Back to Journals » Cancer Management and Research » Volume 13
GSK-3β Regulates the Expression of P21 to Promote the Progression of Chordoma
Authors Chen L, Zuo Y , Pan R, Ye Z, Wei K, Xia S, Li W, Tan J, Xia X
Received 5 November 2020
Accepted for publication 19 December 2020
Published 11 January 2021 Volume 2021:13 Pages 201—214
DOI https://doi.org/10.2147/CMAR.S289883
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Eileen O'Reilly
Li Chen,1,2 Yi Zuo,2 Ru Pan,2 Zhen Ye,1,2 Kailun Wei,1,2 Shaohuai Xia,1 Wencai Li,1 Jie Tan,2 Xuewei Xia1,2
1Department of Neurosurgery, Affiliated Hospital of Guilin Medical University, Guilin, Guangxi 541001, People’s Republic of China; 2Guangxi Key Laboratory of Brain and Cognitive Neuroscience, Guilin, Guangxi 541004, People’s Republic of China
Correspondence: Xuewei Xia
Department of Neurosurgery, Affiliated Hospital of Guilin Medical University, 15 Lequn Road, Guilin, Guangxi 541001, People’s Republic of China
Tel + (86)-(773) 2800672
Fax + (86)-(773) 2812650
Email [email protected]
Purpose: Chordoma is a rare malignant bone tumor transformed from the remnants of notochord. It is characterized as highly aggressive and locally invasive, difficult to be completely removed by surgery, and has a poor clinical prognosis. Glycogen synthase kinase 3 beta (GSK-3β) is involved in many cellular processes. GSK-3β overexpression has been shown to promote the development of many cancers, according to previous studies. However, the role of GSK-3β in chordoma remains unclear.
Methods: Immunohistochemistry (IHC) and Western blotting (WB) were performed on clinical specimens to measure GSK-3β expression in chordoma, and immunofluorescence and quantitative real-time polymerase chain reaction (QRT-PCR) were performed to examine the expression of GSK-3β and P21 in cell lines. Cell proliferation was detected by the CCK-8 assay and colony formation analysis, cell migration and invasion checked by Transwell experiments, and cell apoptosis was determined by Annexin V/propidium iodide staining. P21 was predicted as a downstream target gene of GSK-3β using STRING and UNIHI databases. Moreover, we used immunoprecipitation to confirm that GSK-3β and P21 interacted with each other. The double luciferase reporter gene assay showed that GSK-3β could regulate the promoter activity of P21. Finally, the role of the GSK-3β -P21 pathway in chordoma tumorigenesis was analyzed in vivo in nude mice.
Results: Our study showed that GSK-3β was significantly higher in chordoma tissues than in paracancer tissues, and siRNA knockdown of GSK-3β inhibited chordoma cell proliferation and promoted cell apoptosis. Additionally, our research found that GSK-3β bound and downregulated the expression of the P21 gene, and the expression of silencing P21 partially reversed the inhibitory effect of knockdown GSK-3β on chordoma. Furthermore, xenografts showed that knockdown GSK-3β inhibited the formation of chordomas in vivo.
Conclusion: Our results indicated that the GSK-3β-P21 axis may be an important signaling pathway for the occurrence and development of chordoma, providing a new therapeutic target for the clinical treatment of this disorder.
Keywords: GSK-3β, chordoma, P21, apoptosis
Introduction
Chordoma, considered to be a rare malignant bone tumor transformed from the remnants of the notochord, is aggressive and locally invasive, and is characterized by a poor clinical prognosis.1 Chordoma mainly affects the sacrum (50–60%) and the base of the skull (25–35%), but a small number of cases occur on the vertebral axis (5%).2 The incidence of chordoma in the population is 0.089 per 100,000 people.3 Clinically, due to the localized invasion of chordoma, surgery is generally used to maximize the resection to prevent and treat the second recurrence of the tumor. However, due to the characteristics of its intracranial growth, it is very difficult to achieve complete tumor resection, and surgery is often accompanied by damage to adjacent structures such as important nerves and blood vessels at the base of the skull. As a result, the prognosis of patients with chordoma is poor, and percentage rates of the recurrence and disability are high.4 The overall median survival time is only 6.29 years, and the 5-year and 10-year relative survival percentages are 67.6% and 39.9%,5 respectively. Furthermore, chordoma is not sensitive to conventional radiotherapy and chemotherapy.6 There is therefore an urgent need to identify the molecular mechanisms involving the occurrence and progression of chordoma, which is of great importance for early diagnosis to improve the treatment effects and prognosis of patients.
Glycogen synthase kinase 3β (GSK-3β) is a key enzyme involved in glycogen metabolism.7 However, it has also been reported to regulate many different cell functions, including metabolic and signal transduction pathways and the modification of structural proteins, cancer progression, bipolar disorder, Alzheimer’s disease, and non-insulin-dependent diabetes mellitus.8,9 Previous studies reported that GSK-3β acted on different tumors to regulate Myc and cyclin D1,10 which are considered as potential tumor suppressors. Studies have recently shown that abnormal expression of GSK-3β is closely related to tumorigenesis. For example, abnormally low GSK-3β levels in breast cancer promote cell migration and inhibit autophagy through the AMPK pathway.11 The inhibition of GSK-3β can prevent the cell survival of glioblastomas,12 ovarian cancer,13 osteosarcomas,14 pancreatic cancer,15 and colorectal cancer.16 As a result, GSK-3β is therefore involved in the pathogenesis of a variety of human cancers.
The role of GSK-3β in chordoma and the efficacy of targeted GSK-3β signal transduction therapy have not been reported. We therefore characterized the expression of GSK-3β in the tissues of chordoma patients, and also evaluated its role in the proliferation, migration, and invasion of chordoma cells. Furthermore, we also characterized the potential regulatory mechanism of targeted inhibition of GSK-3β in chordoma cells.
The p21 WAF1/CIP1 gene is located on chromosomes 6p21.2.17 It codes for a specific cyclin-dependent kinase inhibitor belonging to the cyclin-dependent kinase inhibitor CIP family, which plays an important role in various metabolic regulatory processes such as cell proliferation, apoptosis, cell cycle regulation, and DNA repair.18 It has been reported that the target of p21WAF1 is CDK-cyclin, which regulates eukaryotic cell progression and proliferating cell nuclear antigen (PCNA) through the cell cycle. P21 forms a complex with CDK-cyclin to inhibit its kinase activity, and forms a complex with PCNA to affect DNA synthesis, thus suppressing cell cycle progression.19 Studies have reported that P21 is downregulated in most cancers, leading to the continuation of cell cycle processes that causing the development of tumors; and overexpression of P21 in cancer stops tumor progression.20 For example, the targeted activation of P21 inhibits the proliferation of hepatoma cells via RNAi.21 The downregulation of P21 expression targeted by the activation of PI3K/AKT results in the occurrence of colorectal cancer.22 It has been found that the downregulation of P21 expression promotes the development of chordoma.23 However, the specific molecular mechanism of P21 regulation of chordoma remains unknown.
To better understand the function of GSK-3β in chordoma, we determined whether GSK-3β modulated the P21 pathway to promote the progression of chordoma, and whether targeting GSK-3β and/or the P21 pathway might be an effective strategy for the treatment of chordoma. First, we conducted immunohistochemistry analyses of tissue samples to assess whether the expression of GSK-3β was associated with the clinical characteristics of patients with chordoma. Then, cell viability and apoptosis assays were conducted to evaluate the relationship between GSK-3β and chordoma cell survival. Finally, nude mouse models were used to evaluate the in vivo role of GSK-3β during tumorigenesis.
Methods
Chordoma Tissue Samples
A total of 41 pairs of Intracranial clival chordoma which is an intracranial bone chordoma and normal para-cancerous tissue specimens were obtained from patients, which were surgically removed at the Affiliated Hospital of Guilin Medical University. None of the enrolled patients received any radiotherapy or chemotherapy before surgical removal of the tumors. Before participating in this study, each patient signed a written informed consent form. The study was approved by the ethics committee of the Affiliated Hospital of Guilin Medical University, which was performed in accordance with the Declaration of Helsinki.
Immunohistochemistry (IHC) Staining
Paraffin-embedded tissue sections, 3 mm thick, were used to analyze GSK-3β. The sections were dewaxed and rehydrated, then incubated in 3% H2O2 for 30 min to block endogenous peroxidase activity. Next, the sections were put into a pressure cooker to regenerate the antigens using citrate repair solution (pH = 8.0), then treated with 5% goat serum for 1 h to block nonspecific binding, followed by incubation with primary antibody overnight at 4°C. The enhanced secondary antibody was added dropwise and incubated for 30 min at room temperature. After treatment, the slides were washed three times with PBST (phosphate-buffered solution) for 5 min each time. The final reaction used diaminobenzidine tetrahydrochloride, followed by counterstaining with hematoxylin and 1% hydrochloric acid alcohol for differentiation analyses. Three independent pathologists, who were unaware of the patients’ histopathological characteristics and other information, recorded the staining results. The staining results were scored according to the following criteria: 0: staining <5%; 1: staining 6–25%; 2: staining 26–50%; 3: staining 51–75%; and 4: staining >75%. Patients with a score <1 were confirmed as negative staining; otherwise, samples were confirmed as having positive staining.
Cell Culture and Transfection
The human chordoma cell line, U-CH1, was obtained from Johns Hopkins University (Baltimore, MD, USA), which was approved by the ethics committee of the Affiliated Hospital of Guilin Medical University. JHC7 cells were purchased from the American Type Culture Collection (Manassas, VA, USA). U-CH1 cells were cultured in IMDM-RMPI medium supplemented with 10% fetal bovine serum (FBS) and 1% penicillin–streptomycin. JHC7 cells were cultured in DMEM medium containing 10% FBS and 1% penicillin–streptomycin. The two cell lines were maintained in a humid environment with 5% CO2 at 37°C. Lithium chloride (LiCl), a classic GSK-3β inhibitor, was purchased from Sigma-Aldrich (St. Louis, MO, USA). The si-P21, si-GSK-3β, and the negative control were synthesized by Shanghai Biotech Company Gemma Gene (Shanghai, China). The sequences of siRNA were as follows:
The si-GSK-3β-1 sense: 5ʹ-GGUAUAUCAAGCCAAACUUTT-3ʹ and antisense: 5ʹ-AAGUUUGGCUUGAUAUACCTT-3ʹ. The si-GSK-3β-2 sense: 5ʹ-GCUAGAUCACUGUAACAUATT-3ʹ and antisense 5ʹ-UAUGUUACAGUGAUCUAGCTT-3ʹ. The si-GSK-3β-3 sense: 5ʹ-GGAAACAGUAUACAGAGUUTT-3ʹ and-antisense: 5ʹ-AACUCUGUAUACUGUUUCCTT-3ʹ. The si-P21 sense: 5ʹ-GAUGGAACUUCGACUUUGUTT-3ʹ and antisense: 5ʹ-ACAAAGUCGAAGUUCCAUCTT-3ʹ. The negative control sense: 5ʹ-UUCUCCGAACGUGUCACGUTT-3ʹ and antisense: 5ʹ-ACGUGACACGUUCGGAGAATT-3ʹ. Lipofectamine 3000 (lipo3000) (Invitrogen, Carlsbad, CA, USA) was used for the transfections. After transfection for 48 h, the efficiency of lipo3000 for siRNA was evaluated by fluorescence microscopy (Olympus U-RFL-T, Tokyo, Japan), and the silencing effect was further verified by qRT-PCR and Western blotting experiments.
RNA Extraction and qRT-PCR
Total RNA in the cells was extracted with TRIzol reagent (Invitrogen), and reverse transcription was performed using the Thermo Fisher Kit RevertAid First Stand cDNA Synthesis Kit (Invitrogen). The qRT-PCR reaction was amplified using the SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA) with a 7900 Real-time PCR System (Applied Biosystems), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the housekeeping gene. These primers were designed and synthesized by The Beijing Genomics Institute (BGI, Shenzhen, China). The primer sequences were as follows:
The GSK-3β forward: 5ʹ-ATTTTCCAGGGGATAGTGGTGT-3ʹ and reverse: 5ʹ-GGTCGGAAGACCTTAGTCCAAG-3ʹ. The P21 forward: 5ʹ-TGTCCGTCAGAACCCATGC-3ʹ and reverse: 5ʹ-AAAGTCGAAGTTCCATCGCTC-3ʹ. The GAPDH forward: 5ʹ-CAGGAGGCATTGCTGATGAT-3ʹ and reverse: 5ʹ-GAAGGCTGGGGCTCATTT-3ʹ.
Protein Extraction and Western Blotting
Western blotting was used to detect protein expression. A pre-cooled radioimmunoprecipitation assay buffer (RIPA buffer) (Beyotime, Shanghai, China) with protease inhibitors was used to separate protein samples from cells, and the bicinchoninic acid protein assay kit (Thermo Fisher Scientific, San Jose, CA, USA) was used to determine protein concentrations. The proteins were then resolved using 10% SDS-PAGE for 2 h at 100 V and then transferred to a polyvinylidene fluoride membrane (Millipore, Billerica, MA, USA). The membrane was then incubated with 5% bovine serum albumin (BSA) at room temperature for 1 h. After washing with TBST (1× Tris-buffered saline with Tween 20), the membrane was blocked for 60 min, and the primary antibody was added and incubated for 1 h at room temperature. Specific antibodies against GSK-3β (#9315S; Cell Signaling Technology, Danvers, MA, USA), p21 (1: 1,000, #2947S; Cell Signaling Technology), and GAPDH (1:1,000, #2118S; Cell Signaling Technology) were used, followed by horseradish peroxidase-labeled secondary antibody (1: 2,000, #7074S; Cell Signaling Technology). GAPDH was used as an internal control. The protein level was quantified using gray values, and each experiment was repeated three times.
The CCK-8 Assay
A CCK-8 kit (Dojindo Molecular Technologies, Shiga, Japan) was used to detect cell proliferation activity. Transfection was with si-GSK-3β for 48h (Beyotime). Using 2,000 cells per well, seeded into three replicate wells for each sample in a 96-well plate. Ten microliters of CCK-8 reagent was added to each well and mixed with cell culture medium, followed by further incubation for 1–4 h. The absorbance at 450 nm was then measured using a microplate reader (iMark Microplate Reader; Bio-Rad, Hercules, CA, USA), and the proliferation curve was drawn using the absorbance values.
Invasion and Migration Assay
Transwell chambers (Corning, Corning, NY, USA) were used for cell migration and invasion assays. A total of 100 µL Matrigel (Corning) was added to the upper chamber and incubated at 37°C for 1 h for the invasion test. About 0.5 µL of cell suspension was inoculated into the upper chamber with serum-free DMEM, and the complete medium containing 10% FBS was added to the lower chamber. After incubation, the cells were stained with Crystal Violet (Beyotime) for 20 min, and images were captured using an upright microscope (BX53, Olympus, Tokyo, Japan) and digital camera. All experiments were conducted in triplicate.
Analysis of Cells by Flow Cytometry
The cells were transfected with si-GSK-3β or cell-specific disordered si-Scramble for 48 h, then processed and collected according to the kit instructions. The cells were then washed twice with cold phosphate-buffered saline (PBS) and resuspended in 1× saline according to the kit instructions. Five microliters of FITC annexin V (BD Biosciences) was added to stain the cells for 10 min in the dark. Then, 5 μL of propidium iodide (PI) (BD Biosciences) was added and the cells were incubated at room temperature for 15 min in the dark. A total of 400 μL 1x binding buffer was then added to each sample, and cell apoptosis was analyzed by flow cytometry (BD FACSCalibur, BD Biosciences). Each experimental assay was conducted three times.
Colony Formation Assay
After transfection using siRNA for 48 h, 500 cells were inoculated into a 6-well culture plate and incubated at 37°C in 5% CO2 and cultured in complete growth medium without antibiotics for 1–2 weeks. The colonies were fixed with 4% paraformaldehyde and stained with Crystal Violet (Beyotime), and then a colony count was performed to calculate the number of colonies composed of at least 50 cells. Each experiment was conducted three times.
Immunofluorescence
The expression of GSK-3β protein was evaluated by immunofluorescence. Briefly, the cells were transfected with si-GSK-3β or si-Scramble for 48 h. Then, the cells were incubated in 4% paraformaldehyde, fixed in ice-cold paraformaldehyde, blocked with 5% BSA, combined with antibody against GSK-3β or p21 (1:800) and then incubated overnight at 4°C, followed by incubation with 488-conjugated AlexaFluor® IgG and/or 594-conjugated AlexaFluor® IgG (ZSGB-BIO, Beijing, China) for 2 h. The slides were then stained and mounted with 4ʹ,6-diamidino-2-phenylindole containing anti-fluorescence quenching reagent. An Olympus inverted fluorescence microscope was used to capture images, which were analyzed using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Co-Immunoprecipitation (CoIP)
The interaction of P21 and GSK-3β in U-CH1 and JHC7 cells was evaluated by CoIP. The cells were lysed in RIPA buffer containing 1% protease and phosphatase inhibitors. The lysate was incubated with 4 µL each of anti-P21 antibody, anti-IgG antibody, or anti- GSK-3β antibody, followed by addition of 30 µL of protein A agarose beads (Cell Signaling Technology) to capture the antigen-antibody complex. The beads were incubated at 4°C for 3 h, then suspended in 60 μL of loading buffer, followed by collection of the beads and washing four times with 2 mL PBS for 5 min. The suspensions were microcentrifuged for 1 min, followed by Western blotting. The CoIP assay was used to check the association between GSK-3β and P21, and input and IgG were used as positive and negative controls, respectively.
Dual-Luciferase Reporter Assay
The P21 gene promoter sequence from −2000 to +100 was inserted into the reporter plasmid containing firefly luciferase. The specific sequence can be found in the supplementary document. The PCR method was used to amplify this sequence, and then it was cloned into the pPRO-RB-REPORT vector (RIOBIO, Guangzhou, China) containing the firefly luciferase wild-type reporter gene. Based on previous research, we chose U-CH1 for dual-luciferase reporter gene experiment.24 The vector was then transfected into U-CH1 cells with or without the si- GSK-3β vector. After transfection for 48 h, the cells were harvested, and luciferase activity was measured using the dual-luciferase reporter gene detection system (Promega, Madison, WI, USA), and normalized for Renilla activity.
Nude Mouse Xenograft Assay in vivo
BALB/c female nude mice aged 6–8 weeks were purchased from Yunnan Kunming Research Animal Center (Yunnan, China) for in vivo studies. U-CH1 cells transfected with si- GSK-3β or si-NC (suspending 2×106 cells with a mixture of 100 μL DMEM and 100 μL Matrigel) were injected into the subcutaneous left thigh of nude mice. One week after the injection, the mice were randomly divided into two groups with five mice in each group. The first group was the si-NC cell group, and the second group was the si-GSK-3β group. The formation of subcutaneous tumors was observed approximately on the 10th day after injection. The tumors were measured once a day with a Vernier caliper, and tumor volume was calculated using the formula, V = (width2 × length)/2. Euthanize mice by cervical dislocation according to the guidelines of the Animal Experiment Center of Guilin Medical University. All animal experiments were conducted in accordance with the animal welfare guidelines of Guilin Medical University.
Statistical Analysis
All data analysis and graph drawing were performed using SPSS statistical software for Windows, version 22.0 (SPSS, Chicago, IL, USA) and GraphPad Prism, version 6.0 (GraphPad, San Diego, CA, USA). Continuous variables are expressed as the mean ± standard deviation (SD). The experimental group and the control group were compared using Student’s t-test. The Χ2 test was used to study the relationship between the expression of GSK-3β and the clinical characteristics of patients with chordoma, and P < 0.05 was considered statistically significant.
Results
Patient Characteristics
A total of 41 patients with intracranial clival chordoma were included in this study, including 23 males and 18 females. The average age of onset of patients with chordoma was 57.132 ± 10.327 years. There were seven patients with a family history of cancer, 12 patients with recurrence, and four patients with distant metastasis, 29 of which had tumors >3 cm. The clinical characteristics of the patients were provided in Table 1.
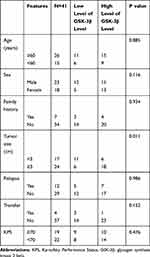 |
Table 1 The Relationships Between the Expression of GSK-3β Relationships Chordoma and Clinical Features |
The Expression of GSK-3β in Chordoma Tissues and Cells
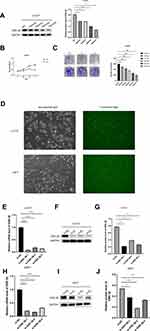
IHC was used to detect the expression of GSK-3β in chordoma and the corresponding adjacent tissues. Figure 1A shows that GSK-3β had strong positive expression in chordoma tissues and weak positive expression in adjacent tissues. The positive percentage of GSK-3β of chordoma tissues was 70.73% (29/41), while in adjacent paracancerous tissues, the positive percentage was only 19.51% (8/41). The protein levels of GSK-3β in chordoma tissues and adjacent tissues were then detected by Western blotting. The results showed that the level of GSK-3β protein in chordoma tissues was significantly higher than that of adjacent tissues (p < 0.01; Figure 1B and C). The expression of GSK-3β protein in chordoma tissues was significantly higher than that in adjacent tissues.
The Relationships Between the Levels of GSK-3β Protein and the Clinical Characteristics of Patients with Chordoma
In our study, we evaluated the relationships between the expression of GSK-3β in patients with chordoma and their clinical parameters. Enrolled patients were divided into a high-level group (n = 24) and a low-level group (n = 17) according to the average expression of the protein GSK-3β in chordoma tissues. The results showed that high levels of GSK-3β positively correlated with tumor size (P = 0.011). However, the level of GSK-3β did not correlate with age, sex, relapse, transfer, family history, or Karnofsky Performance Status (all, p > 0.05; Table 1).
Silencing of GSK-3β Inhibits the Proliferation of Chordoma Cells in vitro
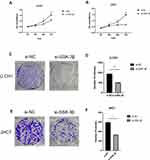
Due to the increasing expression of GSK-3β in chordoma samples, we characterized the biological functions of GSK-3β in chordoma cells. First, for the reliability of the experiment, we select representative U-CH1 cells for LiCl treatment. LiCl is a classical inhibitor of GSK-3β, then confirmed the inhibition using Western blotting, followed by characterizing the proliferation of cells using the CCK-8 and the colony formation assays. The results showed that the expression of GSK-3β, proliferation capacity, and numbers of colonies of cells significantly decreased after lithium chloride treatment (Figure 2A–C). Because of the instability of drugs, we verified the results by silencing GSK-3β with siRNA. We evaluated the efficiency of transfection for 24 h with siRNA in U-CH1 and JHC7 cells using immunofluorescence (Figure 2D). We tested the knockdown efficiency of GSK-3β with qPCR and Western blotting (Figure 2E and F and H and I). We selected the highest efficiency of knockdown to perform the experiments according to these results (Figure 2G and J). We performed the CCK-8 and colony formation assays to determine whether GSK-3β regulated the biological functions of chordoma cells. The results showed that the proliferation capacity and numbers of colonies in U-CH1 and JHC7 cells rapidly decreased after transfection (Figure 3A–F), which supported the conclusion that GSK-3β regulated the proliferation ability of chordoma cells.
Silencing of GSK-3β Accelerates the Apoptosis of Chordoma Cells
To evaluate whether GSK-3β causes apoptosis of chordoma cells, we transfected with si-GSK-3β in U-CH1 and JHC7 to detect the levels of apoptosis. The results showed that silencing of GSK-3β significantly increased the apoptosis rate of these two kinds of cells, suggesting that GSK-3β played an important role in the apoptosis of chordoma cells (Figure 4A and B).
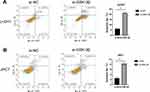 |
Figure 4 The knockdown of GSK-3β induces the apoptosis of chordoma cells. (A and B) Detection of cell apoptosis of U-CH1 and JHC7 cells transfected with si-GSK-3β, **p < 0.01; ***p < 0.001. |
Knockdown of GSK-3β Inhibits the Migration and Invasion of Chordoma Cells
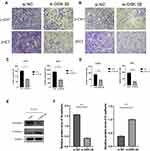
It has been reported that GSK-3β participates in the regulation of the EMT in soft tissue sarcomas,25 endometrial cancer,26 and colorectal cancer.27 However, the relationship between GSK-3β and the EMT in chordoma has not been reported. In the present study, we confirmed that GSK-3β regulated the progression of the EMT in chordoma cells. Compared with the control group transfection with si-NC, the cell migration and invasion significantly decreased in the si-GSK-3β group (Figure 5A–D). Moreover, E-cadherin, a characteristic protein of epithelial cell, was upregulated, while N-cadherin, that of mesenchymal cell, was downregulated after transfection of si-GSK-3β in U-CH1 cells (Figure 5E and F). Our results showed that GSK-3β may play a role in the progress of the EMT in chordoma cells.
GSK-3β Plays a Role in Chordoma by Regulating the Expression of P21
Our preliminary results confirmed the role of GSK-3β in chordoma, but the specific regulation mechanism remained unclear. To further characterize the regulation mechanism, we predicted the protein interaction to identify the target gene regulated by GSK-3β using the Unihi and String online databases.28,29 We filtered P21, a tumor suppressor gene, which is involved in the process of cell cycle apoptosis. According to our previous results, we speculated that GSK-3β may interact with P21 to regulate the expression of P21 in chordoma cells. For this reason, we conducted co-IP to verify whether the two components interacted. We used anti- GSK-3β antibody to precipitate and enrich the P21 protein, and found the expression of it in the GSK-3β complex. The results showed that P21 and GSK-3β interacted. P21 protein also precipitated during the precipitation of GSK-3β (Figure 6A). Next, we transfected chordoma cells with si-GSK-3β followed by Western blotting, qRT-PCR, and immunofluorescence analyses. The results showed that the level of P21 increased in U-CH1 and JHC7 cells after transfection (Figure 6B–E). We knocked down P21 and confirmed the efficiency of this knockdown using qPCR (Figure 6F). In addition, the CCK-8 assay was conducted to confirm that the downregulation of P21 promoted the proliferation of chordoma cells. We also found that the cell viability of the group co-transfected with si-P21 and si-GSK-3β was higher than the group transfected simply with siGSK-3β, so the knockdown of P21 reversed the partial functions of GSK-3β, in chordoma cells (Figure 6G). In addition, the apoptosis of cotransfected cells was distinctly decreased (Figure 6H). Moreover, after the transfection of P21, Western blotting showed that the knockdown of P21 did not affect the level of GSK-3β (Figure 6I). Together, these results showed that P21 suppressed the proliferation of chordoma cells, and that P21 may have been the downstream target of GSK-3β. To further explain how GSK-3β regulated the expression of P21, we performed dual-luciferase reporter assays in U-CH1 and found that silencing GSK-3β increased the promoter activity of P21 (Figure 6J). Combined with the previous results, we concluded that GSK-3β influenced the expression of P21 by regulating the promoter activity of P21. In general, GSK-3β promoted the development of chordomas at least in part by regulating the expression of P21.
Silencing GSK-3β in vivo Inhibits the Development of Chordoma
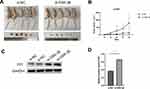
Using a nude mouse xenograft tumor model, we studied the effect of GSK-3β in chordoma cells in vivo. We injected 2 × 106 transfected U-CH1 cells subcutaneously into BALB/c nude mice. Subcutaneous tumor formation was observed on the 10th day, and the tumor volume was measured every 5 days. All nude mice were sacrificed 30 days after injection of U-CH1 cells. The results showed that the tumors in the si-GSK-3β group were much smaller than those in the si-NC group (Figure 7A). The same si-GSK-3β group had a slower tumor growth rate than the si-NC group (Figure 7B). The results of Western blotting showed that the expression of P21 in the si-GSK-3β group was up-regulated compared with the si-NC group (Figure 7C and D).In summary, our experiments showed that silencing GSK-3β expression inhibited the proliferation of chordoma cells in vivo and up-regulated the expression of tumor suppressor gene p21.
Discussion
Chordoma is a rare malignant bone tumor with a high recurrence rate.30 The characteristics of the growth mode and location of chordoma make it difficult to completely remove the tumor. Due to the chemical resistance of chordoma, the clinical effects of radiotherapy and chemotherapy are unsatisfactory because there is no effective drug treatment.4 To improve the prognosis, it is necessary to identify the molecular mechanism of chordoma progression. In the present study, we found that silencing of GSK-3β significantly inhibited proliferation, migration, and invasion of chordoma cells, and promoted cell apoptosis in vitro. The result was also confirmed in animal tumor formation. GSK-3β may therefore participate in the progression of chordoma by regulating the expression of P21.
GSK-3β is often recognized as a key signaling molecule in the PI3K/PTEN/Akt/mTORC1 and Wnt/β-catenin pathways. GSK-3β interacts with Akt and TSC2 to regulate mTORC1. Akt has a negative effect on GSK-3β, because when Akt phosphorylates GSK-3β, GSK-3β cannot phosphorylate TSC2. Because TSC2 does not inhibit mTORC1, it has a proliferative effect. GSK-3β has a negative effect on mTORC1 because it phosphorylates and promotes the activity of TSC2, thereby inhibiting mTORC1, which has an anti-proliferative effect.31 Previous studies have shown that inhibition of GSK-3β suppresses the survival rates of glioblastomas, leukemias, neuroblastomas,12,32 osteosarcomas, melanomas, ovarian cancer,13 prostate cancer,33 and lung cancer.34 These studies further support that increased expression of GSK-3β may be an important indicator of cancer developments, which involves the progression of tumors.
Due to the variety of GSK-3β types involved in specific cell functions, a number of cellular processes may be altered, such as cell proliferation, migration, invasion, and apoptosis. In the present study, we conducted in vitro studies to identify the functions of GSK-3β on the biological behaviors of chordoma cells. The results showed that when the level of GSK-3β decreased, the growth and movement (migration and invasion) of the transfected cells were significantly suppressed, and were accompanied by increasing apoptosis. It has been reported by Ougolkov et al35 that the upregulation of GSK-3β significantly promoted the proliferation and growth of pancreatic cancer cells in vitro. Tang et al36 found that silencing of GSK-3β significantly inhibited the proliferation and growth of glioma cells in vitro. These studies supported our conclusions that GSK-3β may be an oncogene and a therapeutic target for chordoma. In the present study, we also found that P21 gene, the classic tumor suppressor, is the target of GSK-3β, located on human chromosome 6p21.2, with a length of 85 kb, and participates in cellular cycle regulation. There are two specific p53 protein binding sites at 2.4 kb and 8 kb upstream of the P21 gene, one at 1–2 kb upstream of MyoD (myogenic D, myogenic transcription factor), and the other at 50–104 bp upstream of SP1. The expression product of the P21 gene, the P21 protein, is localized to the cell nucleus and consists of 164 amino acids, containing many arginine residues. The N-terminal 21–26 amino acids bind to cyclin D and cyclin E, the C-terminal 124–164 amino acids bind to PCNA, and the intermediate 49–72 amino acids bind to cyclin-dependent kinase 2. A previous study reported that P21 is a potential tumor suppressor.37 It has been reported by Spentzo et al23 that tissue microarrays and immunohistochemical analyses of 50 patients with chordoma cristae showed that SOX9 was widely expressed in chordoma, and higher levels of SOX9 were associated with poor prognoses. Surprisingly, the level of P21 protein was also both high and low in chordoma cristae, and the proliferative ability of high expression of P21 protein in chordoma tissue was significantly lower than that involving low expression of P21 protein; patients with high expression of P21 protein were positively correlated with increased survival. Chen et al38 reported that treatment with MK2206, an AKT-specific inhibitor, mediated the phosphorylation of AKT serine 473, resulting in the overexpression of GSK-3β and the upregulation of its downstream p-p21 (Thr 145), p-p27 (Thr 157), cyclin D1, and inhibition of the expression of p-cyclin D1 (Thr 286), p21, and p27. Our study showed that the knockdown of GSK-3β may lead to high expression of P21 in chordoma cells. In addition, the expression of P21 did not significantly affect that of GSK-3β; thus, it can be seen P21 may be the downstream target gene of GSK-3β in chordomas. Moreover, immunoprecipitation assays, the luciferase reporter gene assays, and mice experiments in vivo have also verified that GSK-3β regulates the expression of P21. In our study, we also found that knocking-out p21 reversed tumor suppression caused by the silencing of GSK-3β in chordomas. GSK-3β may therefore promote the development of chordoma by inhibiting the P21 signaling pathway.
The present study is the first to show the pathway of GSK-3β-P21 in chordomas. However, some limitations still exist. First, the sample size was relatively small, which may reduce the statistical reliability. Second, our experimental results involved xenotransplantation of chordoma cells into nude mice, and the actual clinical status of patients with chordoma may differ. Third, this study did not study the interaction between GSK-3β and the signal transduction pathways that may play a potential role in cancer developments. The progression of chordoma is a complex process regulated by a network composed of various genes, non-coding RNAs, pathways, and proteins. To explain the development and progression of chordoma, further research is needed to identify the genetic regulatory network of chordoma.
Conclusion
In summary, our research shows that GSK-3β can promote the development of chordoma and served as an oncogene in chordoma. The functional role of GSK-3β during chordoma cell proliferation and tumorigenesis was partly elucidated by silencing the expression of p21. The GSK-3β-P21 pathway may therefore become a promising target for the diagnosis and treatment strategy of chordoma.
Acknowledgments
This project was supported by a grant from the National Natural Science Foundation of China (No. 81860449 and No. 81560413) and The Natural Science Foundation for Returned Scholars of Guangxi (2016GXNSFCA380028). We thank International Science Editing (http://www.internationalscienceediting.com) for editing this manuscript.
Author Contributions
Xuewei Xia and Li Chen conceived and designed the study. Li Chen conducted the main experiments. Li Chen and Kailun Wei produced and analyzed the data from the xenograft tumor experiments. Li Chen and Yi Zuo both participated in data analyses and manuscript writing. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Walcott BP, Nahed BV, Mohyeldin A, Coumans JV, Kahle KT, Ferreira MJ. Chordoma: current concepts, management, and future directions. Lancet Oncol. 2012;13(2):e69–76. doi:10.1016/S1470-2045(11)70337-0
2. Stacchiotti S, Casali PG, Lo Vullo S, et al. Chordoma of the mobile spine and sacrum: a retrospective analysis of a series of patients surgically treated at two referral centers. Ann Surg Oncol. 2010;17(1):211–219. doi:10.1245/s10434-009-0740-x
3. Chambers KJ, Lin DT, Meier J, Remenschneider A, Herr M, Gray ST. Incidence and survival patterns of cranial chordoma in the United States. Laryngoscope. 2014;124(5):1097–1102. doi:10.1002/lary.24420
4. Hao S, Song H, Zhang W, et al. Protein phosphatase 2A inhibition enhances radiation sensitivity and reduces tumor growth in chordoma. Neuro Oncol. 2018;20(6):799–809. doi:10.1093/neuonc/nox241
5. McMaster ML, Goldstein AM, Bromley CM, Ishibe N, Parry DM. Chordoma: incidence and survival patterns in the United States, 1973–1995. Cancer Causes Control. 2001;12(1):1–11. doi:10.1023/A:1008947301735
6. Hu W, Yu J, Huang Y, Hu F, Zhang X, Wang Y. Lymphocyte-related inflammation and immune-based scores predict prognosis of chordoma patients after radical resection. Transl Oncol. 2018;11(2):444–449. doi:10.1016/j.tranon.2018.01.010
7. Woodgett JR. Molecular cloning and expression of glycogen synthase kinase-3/factor A. EMBO J. 1990;9(8):2431–2438. doi:10.1002/j.1460-2075.1990.tb07419.x
8. Kaidanovich-Beilin O, Woodgett JR. GSK-3: functional insights from cell biology and animal models. Front Mol Neurosci. 2011;4:40. doi:10.3389/fnmol.2011.00040
9. Domoto T, Pyko IV, Furuta T, et al. Glycogen synthase kinase-3beta is a pivotal mediator of cancer invasion and resistance to therapy. Cancer Sci. 2016;107(10):1363–1372. doi:10.1111/cas.13028
10. Rubinfeld B, Albert I, Porfiri E, Fiol C, Munemitsu S, Polakis P. Binding of GSK3beta to the APC-beta-catenin complex and regulation of complex assembly. Science. 1996;272(5264):1023–1026. doi:10.1126/science.272.5264.1023
11. Guo L, Chen D, Yin X, Shu Q. GSK-3beta promotes cell migration and inhibits autophagy by mediating the AMPK pathway in breast cancer. Oncol Res. 2019;27(4):487–494. doi:10.3727/096504018X15323394008784
12. Dickey A, Schleicher S, Leahy K, Hu R, Hallahan D, Thotala DK. GSK-3beta inhibition promotes cell death, apoptosis, and in vivo tumor growth delay in neuroblastoma Neuro-2A cell line. J Neurooncol. 2011;104(1):145–153. doi:10.1007/s11060-010-0491-3
13. Cao Q, Lu X, Feng YJ. Glycogen synthase kinase-3beta positively regulates the proliferation of human ovarian cancer cells. Cell Res. 2006;16(7):671–677. doi:10.1038/sj.cr.7310078
14. Tang QL, Xie XB, Wang J, et al. Glycogen synthase kinase-3beta, NF-kappaB signaling, and tumorigenesis of human osteosarcoma. J Natl Cancer Inst. 2012;104(10):749–763. doi:10.1093/jnci/djs210
15. Baumgart S, Chen NM, Zhang JS, et al. GSK-3beta governs inflammation-induced NFATc2 signaling hubs to promote pancreatic cancer progression. Mol Cancer Ther. 2016;15(3):491–502. doi:10.1158/1535-7163.MCT-15-0309
16. Park GB, Chung YH, Gong JH, Jin DH, Kim D. GSK-3beta-mediated fatty acid synthesis enhances epithelial to mesenchymal transition of TLR4-activated colorectal cancer cells through regulation of TAp63. Int J Oncol. 2016;49(5):2163–2172. doi:10.3892/ijo.2016.3679
17. el-Deiry WS, Harper JW, O’Connor PM, et al. WAF1/CIP1 is induced in p53-mediated G1 arrest and apoptosis. Cancer Res. 1994;54(5):1169–1174.
18. Wu HL, Li SM, Hu J, et al. Demystifying the mechanistic and functional aspects of p21 gene activation with double-stranded RNAs in human cancer cells. J Exp Clin Cancer Res. 2016;35(1):145. doi:10.1186/s13046-016-0423-y
19. Boulaire J, Fotedar A, Fotedar R. The functions of the CDK-cyclin kinase inhibitor p21WAF1. Pathol Biol (Paris). 2000;48(3):190–202.
20. Abbas T, Dutta A. p21 in cancer: intricate networks and multiple activities. Nat Rev Cancer. 2009;9(6):400–414.
21. Kosaka M, Kang MR, Yang G, Li LC. Targeted p21WAF1/CIP1 activation by RNAa inhibits hepatocellular carcinoma cells. Nucleic Acid Ther. 2012;22(5):335–343. doi:10.1089/nat.2012.0354
22. Hu Z, Long T, Ma Y, et al. Correction to: downregulation of GLYR1 contributes to microsatellite instability colorectal cancer by targeting p21 via the p38MAPK and PI3K/AKT pathways. J Exp Clin Cancer Res. 2020;39(1):125. doi:10.1186/s13046-020-01635-6
23. Chen H, Garbutt CC, Spentzos D, Choy E, Hornicek FJ, Duan Z. Expression and therapeutic potential of SOX9 in chordoma. Clin Cancer Res. 2017;23(17):5176–5186. doi:10.1158/1078-0432.CCR-17-0177
24. Hai B, Pan X, Du C, et al. LncRNA XIST promotes growth of human chordoma cells by regulating miR-124-3p/iASPP pathway. Onco Targets Ther. 2020;13:4755–4765. doi:10.2147/OTT.S252195
25. Abe K, Yamamoto N, Domoto T, et al. Glycogen synthase kinase 3beta as a potential therapeutic target in synovial sarcoma and fibrosarcoma. Cancer Sci. 2020;111(2):429–440. doi:10.1111/cas.14271
26. Chen S, Sun KX, Liu BL, Zong ZH, Zhao Y. The role of glycogen synthase kinase-3beta (GSK-3beta) in endometrial carcinoma: a carcinogenesis, progression, prognosis, and target therapy marker. Oncotarget. 2016;7(19):27538–27551. doi:10.18632/oncotarget.8485
27. Zhang H, Hou W, Wang HL, et al. GSK-3beta-regulated N-acetyltransferase 10 is involved in colorectal cancer invasion. Clin Cancer Res. 2014;20(17):4717–4729. doi:10.1158/1078-0432.CCR-13-3477
28. Chaurasia G, Malhotra S, Russ J, et al. UniHI 4: new tools for query, analysis and visualization of the human protein-protein interactome. Nucleic Acids Res. 2009;37:D657–660. doi:10.1093/nar/gkn841
29. Szklarczyk D, Morris JH, Cook H, et al. The STRING database in 2017: quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 2017;45(D1):D362–D368. doi:10.1093/nar/gkw937
30. Liu L, Wang T, Yang X, et al. MTNR1B loss promotes chordoma recurrence by abrogating melatonin-mediated beta-catenin signaling repression. J Pineal Res. 2019;67(2):e12588. doi:10.1111/jpi.12588
31. Patel S, Woodgett J. Glycogen synthase kinase-3 and cancer: good cop, bad cop? Cancer Cell. 2008;14(5):351–353. doi:10.1016/j.ccr.2008.10.013
32. Carter YM, Kunnimalaiyaan S, Chen H, Gamblin TC, Kunnimalaiyaan M. Specific glycogen synthase kinase-3 inhibition reduces neuroendocrine markers and suppresses neuroblastoma cell growth. Cancer Biol Ther. 2014;15(5):510–515. doi:10.4161/cbt.28015
33. Kroon J, In ‘T Veld LS, Buijs JT, Cheung H, van der Horst G, van der Pluijm G. Glycogen synthase kinase-3beta inhibition depletes the population of prostate cancer stem/progenitor-like cells and attenuates metastatic growth. Oncotarget. 2014;5(19):8986–8994. doi:10.18632/oncotarget.1510
34. Zeng J, Liu D, Qiu Z, et al. GSK3beta overexpression indicates poor prognosis and its inhibition reduces cell proliferation and survival of non-small cell lung cancer cells. PLoS One. 2014;9(3):e91231. doi:10.1371/journal.pone.0091231
35. Ougolkov AV, Fernandez-Zapico ME, Savoy DN, Urrutia RA, Billadeau DD. Glycogen synthase kinase-3beta participates in nuclear factor kappaB-mediated gene transcription and cell survival in pancreatic cancer cells. Cancer Res. 2005;65(6):2076–2081. doi:10.1158/0008-5472.CAN-04-3642
36. Tang Z, Yang G, Wang X, et al. AKT/GSK-3β/β-catenin signaling pathway participates in erythropoietin-promoted glioma proliferation. J Neurooncol. 2020;149(2):231–242. doi:10.1007/s11060-020-03602-9
37. Luo Y, Hurwitz J, Massague J. Cell-cycle inhibition by independent CDK and PCNA binding domains in p21Cip1. Nature. 1995;375(6527):159–161. doi:10.1038/375159a0
38. Chen Y, Liu X, Wang H, Liu S, Hu N, Li X. Akt regulated phosphorylation of GSK-3beta/Cyclin D1, p21 and p27 contributes to cell proliferation through cell cycle progression from G1 to S/G2M phase in low-dose arsenite exposed HaCat cells. Front Pharmacol. 2019;10:1176. doi:10.3389/fphar.2019.01176
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.