasis, and cytoskeleton reorganization.11 GEF-H1 is overexpressed in hepatocellular cancer, and it is a transcriptional target of gain-of-function p53 mutants and was found to be associated with metastasis.12–14 Brecht et al15 showed that GEF-H1 could induce tumor formation in nude mice. Frolov et al16 found that when gastrointestinal tumors were treated with imatinib, GEF-H1 was significantly downregulated in response. In addition, researchers found that GEF-H1 was required for the survival of many cancer cells, such as breast cancer, colon cancer, lung cancer, and ovarian cancer.17 All these data indicate that GEF-H1 has a significant contribution to cancer progression.18 However, there was little research with regard to the role of GEF-H1 in melanoma. We speculated whether the expression of GEF-H1 in melanoma is correlated with the clinical characteristics and mechanisms of action.
In this study, we detected the expression of GEF-H1 in melanoma specimens, clarified the correlation between survival rate of melanoma patients and GEF-H1 expression, and confirmed the function of GEF-H1 in melanoma cells.
Patients and methods
Patients and tissue samples
The study protocol and acquisition of tissue specimens were approved by the Ethics Committee of Biomedicine Research, General Hospital of Shenyang Military Command and complied with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Clinical trial registration: Clinical and Basic Research of Melanoma Cancer Number 2008R18. Written informed consent was obtained from all individual participants included in the study. All cases were diagnosed with melanoma and treated between June 2009 and May 2010 at the General Hospital of Shenyang Military Command. All cases were classified as shown in Table 1. All samples were diagnosed according to the TNM stage for tumor by two senior pathologists. Following surgery, all the tissues were frozen in liquid nitrogen for research.
Laser capture microdissection was performed on melanoma tumors that were selected out of convenience, that is, tumor blocks were readily available for the tissue sectioning required for laser capture microdissection.19
Cell culture
Human melanoma cell line A375 and A875 cells (Procell, Wuhan, People’s Republic of China) were starved in Dulbecco’s Modified Eagle’s Medium (Thermo Fisher Scientific, St Louis, MO, USA) containing 10% fetal calf serum (Thermo Fisher Scientific).
MTT assay
A total of 1×104 cells were mixed with 0.1 mL DMEM with 10% fetal calf serum and then plated onto 96-well plates; 12 hours later, transfection was performed, and after 24 hours, the cells were incubated for 0 hour, 12 hours, 24 hours, 36 hours, and 48 hours. We used 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay to measure the capability of cellular proliferation. We added 5 mg/mL of MTT to each well. After 4 hours, the medium was replaced by 0.1 mL dimethyl sulfoxide. Microplate Reader (Bio-Rad Laboratories Inc., Hercules, CA, USA) was used to measure the optical densities at 490 nm as described previously.20
Metastasis assay
The matrigel invasion chamber was used to assess the cell invasion ability (24-well plates, 8 μm pore size; Corning Incorporated, Corning, NY, USA). In brief, 1×105 cells in serum-free media were seeded in transwell chambers with matrigel membrane covered or uncovered with the media containing 0.1% bovine serum albumin, while the media containing 30% fetal bovine serum was placed in the lower well. After 24 hours of different treatments, the noninvading cells were removed using cotton swabs. Cells at the bottom of the membrane were stained with 0.1% crystal violet and were counted under microscopic observation.
Reverse transcription and quantitative real-time PCR
Total RNA of A375 cells and tissues was isolated by TRIzol (Thermo Fisher Scientific) according to the manufacturer’s protocol. Total RNA was reverse transcribed to complementary DNA by an RT reaction kit (Promega Corporation, Fitchburg, WI, USA). Real-time polymerase chain reaction (PCR) was performed by an Mx 3000P real-time PCR system (Thermo Fisher Scientific) and SYBR Premix Ex Taq (TaKaRa) as a DNA-specific flouorescent dye. Primer sequences for detection of messenger RNA (mRNA) expression were synthesized as shown in Table 2.
  | Table 2 Primer sequences for detection of messenger RNA expression Abbreviation: GEF-H1, guanine nucleotide exchange factor H1. |
All the reactions were repeated at least three times. Gene expression levels were calculated relative to GAPDH by using StratageneMx 3000P software.
Western blot analyses
To determine the expression of protein, 30 μg of protein from each sample was subjected to 12% sodium dodecyl sulfate polyacrylamide gels and transferred on to a nitrocellulose membrane. Target proteins were probed with specific antibodies – GEF-H1 (sc-134827), RhoA (sc-119), p21 (sc-21532), matrix metallopeptidase 9 (MMP9; sc-21733200), mouse anti-human IgG (sc-2005), rabbit anti-human IgG (sc-2775), and GAPDH (sc-365062) (Santa Cruz Biotechnology Inc., Dallas, TX, USA).
Overexpress GEF-H1 cells
We construct the GEF-H1 plasmid (forward primer [5´→3´] CAGACTTCCTGTCCCCGAGA; reverse primer [5´→3´] TCAGTGTCCTCACATGGTGC). GEF-H1 overexpression cell line was obtained by transfection of empty vector plasmids and GEF-H1 plasmids.
Transfection of short hairpin RNA
To stably silence GEF-H1, cells were transfected with a set of short hairpin RNA constructs against human GEF-H1 and pRS-shGEF-H1 (Shanghai GeneChem Company, Shanghai, People’s Republic of China). pRS vector was used as a control.
Immunofluorescence staining
After fixing in 10% formaldehyde, the pathological slides were deparaffinized in xylene and rehydrated in decreasing concentrations of ethanol to water. Then all the reactions were carried out as described.21 Sections were immunostained with GEF-H1 antibody (sc-134827) (1:200). All slices were independently assessed by two experienced pathologists who were ignorant of patients’ clinical pathology and other information. GEF-H1 expression level was evaluated via positive staining proportion and intensity of tumor cells. Slices with inconsistent results were reexamined by the original two pathologists and a senior pathologist until a consensus.
Statistical analysis
Chi-square test was used to analyze the relationship between GEF-H1 overexpression and clinicopathological variables. All data were analyzed with SPSS17.0 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism Version 6.0 (GraphPad Software, Inc., La Jolla, CA, USA). Difference was analyzed by analysis of variance test. Statistical significance was defined as P<0.05. All experiments were repeated three times.
Results
mRNA and protein expression of GEF-H1 in melanoma tissues and adjacent tissues
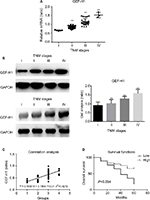
To test if there was a difference in GEF-H1 in melanoma tissues and adjacent tissues, the mRNA levels were measured by real-time PCR and the protein levels were measured by Western blot (Figure 1A and B). Also we did the immunohistochemical staining for GEF-H1 expression in melanoma (Figure 1C). We found that the expression of GEF-H1 in melanoma tissues was much higher than that in adjacent tissues of the same person. We classified 60 patients with melanoma according to the literature,22,23 and the results showed that the expression of GEF-H1 was correlated with melanoma prognosis (Table 1).
mRNA and protein expression of GEF-H1 in melanoma tissues
To determine whether there was a difference of GEF-H1 expression in melanoma tissues, the mRNA levels were measured by real-time PCR and the protein levels were measured by Western blot (Figure 2A and B). Also by correlation analyses we found that the expression of GEF-H1 was related to the prognosis of melanoma (Figure 2C). As shown, the expression levels of GEF-H1 were distinctly increased with the development of melanoma. It means that melanoma tissue samples with high expression of GEF-H1 tend to be with higher pathological stage. We also evaluated in the 60 cases the prognostic value of GEF-H1 positive on overall survival. As shown by the Kaplan–Meier analysis and log-rank test (Figure 2D), we found that patients with high expression of GEF-H1 had a significantly worse overall survival than those with low expression. The results indicated that the expression of GEF-H1 was increased with the shortened survival, which suggested that GEF-H1 may play an important role in the progression of melanoma.
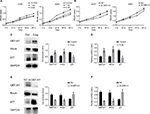
GEF-H1 promoted the proliferation of melanoma cells by GEF-H1/RhoA pathway
As we all know, GEF-H1/RhoA pathway is associated with cancer cells proliferation. We were interested that if GEF-H1 played an important role in melanoma cells growth. By MTT assay, the effects of GEF-H1 on A375 and A875 cell growth were detected. We found that the proliferation of cells was induced in a concentration-dependent manner of GEF-H1 (Figure 3A). Then, the effect of GEF-H1 knockdown was observed in cells/si-GEF-H1 and cells/si-NC (negative control). Results showed that there was a significant difference between si-NC groups and si-GEF-H1 groups in cells proliferation (Figure 3B). These indicated that GEF-H1 could promote proliferation of A375 and A875 cells in a concentration-dependent manner. Since we know p21 is a key downstream protein of GEF-H1/RhoA pathway, we examined the expression of RhoA and p21 by Western blot and real-time reverse transcription PCR. Results showed that when A375 cells were transfected with GEF-H1, RhoA was increased and p21 was decreased in both mRNA and protein levels (Figure 3C–F). These results mean that GEF-H1 can promote melanoma cells proliferation.
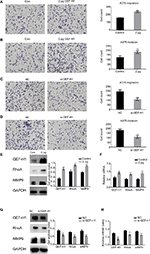
GEF-H1 promoted the metastasis of melanoma cells by GEF-H1/RhoA pathway
To study whether GEF-H1 is involved in migration and invasion of tumor cells, transwell assay (with or without matrigel) was performed. Results showed that GEF-H1 significantly promoted the invasion and migration potential of A375 cells in a dose-dependent manner (Figure 4A and B). In addition, we found the cells metastasis was inhibited after transfected with si-GEF-H1 (Figure 4C and D). Subsequently, MMP9, the indicator of metastasis, was tested at protein and mRNA levels, respectively. With the increase of GEF-H1, MMP9 expression significantly increased, which implied that GEF-H1 promoted the metastasis potential of A375 cells (Figure 4E and F). Then, the effect of GEF-H1 knockdown was observed with A375/si-GEF-H1 and A375/si-NC (negative control) cell lines. Results showed that, compared with A375/si-NC (negative control) cell lines, there was a significant difference in mRNA and protein levels of MMP9 (Figure 4G and H). These results confirmed that GEF-H1 can promote the metastasis of melanoma cells.
The earlier results indicated that GEF-H1 may be a valuable biomarker in degree of melanoma and evaluation prognostic following surgery. Also GEF-H1 can promote the proliferation and metastasis of melanoma.
Discussion
Worldwide, the rate of new cases of melanoma has been rapidly increasing for many years, and the current 5-year survival rate for patients is very low.24–26 Surgical resection is considered to be the most effective method of treatment; however, it cannot prolong the survival of patients significantly.27,28 Consequently, it is necessary to explore sensitive and specific molecular markers associated with diagnosis and progression of melanoma.
Since activating mutations in the BRAF oncogene are present in >70% of melanomas and >90% of which are BRAFV600E, BRAFV600E has been known as a target of therapy of melanoma.29–32 Moreover, reports showed that GEF-H1 expression increased in BRAFV600E-transformed cells.18 We wonder if GEF-H1 plays an important role in melanoma.
GEF-H1 is known to be associated with cytoskeletal structure, microtubules, and actin cytoskeleton.33,34 GEF-H1 promotes Rho activity through catalyzing the exchange of GDP for GTP to generate the activated form of Rho and is involved in the regulation of RhoA; hence, it has been characterized as a RhoA-specific GEF.35–41 GEF-H1 was reported to be highly expressed in several human malignancies.14,42,43 However, only one report showed that modulation of GEF-H1 can induce signaling in brain metastatic melanoma cells.44 In this study, we first found that GEF-H1 showed a dramatically higher expression in melanoma. Real-time PCR and Western blot analyses of the 60 cases we detected showed that the mRNA and protein levels of GEF-H1 in melanoma tissues were 35.87% (P<0.01) and 25.86% (P<0.01), respectively, higher than those in normal tissues. Through the literature,22,23 we learned that the development and prognosis of melanoma are closely related to primary tumor ulceration, lymph node metastases, distant metastases, and tumor thickness, and melanoma can be grouped into four stages based on these clinical features. Through the chi-square test, we found that there was a correlation between GEF-H1 and the TNM stage of melanoma. Our study also identified that GEF-H1 is associated with the grades and overall survival of melanoma patients.
GEF-H1 has been reported to contribute to the growth and survival of cancer cell lines by harboring stabilizing p53 mutations,14 serving to coordinate Rho-, Rac-, and Cdc42-mediated signaling pathways45 and regulating the endomitosis in megakaryocytes.46 In our experiments, we found that GEF-H1 can promote the proliferation of melanoma cell lines – A375 and A875. This regulation function was achieved by changing the mRNA and protein contents of RhoA and p21. In addition, many reports showed that GEF-H1 is activated by stiff collagen matrices, and its exchange activity is required for RhoA activation.47,48 GEF-H1 expression is associated with cell migration and invasion in two-dimensional and three-dimensional matrices.48,49 GEF-H1 has been reported to contribute to directional cell migration by regulating β1 integrin surface levels and activity50; overexpression in hepatocellular carcinoma promotes cell motility via activation of RhoA signaling12 and is related to metastasis of mouse neuroblastoma cells51 and prostate cancer cells.52 MMP9 is closely related to the invasion and metastasis of cells, which can be regulated by RhoA.53 In our report, we found that GEF-H1 can promote the expression of MMP9. Hence, we considered that GEF-H1 can promote melanoma cell metastasis through MMP9.
Conclusion
To the best of our knowledge, this is the first study to detect the expression patterns and clinical significance of GEF-H1 at transcriptional and translational levels in 60 cases of melanoma tissues. Moreover, we also found that GEF-H1 can promote the proliferation and metastasis of melanoma. Therefore, GEF-H1 may be a valuable biomarker for assessing the degree and prognosis of melanoma following surgery.
Disclosure
The authors report no conflicts of interest in this work.
References
Tsao H, Chin L, Garraway LA, Fisher DE. Melanoma: from mutations to medicine. Genes Dev. 2012;26(11):1131–1155. | ||
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. | ||
Grazia G, Penna I, Perotti V, Anichini A, Tassi E. Towards combinatorial targeted therapy in melanoma: from pre-clinical evidence to clinical application (review). Int J Oncol. 2014;45(3):929–949. | ||
Ascierto PA, Grimaldi AM, Acquavella N, et al. Future perspectives in melanoma research. Meeting report from the “melanoma bridge. Napoli, December 2nd-4th 2012”. J Transl Med. 2013;11:137. | ||
Leong SP, Mihm MC Jr, Murphy GF, et al. Progression of cutaneous melanoma: implications for treatment. Clin Exp Metastasis. 2012;29(7):775–796. | ||
Seliger B. The non-classical antigens of HLA-G and HLA-E as diagnostic and prognostic biomarkers and as therapeutic targets in transplantation and tumors. Clin Transpl. 2013:465–472. | ||
von Thun A, Preisinger C, Rath O, et al. Extracellular signal-regulated kinase regulates RhoA activation and tumor cell plasticity by inhibiting guanine exchange factor H1 activity. Mol Cell Biol. 2013;33(22):4526–4537. | ||
Krendel M, Zenke FT, Bokoch GM. Nucleotide exchange factor GEF-H1 mediates cross-talk between microtubules and the actin cytoskeleton. Nat Cell Biol. 2002;4(4):294–301. | ||
Matsuzawa T, Kuwae A, Yoshida S, Sasakawa C, Abe A. Enteropathogenic Escherichia coli activates the RhoA signaling pathway via the stimulation of GEF-H1. EMBO J. 2004;23(17):3570–3582. | ||
Ren Y, Li R, Zheng Y, Busch H. Cloning and characterization of GEF-H1, a microtubule-associated guanine nucleotide exchange factor for Rac and Rho GTPases. J Biol Chem. 1998;273(52):34954–34960. | ||
Aznar S, Fernández-Valerón P, Espina C, Lacal JC. Rho GTPases: potential candidates for anticancer therapy. Cancer Lett. 2004;206(2):181–191. | ||
Cheng IK, Tsang BC, Lai KP, et al. GEF-H1 over-expression in hepatocellular carcinoma promotes cell motility via activation of RhoA signalling. J Pathol. 2012;228(4):575–585. | ||
Mizuarai S, Yamanaka K, Kotani H. Mutant p53 induces the GEF-H1 oncogene, a guanine nucleotide exchange factor-H1 for RhoA, resulting in accelerated cell proliferation in tumor cells. Cancer Res. 2006;66(12):6319–6326. | ||
Liao YC, Ruan JW, Lua I, et al. Overexpressed hPTTG1 promotes breast cancer cell invasion and metastasis by regulating GEF-H1/RhoA signalling. Oncogene. 2012;31(25):3086–3097. | ||
Brecht M, Steenvoorden AC, Collard JG, et al. Activation of gef-h1, a guanine nucleotide exchange factor for RhoA, by DNA transfection. Int J Cancer. 2005;113(4):533–540. | ||
Frolov A, Chahwan S, Ochs M, et al. Response markers and the molecular mechanisms of action of Gleevec in gastrointestinal stromal tumors. Mol Cancer Ther. 2003;2(8):699–709. | ||
Marcotte R, Brown KR, Suarez F, et al. Essential gene profiles in breast, pancreatic, and ovarian cancer cells. Cancer Discov. 2012;2(2):172–189. | ||
Cullis J, Meiri D, Sandi MJ, et al. The RhoGEF GEF-H1 is required for oncogenic RAS signaling via KSR-1. Cancer Cell. 2014;25(2):181–195. | ||
Kemper K, Krijgsman O, Cornelissen-Steijger P, et al. Intra- and inter-tumor heterogeneity in a vemurafenib-resistant melanoma patient and derived xenografts. EMBO Mol Med. 2015;7(9):1104–1118. | ||
Yang S, Evens AM, Prachand S, et al. Mitochondrial-mediated apoptosis in lymphoma cells by the diterpenoid lactone andrographolide, the active component of Andrographis paniculata. Clin Cancer Res. 2010;16(19):4755–4768. | ||
Patel D, Chaudhary J. Increased expression of bHLH transcription factor E2A (TCF3) in prostate cancer promotes proliferation and confers resistance to doxorubicin induced apoptosis. Biochem Biophys Res Commun. 2012;422(1):146–151. | ||
Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27(36):6199–6206. | ||
Guo J, Qin S, Liang J, et al; written on behalf of Chinese Society of Clinical Oncology (CSCO) Melanoma Panel. Chinese guidelines on the diagnosis and treatment of melanoma (2015 edition). Chin Clin Oncol. Epub 2015 Dec 17. | ||
Becker D, Mihm MC, Hewitt SM, Sondak VK, Fountain JW, Thurin M. Markers and tissue resources for melanoma: meeting report. Cancer Res. 2006;66(22):10652–10657. | ||
Balch CM, Buzaid AC, Soong SJ, et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol. 2001;19(16):3635–3648. | ||
Schuchter LM. Review of the 2001 AJCC staging system for cutaneous malignant melanoma. Curr Oncol Rep. 2001;3(4):332–337. | ||
Masci P, Borden EC. Malignant melanoma: treatments emerging, but early detection is still key. Cleve Clin J Med. 2002;69(7):529, 33–34, 36–38 passim. | ||
Schmid-Wendtner MH, Wendtner CM, Sander C, Thetter O, Volkenond M. Early detection of lymph node metastases by 7.5 MHz-ultrasound examination in a patient with primary malignant melanoma of the lung. Eur J Dermatol. 2000;10(2):143–145. | ||
Puntervoll HE, Molven A, Akslen LA. Frequency of somatic BRAF mutations in melanocytic lesions from patients in a CDK4 melanoma family. Pigment Cell Melanoma Res. 2014;27(1):149–151. | ||
Jang S, Atkins MB. Treatment of BRAF-mutant melanoma: the role of vemurafenib and other therapies. Clin Pharmacol Ther. 2014;95(1):24–31. | ||
Koop A, Satzger I, Alter M, Kapp A, Hauschild A, Gutzmer R. Intermittent BRAF-inhibitor therapy is a feasible option: report of a patient with metastatic melanoma. Br J Dermatol. 2014;170(1):220–222. | ||
Stadelmeyer E, Heitzer E, Resel M, Cerroni L, Wolf P, Dandachi N. The BRAF V600K mutation is more frequent than the BRAF V600E mutation in melanoma in situ of lentigo maligna type. J Invest Dermatol. 2014;134(2):548–550. | ||
Yu L, Quinn DA, Garg HG, Hales CA. Heparin inhibits pulmonary artery smooth muscle cell proliferation through guanine nucleotide exchange factor-H1/RhoA/Rho kinase/p27. Am J Respir Cell Mol Biol. 2011;44(4):524–530. | ||
Rossman KL, Der CJ, Sondek J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol.2005;6(2):167–180. | ||
Abiko H, Fujiwara S, Ohashi K, et al. Rho guanine nucleotide exchange factors involved in cyclic-stretch-induced reorientation of vascular endothelial cells. J Cell Sci. 2015;128(9):1683–1695. | ||
Momboisse F, Ory S, Ceridono M, et al. The Rho guanine nucleotide exchange factors Intersectin 1L and beta-Pix control calcium-regulated exocytosis in neuroendocrine PC12 cells. Cell Mol Neurobiol. 2010;30(8):1327–1333. | ||
Lutz S, Freichel-Blomquist A, Rümenapp U, Schmidt M, Jakobs KH, Wieland T. p63RhoGEF and GEFT are Rho-specific guanine nucleotide exchange factors encoded by the same gene. Naunyn Schmiedebergs Arch Pharmacol. 2004;369(5):540–546. | ||
Ishimaru S, Hama C. [Guanine nucleotide exchange factors for Rho family GTPases: specific mediators for a variety of signals]. Tanpakushitsu Kakusan Koso. 2004;49(3 suppl):324–330. Japanese. | ||
Schmidt A, Hall A. Guanine nucleotide exchange factors for Rho GTPases: turning on the switch. Genes Dev. 2002;16:1587–1609. | ||
Li R, Zheng Y. Residues of the Rho family GTPases Rho and Cdc42 that specify sensitivity to Dbl-like guanine nucleotide exchange factors. J Biol Chem. 1997;272(8):4671–4679. | ||
Olson MF. Guanine nucleotide exchange factors for the Rho GTPases: a role in human disease? J Mol Med (Berl). 1996;74(10):563–571. | ||
Biondini M, Duclos G, Meyer-Schaller N, Silberzan P, Camonis J, Parrini MC. RalB regulates contractility-driven cancer dissemination upon TGFbeta stimulation via the RhoGEF GEF-H1. Sci Rep. 2015;5:11759. | ||
Ridgway LD, Wetzel MD, Ngo JA, Erdreich-Epstein A, Marchetti D. Heparanase-induced GEF-H1 signaling regulates the cytoskeletal dynamics of brain metastatic breast cancer cells. Mol Cancer Res. 2012;10(6):689–702. | ||
Ridgway LD, Wetzel MD, Marchetti D. Modulation of GEF-H1 induced signaling by heparanase in brain metastatic melanoma cells. J Cell Biochem. 2010;111(5):1299–1309. | ||
Zenke FT, Krendel M, DerMardirossian C, King CC, Bohl BP, Bokoch GM. p21-activated kinase 1 phosphorylates and regulates 14-3-3 binding to GEF-H1, a microtubule-localized Rho exchange factor. J Biol Chem. 2004;279(18):18392–18400. | ||
Gao Y, Smith E, Ker E, et al. Role of RhoA-specific guanine exchange factors in regulation of endomitosis in megakaryocytes. Dev Cell. 2012;22(3):573–584. | ||
Wozniak MA, Desai R, Solski PA, Der CJ, Keely PJ. ROCK-generated contractility regulates breast epithelial cell differentiation in response to the physical properties of a three-dimensional collagen matrix. J Cell Biol. 2003;163(3):583–595. | ||
Heck JN, Ponik SM, Garcia-Mendoza MG, et al. Microtubules regulate GEF-H1 in response to extracellular matrix stiffness. Mol Biol Cell. 2012;23(13):2583–2592. | ||
Tsapara A, Luthert P, Greenwood J, Hill CS, Matter K, Balda MS. The RhoA activator GEF-H1/Lfc is a transforming growth factor-beta target gene and effector that regulates alpha-smooth muscle actin expression and cell migration. Mol Biol Cell. 2010;21(6):860–870. | ||
Pollock JK, Verma NK, O’Boyle NM, Carr M, Meegan MJ, Zisterer DM. Combretastatin (CA)-4 and its novel analogue CA-432 impair T-cell migration through the Rho/ROCK signalling pathway. Biochem Pharmacol. 2014;92(4):544–557. | ||
Tsuji T, Ohta Y, Kanno Y, Hirose K, Ohashi K, Mizuno K. Involvement of p114-RhoGEF and Lfc in Wnt-3a- and dishevelled-induced RhoA activation and neurite retraction in N1E-115 mouse neuroblastoma cells. Mol Biol Cell. 2010;21(20):3590–3600. | ||
Wells CM, Whale AD, Parsons M, Masters JR, Jones GE. PAK4: a pluripotent kinase that regulates prostate cancer cell adhesion. J Cell Sci. 2010;123(pt 10):1663–1673. | ||
Bhattacharyya S, Tobacman JK. Arylsulfatase B regulates colonic epithelial cell migration by effects on MMP9 expression and RhoA activation. Clin Exp Metastasis. 2009;26(6):535–545. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.