Back to Journals » Therapeutics and Clinical Risk Management » Volume 17
Growth Promoting Effect of Vacuum Sealing Drainage in the Healing Processes of Diabetic Foot Ulcers
Authors Yang H, Liu L, Li G, Chen Y, Jiang D, Wang W, Wang T, Sun J, Che J, Gu D, Lu M, Wang A
Received 23 September 2020
Accepted for publication 3 January 2021
Published 19 January 2021 Volume 2021:17 Pages 65—71
DOI https://doi.org/10.2147/TCRM.S282840
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Deyun Wang
Hui Yang,* Lan Liu,* Gai Li, Yinchen Chen, Dong Jiang, Wei Wang, Tianyuan Wang, Jinshan Sun, Jianfang Che, Dongmei Gu, Meng Lu, Aiping Wang
Department of Endocrinology, The Air Force Hospital from Eastern Theater of PLA, Nanjing 210029, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Aiping Wang; Meng Lu
Department of Endocrinology, The Air Force Hospital from Eastern Theater of PLA, 1 Malujie Road, Nanjing 210029, People’s Republic of China
Tel +86-25-80865162
Email [email protected]; [email protected]
Aim: To explore the growth-promoting effect of vacuum sealing drainage (VSD) during the healing processes of diabetic foot ulcers (DFUs).
Methods: From November 2018 to December 2019, 38 patients with unilateral DFUs were enrolled in this retrospective study. All patients were divided into two groups according to the use of VSD or not: the VSD group (n=20) and the control group (n=18). The following parameters were used to evaluate the healing process: changes in the mean areas of the ulcers; healing rate (HR); epithelial hyperplasia and angiogenesis as determined by hematoxylin-eosin staining (HE staining); and expression of CD34, CD68 and VEGF as assessed through immunohistochemistry. Perioperative side effects and complications were also recorded.
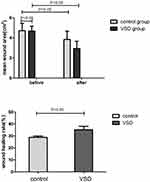
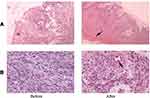
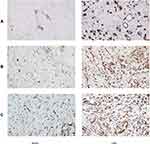
Results: All patients received follow-up and eventually healed. The mean area of wounds was reduced in the VSD group compared to the control group (1.75± 0.64 cm2 vs 0.88± 0.54 cm2, P=0.031). The mean HR of the ulcers in the VSD group was significantly higher than that in the control group (35.23± 2.87% vs 28.78± 1.09%, P=0.017). HE staining showed that the amount of epithelial hyperplasia and angiogenesis increased significantly after VSD, and the immunohistochemistry results showed that the expression of CD34, CD68 and VEGF increased significantly in the VSD group.
Conclusion: VSD could significantly accelerate the wound healing process, probably by enhancing the inflammatory response and promoting granulation and angiogenesis in DFUs.
Keywords: vacuum sealing drainage, diabetic foot ulcers, wound healing, CD34, CD68, VEGF
Introduction
Diabetic foot ulcers (DFUs) are ulcers or deep tissue damage due to peripheral nerve abnormalities and/or various degrees of peripheral vascular disease in the feet of patients with diabetes. DFUs occur in millions of people all over the world and impose tremendous medical and psychosocial burdens, financial loss, and even lower limb amputations or death.1
Abnormal pathologic changes in DFUs, such as ischemia, slowed growth and delayed healing, are often observed in patients. Angiogenesis and functional vessels play important roles during the formation of granulation tissue and wound healing.2 Vacuum sealing drainage (VSD) is a novel technology and is used as standard care in the treatment of wounds, including DFUs.3 It is widely known that VSD can induce much more rapid and robust responses of granulation tissue in a wound4 and reduce the risk of amputations in patients with DFU.5 However, little is known about the mechanisms of VSD, especially how VSD promotes tissue growth in DFUs.
The aim of the present study was to explore the molecular mechanisms of the growth-promoting effect of VSD upon the wound healing processes of DFUs. We also analyzed the healing rates (HRs) and pathological features in DFUs treated with VSD.
Patients and Methods
The study protocol was approved by the Ethics Review Committee of Nanjing Jun Xie Hospital. Written informed consent was obtained from patients before this study. The study was carried out in accordance with the approved guidelines and we promise that this study was conducted in accordance with the Declaration of Helsinki.
Patients
From November 2018 to December 2019, a total of 38 patients (20 male and 18 female), aged 42–69 years (mean, 55.47 ± 12.19 years), with unilateral DFUs (Wagner grade 2 or 3) were enrolled in this retrospective study. According to whether or not VSD was used, all patients were divided into two groups: the VSD group (20 patients treated with VSD) and control group (18 patients treated without VSD). The exclusion criteria applied were foot ulcers of other causes (tumor or others), no history of diabetes, or severe mental or psychiatric illness interfering with follow-up. No significant differences were found in the severity of DFUs, gender, smoking, duration of diabetes mellitus (DMZ), duration of DFU, body mass index (BMI), glycosylated hemoglobin (HbA1c), ankle brachial index (ABI) or area of ulcer between the two groups before treatment (P > 0.05) (Table 1).
 |
Table 1 Demographic and Clinical Characteristic of DFU |
Therapeutic Regimens
All surgeries were done by a dedicated orthopedic team experienced in treating DFUs. The anesthesia methods were epidural anesthesia (23 patients, 60.5%) or general anesthesia (15 patients, 39.5%). To achieve the optimal fasting blood glucose (<8.0 mmol/L), the four-needle regime was used during the perioperative period. The antibiotic therapy was empiric and based on the suspected pathogen, with later modification according to microbiological culture and susceptibility results. Its duration varied from 1 to 4 weeks, according to the severity of the infection. The duration of antibiotic therapy was longer if there was osteomyelitis.
Control group: Regular thorough debridement was used, and only the healthy tissue was preserved. The bone prominences and calluses were also debrided, which can reduce the plantar pressure and subcutaneous bleeding as well as ulcer formation. Control of the bacterial load was essential for earlier closure of the wound through conservative treatment or a skin graft.
VSD group: In addition to the regime applied in the control group, these patients received VSD to promote the repair of ulcers. For VSD using VAC, a negative pressure system provided by KCI company (San Antonio, TX, USA) was used. All procedures were carried out according to the Negative Pressure Wound Therapy Guideline With Installation: Review of Evidence and Recommendations.6 After debridement, sterile polyurethane foam dressing was designed according to the shape of ulcers and was covered with adhesive drape to create an airtight seal, which was applied in accordance with the manufacturer’s protocol and changed every 48 hours. A vacuum is created by aspirating the air for continuous suction at negative 125 mmHg for a total of 7 days. After 7 days of therapy, the dressing is removed. Measurements and photos were obtained to document wound progress.
Study Parameters
After 7 days, the following parameters were compared: changes in the mean areas of the ulcers; HR; epithelial hyperplasia and angiogenesis in HE staining; and expression of CD34, CD68 and VEGF as assessed through immunohistochemistry. Perioperative side effects and complications were also recorded.
Areas of the Ulcers, and HR
We took pictures of the wound and uploaded them to Image J (developed by Wayne Rasband), which was used to measure the wound area. The HR was calculated using the following formula: HR = (surface area in the end stage (cm2) – surface area in the initial stage (cm2)/surface area in the initial stage (cm2).
HE Staining
All the samples were obtained from the borders of normal and necrotic tissues of the patients’ feet and fixed in formalin fluid. Epithelial hyperplasia and angiogenesis were observed using HE staining; 5 µm thick paraffin-embedded sections were used for HE staining. Semi-quantitative analysis was used to account for the amount of epithelial hyperplasia and angiogenesis measured by HE staining based on the number of cells in a visual field. The amount of epithelial hyperplasia and angiogenesis were quantified using Image J (developed by Wayne Rasband) as previously described.7
Immunohistochemistry
The expression levels of CD34, CD68 and VEGF were detected by immunohistochemistry. For immunohistochemistry, the sections were briefly immersed in xylene, hydrated through graded ethanol solutions, and incubated in 3% hydrogen peroxide for 5 minutes to eliminate intrinsic peroxidase activity. Then, the sections were incubated overnight at 4°C with anti-CD34, anti-CD68 and anti-VEGF antibodies (1:100). Next, the sections were incubated for 30 minutes in anti-goat horseradish peroxidase (HRP) antisera, then incubated for 1 hour in species-specific peroxidase antiperoxidase complex. 3,30-Diaminobenzidine (DAB) was used as the chromogen with sections developed in 0.75 mg/mL DAB with 0.015% hydrogen peroxide in Tris buffer. The expression level of CD34, CD68 and VEGF were quantified using Image J (developed by Wayne Rasband) as previously described.7
Statistical Analysis
Data processing was performed using the SPSS statistical package (Version 18.0, SPSS Inc., Chicago, IL, USA). The normal distribution of the variables was evaluated by Kolmogorov–Smirnov test, and the variables were presented as the means±SDs or medians ± interquartile ranges (95% confidence intervals [95% CIs]). Student’s t-test was used for normally distributed continuous variables, while Pearson’s χ2 test or Wilcoxon’s rank-sum test was used for nonnormally distributed variables. P < 0.05 was considered statistically significant.
Results
All patients received follow-up and eventually healed. Of them, two patients had two operations and finally healed; one patient had osteomyelitis of the third metatarsal bone and was healed after comprehensive debridement. No lower limb amputations occurred. No serious perioperative side effects or complications were observed.
Wound Healing
No significant difference was found before surgery between the two groups.
The mean wound area was significantly reduced in the VSD group compared to the control group (1.75±0.64 cm2 vs 0.88±0.54 cm2, P=0.031) (Figure 1). The mean HR of the ulcers in the VSD group was significantly higher than that in the control group (35.23±2.87% vs 28.78±1.09%, P=0.017) (Figure 1).
Epithelial Hyperplasia, and Angiogenesis
HE staining was used to observe the expression of epithelial hyperplasia and angiogenesis. The amount of epithelial hyperplasia and angiogenesis increased significantly after VSD (Figure 2).
Expression Levels of CD34, CD68 and VEGF
Immunohistochemistry was used to detect the expression levels of CD34, CD68 and VEGF, and the results indicated that the expression levels of CD34, CD68 and VEGF increased significantly in the VSD group (Figure 3).
Discussion
DFUs are characterized by peripheral neuropathy, ischemia, foot deformity and infection. The wound healing process is impaired in DFUs, due to both intrinsic factors (neuropathy and vascular problems and other complex systemic alterations due to diabetes) and extrinsic factors (wound infection, callus formation, and excessive pressure at the site).8 The infections are usually mixed infections (gram-positive cocci bacteria, gram-negative anaerobic bacteria and fungi), which make the wound more difficult to manage. In an attempt to reduce the wound burden, much effort has been focused on understanding the physiology of healing and wound care with an emphasis on new therapeutic approaches and the continuing development of technologies for acute and long-term wound management.9 Due to the advantages of being inexpensive and having few adverse effects, VSD therapy seems to be a good choice in treating DFUs. It can be used in a smaller space in a timely manner to facilitate thorough exudation of the fluid, pus, and necrotic tissue from the wound without leaving a cavity in the wound areas, thereby eliminating the availability of a purulent medium for bacteria to flourish.10 Furthermore, VSD simultaneously allows wound closure with a sealed wet dressing that contains immune cells, inducing phagocytosis and promoting an aseptic environment, which is also conducive to preventing the spread of infection.11 On the other hand, VSD could decrease the permeability of blood vessels via suction and reduce the extent of the inflammatory response,12 thereby improving edema of the affected extremity. It has also been reported that early use of VSD therapy can prevent the expansion of stenosis and occlusion of capillaries in foot ulcers, marginally increase capillary number, improve blood supply to the foot, and increase the local tissue oxygen pressure.13 Many studies have pointed out that VSD can promote the repair of the wound, but they have not further explored the mechanism by which VSD promotes wound growth.14–17 In the present study, comprehensive treatments were given to the patients, including glucose control, anti-infection medication and debridement of the wounds. VSD therapy was used after the performance of surgical debridement. The mean area of wounds was significantly reduced after VSD therapy, and the HR was significantly higher in the VSD group, and the expression levels of CD34, CD68, VEGF increased significantly in VSD group. These results indicated the efficacy of VSD therapy in accelerating wound healing in DFUs.
CD34, a glycosylated type I transmembrane glycoprotein, is selectively expressed on the surfaces of hematopoietic stem/progenitor cells in humans and other mammals and gradually disappears following the maturation of cells. More and more research has shown that the CD34 molecule plays an important role in mediating intercellular adhesion, transportation and engraftment of hematopoietic stem cells, inflammatory response, and homing of lymphocytes. The CD34 molecule could bind to the e and p elements by connecting the side chain with the surface receptors of white blood cells, mediating leukocyte aggregation and enhancing the inflammatory response. On the other hand, activation of endothelial cell adhesion molecules and chemokines under the joint action of migration is advantageous to endothelial repair and vascular remodeling. In the present study, we showed that the expression of CD34 significantly increased after VSD therapy, indicating that VSD therapy may exert effects in enhancing the inflammatory response and promoting the revascularization in DFUs.
Cluster of Differentiation 68 (CD68) is a protein highly expressed by cells in the monocyte lineage (eg, monocytic phagocytes and osteoclasts), by circulating macrophages and by tissue macrophages (eg, Kupffer cells and microglia).18 It is well known that macrophages exert crucial effects on the modulation of the inflammatory response since they can be phenotypically polarized to the classical activated macrophages that stimulate the inflammatory process or to the alternatively activated macrophages that play a role in the resolution of inflammation.19 Macrophages also contribute to wound healing by releasing cytokines, interleukins and growth factors, modulating the inflammatory phase of wound healing.20–22 In the present study, we observed that VSD therapy significantly elevated the expression of CD68 in the wound, indicating that it may play important roles in increasing macrophages and strengthening the inflammatory process in the wound area.
Previous studies have shown that angiogenesis plays a critical role in wound healing.23 Angiogenesis, in addition to assisting granulation tissue formation, also transfers nutrition and oxygen to wounds and promotes tissue regeneration.23 Therefore, inadequate angiogenesis could lead to a prolonged time to wound healing. In addition, VEGF (originally known as the vascular permeability factor [VPF]),24 is a signaling protein produced by cells that stimulates the formation of blood vessels. To be specific, VEGF is a sub-family of growth factors, specifically the platelet-derived growth factor family of cystine-knot growth factors. They are important signaling proteins involved in both vasculogenesis (the de novo formation of the embryonic circulatory system) and angiogenesis (the growth of blood vessels from pre-existing vasculature). VEGF binds to receptor complexes consisting of both neuropilins and VEGFRs. These receptor complexes increase VEGF signalling activity in endothelial cells (blood vessels).25,26 The main functions of VEGF includes immigration, proliferation and vascularization of endothelial cells. In the present study, HE staining demonstrated that VSD therapy can both increase the amount of granulation tissue and angiogenesis in DFUs. In addition, the expression of VEGF in the wound area also significantly increased after VSD therapy, implying that VSD therapy could promote granulation and angiogenesis in DFUs, at least partly through increasing expression of the VEGF.
Some limitations were found in the present study. First, it was a retrospective study in design, and strict inclusion and exclusion criteria were needed. Second, the follow-up period was just 1 week, so it does not provide information on possible long-term effects. Third, we just conducted an initial analysis of a relatively small sample size with limited statistical power.
In summary, we believe that VSD is a safe and efficacious treatment for DFUs. It could accelerate the healing process by helping to restore normal morphology, control infections, enhance the inflammatory response, and promote wound granulation and angiogenesis.
Acknowledgments
This work was supported by research-funded projects of PLA 454 hospital (10Z014) and the National Natural Science Foundation of China (NO. 81770810). And the authors gratefully acknowledge the contribution of the participants in this study and their families. Hui Yang and Lan Liu are co-first authors in this study.
Disclosure
The authors declare no competing financial interests.
References
1. Zelen CM, Stover B, Nielson D, et al. A prospective study of negative pressure wound therapy with integrated irrigation for the treatment of diabetic foot ulcers. Eplasty. 2011;11:e5.
2. Erba P, Ogawa R, Ackermann M, et al. Angiogenesis in wounds treated by microdeformational wound therapy. Ann Surg. 2011;253(2):402–409. doi:10.1097/SLA.0b013e31820563a8
3. Guffanti A. Negative pressure wound therapy in the treatment of diabetic foot ulcers: a systematic review of the literature. Wound Ostomy Continence Nurs. 2014;41(3):233–237. doi:10.1097/WON.0000000000000021
4. Jacobs S, Simhaee DA, Marsano A, et al. Efficacy and mechanisms of vacuum-assisted closure (VAC) therapy in promoting wound healing: a rodent model. J Plast Reconstr Aesthet Surg. 2009;62(10):1331–1338. doi:10.1016/j.bjps.2008.03.024
5. Armstrong DG, Lavery LA. Negative pressure wound therapy after partial diabetic foot amputation: a multicentre, randomised controlled trial. Lancet. 2005;366(9498):1704–1710. doi:10.1016/S0140-6736(05)67695-7
6. Kim PJ, Attinger CE, Crist BD, et al. Negative pressure wound therapy with instillation: review of evidence and recommendations. Wounds. 2015;27(12):S2–S19.
7. Jensen EC. Quantitative analysis of histological staining and fluorescence using ImageJ. Anat Rec. 2013;296(3):378–381. doi:10.1002/ar.22641
8. Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366(9498):1736–1743. doi:10.1016/S0140-6736(05)67700-8
9. Robson MC, Steed DL, Franz MG. Wound healing: biologic features and approaches to maximize healing trajectories. Curr Probl Surg. 2001;38(2):72–140. doi:10.1067/msg.2001.111167
10. Lavery LA. Effectiveness and safety of elective surgical procedures to improve wound healing and reduce re-ulceration in diabetic patients with foot ulcers. Diabetes Metab Res Rev. 2012;28(Suppl 1):60–63. doi:10.1002/dmrr.2241
11. Lerman B, Oldenbrook L, Ryu J, et al. The SNaP wound care system: a case series using a novel ultraportable negative pressure wound therapy device for the treatment of diabetic lower extremity wounds. J Diabetes Sci Technol. 2010;4(4):825–830. doi:10.1177/193229681000400409
12. Qunaibi EA, Disi AM, Taha MO. Phenytoin enhances collagenization in excision wounds and tensile strength in incision wounds. Pharmazie. 2009;64(9):584–586.
13. Rezvani O, Shabbak E, Aslani A, et al. A randomized, double-blind, placebo-controlled trial to determine the effects of topical insulin on wound healing. Ostomy Wound Manage. 2009;55(8):22–28.
14. Huang Q, Wang JT, Gu HC, et al. Comparison of vacuum sealing drainage and traditional therapy for treatment of diabetic foot ulcers: a meta-analysis. J Foot Ankle Surg. 2019;58(5):954–958. doi:10.1053/j.jfas.2018.12.020
15. Kirsner R, Dove C, Reyzelman A, et al. A prospective, randomized, controlled clinical trial on the efficacy of a single-use negative pressure wound therapy system, compared to traditional negative pressure wound therapy in the treatment of chronic ulcers of the lower extremities. Wound Repair Regen. 2019;27(5):519–529. doi:10.1111/wrr.12727
16. Mohseni S, Aalaa M, Atlasi R, et al. The effectiveness of negative pressure wound therapy as a novel management of diabetic foot ulcers: an overview of systematic reviews. J Diabetes Metab Disord. 2019;18(2):625–641. doi:10.1007/s40200-019-00447-6
17. Borys S, Hohendorff J, Koblik T, et al. Negative-pressure wound therapy for management of chronic neuropathic noninfected diabetic foot ulcerations - short-term efficacy and long-term outcomes. Endocrine. 2018;62(3):611–616. doi:10.1007/s12020-018-1707-0
18. Holness CL, Simmons DL. Molecular cloning of CD68, a human macrophage marker related to lysosomal glycoproteins. Blood. 1993;81(6):1607–1613. doi:10.1182/blood.V81.6.1607.1607
19. Martinez FO, Sica A, Mantovani A, et al. Macrophage activation and polarization. Front Biosci. 2008;13:453–461. doi:10.2741/2692
20. Brem H, Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest. 2007;117(5):1219–1222. doi:10.1172/JCI32169
21. Bagdade JD, Root RK, Bulger RJ. Impaired leukocyte function in patients with poorly controlled diabetes. Diabetes. 1974;23(1):9–15. doi:10.2337/diab.23.1.9
22. Maruyama K, Asai J, Ii M, et al. Decreased macrophage number and activation lead to reduced lymphatic vessel formation and contribute to impaired diabetic wound healing. Am J Pathol. 2007;170(4):1178–1191. doi:10.2353/ajpath.2007.060018
23. Li J, Zhang YP, Kirsner RS. Angiogenesis in wound repair: angiogenic growth factors and the extracellular matrix. Microsc Res Tech. 2003;60(1):107–114. doi:10.1002/jemt.10249
24. Senger DR, Galli SJ, Dvorak AM, et al. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219(4587):983–985. doi:10.1126/science.6823562
25. Soker S, Takashima S, Miao HQ, et al. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92(6):735–745. doi:10.1016/S0092-8674(00)81402-6
26. Herzog B, Pellet-Many C, Britton G, et al. VEGF binding to NRP1 is essential for VEGF stimulation of endothelial cell migration, complex formation between NRP1 and VEGFR2, and signaling via FAK Tyr407 phosphorylation. Mol Biol Cell. 2011;22(15):2766–2776. doi:10.1091/mbc.e09-12-1061
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.