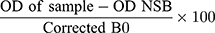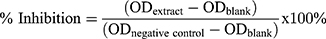Back to Journals » Journal of Inflammation Research » Volume 13
Grewia mollis Leaf Extracts and Fractions Demonstrated Good Inhibitory Activity on Pro-Inflammatory Enzymes and with Lower Cytotoxicity in vitro
Authors Adamu II , Adebayo SA, Al-Shahrani MS
Received 9 August 2020
Accepted for publication 22 September 2020
Published 23 October 2020 Volume 2020:13 Pages 765—772
DOI https://doi.org/10.2147/JIR.S271254
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Isa I Adamu,1,2 Salmon A Adebayo,3 Mohammad S Al-Shahrani4
1Physiology Unit Department of Basic Medical Sciences, College of Medicine, University of Bisha, Bisha, 61922, Saudi Arabia; 2Department of Human Physiology, Faculty of Medicine, Ahmadu Bello University, Zaria, Nigeria; 3Clinical Research, Neoteriks Health Research and Innovation, Indianapolis, IN 46254-2878, USA; 4Department of Family Medicine, College of Medicine, University of Bisha, Bisha, 61922, Saudi Arabia
Correspondence: Salmon A Adebayo Tel +1 (347) 744 4294
Email [email protected]
Introduction: Plant extracts are used to treat illnesses, promote health, and maintain general well-being in traditional medicine. Grewia mollis Juss (Malvaceae) is one of the medicinal herbs that is used traditionally to treat chronic diseases and related pain because currently used anti-inflammatory drugs may cause severe side effects, and naturally occurring compounds with reduced cytotoxicity could be explored for therapeutic goals.
Materials and Methods: Dried leaf of G. mollis was extracted with aqueous and organic solvents and partitioned based on polarity using solvent-solvent methods. The extracts were tested in anti-inflammatory assays against cyclooxygenases and lipoxygenase, and the safety profile was determined in a cell-based in-vitro assay.
Results: The n-hexane fraction of G. mollis leaf extracts had significant activity against both COX-1 (IC50 =0.97± 1.9 μg/mL) and COX-2 (IC50 =1.13± 0.2 μg/mL) better than the indomethacin positive control (IC50 =1.3± 0.6 and 1.52± 0.2 μg/mL), respectively (p≤ 0.05). Also, all the extracts and fractions of G. mollis tested inhibited the activity of 15-LOX (IC50 =12.48± 2.9 to 29.43± 9.9 μg/mL) better than the quercetin reference control (IC50 =61.82± 5.5 μg/mL), with the butanol fraction demonstrating the best anti-15 LOX action (IC50 =12.48± 2.9 μg/mL). Furthermore, all the extracts and fractions of G. mollis had relatively lower cytotoxicity on vero monkey kidney cells (LD50 =30.56– 479± 0.07 μg/mL) compared to the doxorubicin positive control (LD50 =2.59 μg/mL), but the selectivity index (SI=1.04– 1.89) determination suggested that some of the extracts may contain toxic constituents.
Conclusion: Organic extracts of the leaves of Grewia mollis contained bioactive molecules with potent action on COX-2 and 15-LOX. Targeted high-resolution high-performance liquid chromatographic (HPLC) methods have streamlined and enhanced bioactive compound isolation and purification process. This allows for the separation of undesirable compounds that could cause metabolic cytotoxicity in the plant extract mixtures. The method could be used to develop an alternative therapeutic strategy to manage pain associated with chronic inflammation where the use of NSAID is problematic.
Keywords: Grewia mollis, anti-inflammatory, pain, plant extracts, COX, 15-LOX
Introduction
Pathogens and other exogenous stimuli are capable of activating inflammation-promoting mediators including cyclooxygenases (COX) and lipoxygenases (LOX). Cyclooxygenases and 15-LOX are the key enzymes responsible for the synthesis and release of prostanoids and eicosanoids from poly-unsaturated fatty acids (PUFAs), respectively, that exacerbate chronic inflammation and other pathogenic and allergic disorders.1,2
Among the many mediators of chronic inflammation, the prostaglandins (PGs) are of great importance.2,3 They are released as a response to most types of pathological, chemical or mechanical stimuli. Prostaglandins act to protect the stomach mucosa and prevent platelets aggregation3 or may serve as a signalling molecule to perpetuate inflammation.4,5 Because COX-2 promotes the synthesis of PGs during chronic inflammation,6 strategies aimed at inhibiting its activity are used to treat these conditions.5 However, the use of COX-2 inhibitors (Non-Steroidal Anti-Inflammatory Drugs and specific COX-2 inhibitors) may result in unintended adverse consequences such as renal and cardiac problems.5 Therefore, innovative and sustained efforts are needed to identify alternative approaches with better efficacy, lower toxicity and reduced adverse effects.6 Naturally occurring secondary metabolites are a huge source of biologically active molecules that remained largely untapped for therapeutic advantage.7
Medicinal herbs are used traditionally to treat illnesses, promote health and maintain well-being.8 The aqueous extracts of various plant parts (leaf, stem, root and bark) are used for medicine preparation because they contain biologically active ingredients. Grewia mollis Juss (Malvaceae) is one of the medicinal herbs that is used traditionally to treat chronic diseases and related pain by the Hausa tribe in Nigeria.9 It is widely distributed in northern Nigeria,10 known as Dargaza in the local Hausa language (Figure 1). G. mollis also grow in other parts of tropical Africa, from Senegal on the west coast to Zimbabwe on the south coast (Figure 2).10,11
 |
Figure 1 Grewia mollis plant growing in its natural habitat (Source: self). |
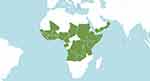 |
Figure 2 The geographical distribution of G. mollis in sub-sahara Africa and the middle east. The plant is native to the following countries in alphabetical order: Angola, Benin, Burkina, Burundi, Cameroon, Chad, Ethiopia, Gabon, Ghana, Guinea, Guinea-Bissau, Ivory Coast, Kenya, Liberia, Mali, Nigeria, Oman, Rwanda, Senegal, Sierra Leone, Somalia, Sudan, Tanzania, Togo, Uganda, Yemen, Zambia, Zaïre (The International Plant Names Index and World Checklist of Selected Plant Families 2020, www.ipni.org and www.apps.kew.org/wcsp/). |
Extracts of the bark and leaves are applied to small skin cuts, body sores and snakebites.10 Aqueous preparations of the leaf, bark and root are taken to relieve cough and fever.11 In addition, the mucilage of the bark is used traditionally by the Yoruba people of Nigeria to facilitate childbirth.11 However, very few studies have attempted to validate the anecdotal claims or at least provide a partial valorisation of its traditional uses. This study was the initial step to validate the biological activity of the extracts to identify the bioactive molecules and explore the basis of their molecular action.
Materials and Methods
Plant Materials
Fresh leaves of G. mollis were collected in April 2015 from Babari village, Zaria, Nigeria. Plant verification was provided by Dr Bala Muhammad, the botanist at the herbarium section of the Department of Biological Sciences, Ahmadu Bello University (ABU), Zaria. Voucher specimen number 161 was deposited at the herbarium at ABU for citations. The collected leaves were dried in a sterile and ventilated room for 2 weeks. The dried leaves were milled to a fine powder using a Mac Salab equipment (Model 2000 LAB Eriez) and kept in a glass container which was then stored in a dark at room temperature (25 ± 3°C).
Extraction and Liquid-Liquid Fractionation
A variation of a previous method12 was used to segregate the constituents of the leaf extracts based on polarity. One kilogram (1 kg) of grounded leaf was macerated three times in 70% acetone in water (l L), filtered and dried in vacuum to obtain the acetone extract (AcetE, 75 g). Seventy grams (70 g) of the dried AcetE was added in a mixture of chloroform and water (1:1, v/v) to yield the water and chloroform fractions. Then, n-butanol was added to the water fraction to yield dried 25.1 g of n-butanol (nButF) and 5 g of water (Wat1) fractions, respectively. The chloroform fraction was dried and dissolved in 10% water in methanol. This mixture yielded 23.9 g of dried n-hexane fraction (HexF). The proportion of water in methanol was increased to 35% water in methanol component that finally yielded 7.05 g of dried chloroform (ChlF) and 2.8 g of 35% water (Wat2) fractions. Based on the preliminary results of comparative Thin Layer Chromatography (TLC) experiments, the water (Wat1) and 35% water in methanol (Wat2) fractions were combined and dried to yield 7.8 g of combined water fraction (WatF). The stock solution of the extracts and fractions were dissolved in dimethyl sulphoxide (DMSO) and the final concentration used for the assay was 10 mg/mL. A schematic representation of the extraction process is shown in Figure 3.
 |
Figure 3 The flow chart indicating the sequential extraction and partitioning of Grewia mollis leaf crude extracts. |
Methods for the Anti-Inflammatory Assessments of the Fractions
Cyclooxygenase Inhibition (COX 1 and 2) Assay
The modulation of the activities of COX-1 and COX-2 is considered as one of the strategies to combat chronic inflammation. The anti-inflammatory action of the extracts and fractions were evaluated using a COX-inhibitor screening kit (Cayman Chemical, USA) following the manufacturer’s recommendation. The EIA kit used to determine the COX-1 and COX-2 inhibitory activity has been used in previous studies.13 The principle of the test kit is based on the inhibition of the biosynthesis of prostaglandins, thromboxanes, and prostacyclins from arachidonic acid by COX. The assay determines the production of prostaglandins (PGF2α) that is generated by stannous chloride (SnCl2), in the presence of PGH2. The initial reactions occurred in test tubes (initial reaction test tubes) at 37°C. In the background test-tubes, reaction buffer and heme were mixed together. Then, in the 100% activity test-tubes, reaction buffer, heme, enzyme (either COX-1 or −2) and the solvent used for dissolving the extract/fraction were added. In the sample test-tubes, reaction buffer, heme, inhibitor at different concentrations and enzymes were added. All test-tubes (initial reaction, 100% activity, background and samples) were incubated for 15 min at 37°C. Then, arachidonic acid (substrate) was added to all the tubes and incubated for 2 min. One molar hydrochloric acid (1 M HCl) was added to each test-tube to stop the reaction and thereafter, SnCl2 was added to stabilize the reaction product. All the test-tubes were incubated for a further 5 min at room temperature. A 96-well microtitre plate coated with mouse anti-rabbit IgG was provided with the kits. In the 96-well microtitre plate, non-specific binding, maximum binding, standards, and the inhibitor dilutions from the test tubes were added with a tracer and antiserum. The 96-well microtitre plate was then incubated at room temperature for 18 h, washed 5 times with the wash buffer and developed with the Ellman’s reagent. The Ellman’s reagent is made up of acetylthiocholine and 5, 5ʹ-dithio-bis-(−2-nitrobenzoic acid) or DTNB. Hydrolysis of acetylthiocholine by acetylcholine esterase (tracer) cleaves thiocholine, which reacts with DTNB to yield 5-thio-2-nitrobenzoic acid or NTB, a yellow product that could be measured at 410 nm. Afterwards, the 96-well microtitre plate was placed on a microplate reader and read at 410 nm. The amounts of the product (yellow colour) are inversely proportional to the concentrations of the PG in the wells.
Detailed data preparation, analysis and interpretation were provided by the kits’ manufacturer (Cayman’s COX inhibitor screening assay kit, item no. 560,131). In brief, the 96-well microtitre plate was set up to include two blank wells (blk), two non-specific binding wells (NSB), two maximum binding (B0) wells, one total activity (TA) well, eights wells for varying concentrations of standards run in duplicates, one background (BC) well for each COX, one 100% initial activity (IA) well for each COX and the remaining wells for varying concentrations of COX-inhibitor samples. The 96-well sample plate map is presented in Figure 4.
 |
Figure 4 A sample 96-well plate format for the COX-inhibitor screening ELISA assay (Cayman’s screening kit item no. 560,131). |
The average absorbance readings from the NSB and the B0 wells were determined. The NSB values were subtracted from the B0 to obtain the corrected B0. The ratio of the absorbance of each sample or standard wells compared to that of the maximum binding (%B/B0) well was calculated by using the equation;
where OD represents the average optical density or absorbance of the sample.
A standard curve using the logit (B/B0) versus the log of the concentrations of the known standards and then linearized with a regression fit. The %B/B0 for the tested extracts or fraction was read off the standard curve and multiplied by the appropriate dilution factor to calculate the concentrations of the sample.
The percentage enzyme inhibition (EI) by the extracts/fraction or control was calculated using the equation;
The %EI values were plotted against the inhibitor concentrations to determine the concentrations that resulted in 50% inhibition (IC50) by the extracts/fraction and the controls. The IC50 values are presented in Table 1, where indomethacin was used as the reference compound.
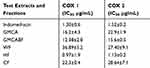 |
Table 1 The Bioactivity of the Leaf Extracts and Fractions of G. mollis on COX-1 and COX-2 |
Assessments of the Extracts/Fractions on 15-LOX
The method used for evaluating the anti-15 LOX action of the extracts/fractions was adapted from a previous study.14 In brief, an aliquot of 12.5 µL of the extracts/fractions dissolved in DMSO was added to 487.5 µL of 15-LOX (Sigma-Aldrich, South Africa) in a 96-well microtitre plate and incubated at room temperature for 5 min. After incubation, 500 µL of the substrate solutions that was made up of 10 µL linoleic acid dissolved in 30 µL ethanol and made up to 120 mL with 2 M borate buffer at pH 9.0 was added to the mixture. The mixture was further incubated for 5 min at room temperature and the absorbance was measured thereafter with SpectraMax 190 microplate reader set at 234 nm (Molecular devices, USA). Quercetin (1 mg/mL) was used as a positive control, while DMSO was used for negative control. The negative control represents the 100% enzyme activity or the activity of the enzyme without inhibition. The percentage enzyme inhibition by the test extract/fraction compared with the negative control was calculated using the equation;
The results were expressed as IC50, ie concentration of the extracts and controls that resulted in 50% 15-LOX inhibition plotted on a graph.
Evaluation of the Extracts/Fractions for Cytotoxicity
The assessment of the extracts and fractions for cytotoxicity was done on vero monkey kidney cells by using the 3(4–5 dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) cell viability assay as previously described15 but with slight modifications. The vero monkey (African green monkey) kidney cells (CCL-81) were bought from American Type Culture Collections (ATCC), Rockville, MD, USA.
The vero monkey kidney cells were seeded at a density of 1 × 105 cells/mL (100 μL) in 96-well microtitre plates and incubated at 37°C in 5% CO2 and a humidified atmosphere. After 24 h incubation, 100 μL of extracts/fractions at varying final concentrations (10, 5, 2.5, 1.25, and 0.625 mg/mL) were added to the wells containing the cells and then incubated for 12 hr. Thereafter, the medium in each well was aspirated from the cells, which were then washed with phosphate buffered saline (PBS), and finally fresh medium (200 μL) was added to each well and incubated for a further 48 hr. Then, 30 μL of MTT (5 mg/mL in PBS) was added to each well and the plates were incubated at 37°C in 5% CO2 humidified environment for 4 h. After incubation, the medium was aspirated from the wells and DMSO was added to solubilize the formed formazan crystals. The absorbance of the mixtures in the 96-well microtitre plates was measured on a BioTek Synergy (BioTek, USA) microplate reader set at 570 nm. Doxorubicin was used as a positive reference and the blank control with equivalent concentrations of acetone was also included.
The cell growth inhibition for each extract was expressed in terms of lethal dose (LD50) values, which was defined as the concentration that caused 50% of inhibition of cell viability. Also, the selectivity index (SI) values were calculated by dividing cytotoxicity LD50 values by the IC50 values of relevant bioactivity (SI=LD50/IC50). The higher the value of SI, the greater the probability that the observed bioactivity (anti-inflammatory) was not as a result of metabolic toxicity of the test extracts.1
Statistical Analysis
The experiments were conducted three times and in triplicates. The data obtained were recorded as mean ± standard deviation (SD). The significant differences between the values were calculated using analysis of variance and the Fisher’s least significant difference (LSD) at 5% significance level.
Results and Discussion
The results indicated that the n-hexane fraction (HF) of G. mollis leaf extracts significantly (p≤ 0.05) inhibited COX-1 (IC50 =0.97±1.9 µg/mL) and COX-2 (IC50 =1.13±0.2 µg/mL) better than the indomethacin reference compound (IC50 =1.3±0.6 and 1.52±0.2 µg/mL) respectively (Table 1). In particular, the inhibition of COX-2 is preferred because COX-1 has beneficial physiological functions in the gastric mucosa and on platelet aggregation.3 There is little information on the anti-COX activity of n-hexane fraction of G. mollis, and this probably represents the first report. Furthermore, the presence of triterpenes in the n-hexane extracts of the roots was confirmed in our study and has been reported elsewhere.16
Triterpenes are known to possess anti-inflammatory, antioxidant and anti-neoplasm action17 and could be responsible for the observed activity on COX. In addition, the presence of other bioactive secondary metabolites in the extracts cannot be ignored. Pharmacologically active constituents, such as tannins, saponins, glycosides and steroids are known to exert beneficial effects including anti-inflammatory and antioxidant activities in humans.18 All the extracts and fractions of G. mollis tested also inhibited the activity of 15-LOX better than the quercetin positive control (Table 2), with the GMBC fraction exhibiting the best anti-15 LOX action. 15-LOX is among the targets in the treatment of certain types of cardiovascular diseases, neuronal inflammation and cancer.19 Bioactive molecules with demonstrated activity and reduced side effects could be exploited as an alternative strategic option to treat such ailments.
 |
Table 2 The Activity of the Leaf Extracts and Fractions of G. Mollis on 15-LOX |
Medicinal plants are traditionally used as extracts containing several hundreds of therapeutically active molecules that often work either in synergy, additively or antagonistically.20 This mode of application is holistic and has been proposed as a possible explanation for the increased cytotoxicity observed during bioassay-guided isolation of active compounds.20,21
In Table 3, all the extracts and fractions of G. mollis had relatively lower cytotoxicity compared to the doxorubicin positive control. This result supports earlier reports that investigated the in vitro and in vivo cytotoxicity of the extracts of G. mollis respectively10,22,23 In addition, the selectivity index of some of the extracts and fractions further suggested that the observed activity could be as a result of metabolic effects rather than bioactivity. For example, the crude acetone fraction (GMCA) had an SI value range of 1.04–1.89 on vero monkey kidney cells. This raised a serious safety issue and suggests that the fraction may contain compounds that were cytotoxic to the cells. This observation has been reported previously by others.24
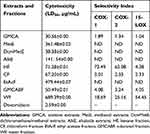 |
Table 3 The Cytotoxicity and Selectivity Index of Extracts of G. Mollis on Vero Monkey Kidney Cells |
Conclusions
This study provided the preliminary evidence that the n-hexane extracts of Grewia mollis leaf extracts contained bioactive molecules with potent action on COX-2 and 15-LOX. Plant-sourced remedies are not ingenious and have continued to remain relevant in drug development programs. Recent technological advances such as targeted high-resolution HPLC have streamlined and enhanced the bioactive compound isolation and purification processes. Newer methods could assist in the separation of undesirable compounds that could cause metabolic cytotoxicity in the extract mixtures. This information could be exploited to develop an alternative therapeutic strategy to treat diseases where the use of NSAID is problematic.
Data Sharing Statement
All data related to this work are available upon authorised requests from the corresponding author.
Acknowledgment
Our gratitude to Dr Bala Muhammad at the Department of Biological Sciences, Ahmadu Bello University, Zaria, Nigeria for plant identification. The Department of Paraclinical Sciences, University of Pretoria, South Africa is thanked for providing the facilities for the bioassay work.
Author Contributions
The manuscript as well as the biological assays, data entry and analysis were done by SAA and IIA. IIA collected the plant materials and performed the plant extraction and fractionation. MSA critically reviewed the draft manuscript and participated in the study design and data analysis. All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
The authors disclosed that no financial support was received for this work.
Disclosure
We declare that there is no conflict of interest in terms of the study design, authorship, or the decision on publication.
References
1. Adebayo SA, Steel HC, Shai LJ, Eloff JN. Investigation of the mechanism of anti-inflammatory action and cytotoxicity of a semi-purified fraction and isolated compounds from the leaf of Peltophorum africanum (Fabaceae). J Evid Based Complementary Altern Med. 2017;22(4):840–845. doi:10.1177/2156587217717417
2. Abdullahi MH, Anuka JA, Yaro AH, Musa A. Analgesic and anti-inflammatory effects of aqueous leaf extract of Combretum micranthum G. Don (Combretaceae). Bayero J Pure Appl Sci. 2014;7(2):78–82. doi:10.4314/bajopas.v7i2.15
3. Abdullahi MH, Anuka JA, Yaro AH, Musa A. Effect of aqueous leaf extract of Combretum micranthum G. Don (Combretaceae) on gastro-intestinal smooth muscle. Bayero J Pure Appl Sci. 2014;7(2):21–25. doi:10.4314/bajopas.v7i2.5
4. Gad SE. Prostaglandins. Reference Modules in Biomedical Sciences; Encyclopedia of Toxicology.
5. Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol. 2011;31(5):986–1000. doi:10.1161/ATVBAHA.110.207449
6. Cerella C, Mario D, Marc D, Diederich M. Targeting COX-2 expression by natural compounds: a promising alternative strategy to synthetic COX-2 inhibitors for cancer chemoprevention and therapy. Biochem Pharmacol. 2010;80(12):1801–1815. doi:10.1016/j.bcp.2010.06.050
7. Botting RM. Inhibitors of cyclooxygenases: mechanisms, selectivity and uses. J Physiol Pharmacol. 2006;57(5):113–124.
8. Sani HD, Aliyu BS. A survey of major ethno-medicinal plants of Kano North, Nigeria, their knowledge and uses by traditional healers. Bayero J Pure Appl Sci. 2011;4(2):28–34.
9. Nep E, Odumosu P, Ngwuluka N, Olorunfemi P, Ochekpe N. Pharmaceutical properties and applications of a natural polymer from Grewia mollis. J Polym. 2013;2013:1–8. doi:10.1155/2013/938726
10. Sambo SH, Olatunde A, Shaltoe SM. Phytochemical screening and mineral analysis of Grewia mollis stems bark. Int J Biochem Res Rev. 2015;6(2):75–81. doi:10.9734/IJBCRR/2015/14162
11. Asuku O, Atawodi SE, Onyike E. Antioxidant, hepatoprotective, and ameliorative effects of methanolic extract of leaves of Grewia mollis juss on carbon tetrachloride–treated albino rats. J Med Food. 2012;15(1):83–88. doi:10.1089/jmf.2010.0285
12. Suffness M, Douros J. Current status of the national cancer institute plant and animal product program. J Nat Prod. 1982;45(1):1–14. doi:10.1021/np50019a001
13. Gautam R, Jachak SM, Saklani A. Anti-inflammatory effect of Ajuga bracteosa wall ex benth. J Ethnopharmacol. 2011;133(2):928–930.
14. Adebayo SA, Dzoyem JP, Shai LJ, Jacobus N, Eloff JN. The anti-inflammatory and antioxidant activity of 25 plant species used traditionally to treat pain in southern Africa. BMC Complement Altern Med. 2015;15(1):159–168. doi:10.1186/s12906-015-0669-5
15. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1–2):18. doi:10.1016/0022-1759(83)90303-4
16. Efiom OO, Oku E. Isolation and characterisation of triterpene lup-20-en-3-ol and 1, 3-hexyloxacyclotridec-10-en-2-one from the root of Grewia mollis. N Y Sci J. 2012;5(11):138–141.
17. de Almeida PDO, de a Boleti AP, Rüdiger AL, Lourenço GA, da Veiga VF, Lima ES. Anti-inflammatory activity of triterpenes isolated from Protium paniculatum oil-resins. Evid Based Complement Alternat Med. 2015;2015:1–10. doi:10.1155/2015/293768
18. Owolabi OO, James DB, Sani I, Andongma BT, Fasanya OO, Kure B. Phytochemical analysis, antioxidant and anti-inflammatory potential of Ferentia apodanthera root bark extracts. BMC Complement Altern Med. 2018;18(1):12–20. doi:10.1186/s12906-017-2070-z
19. Karatas H, Cakir-Aktas C. 12/15 Lipoxygenase as a therapeutic target in brain disorders. Arch Neuropsychiatry. 2019;56(4):288–291.
20. Rasoanaivo P, Wright PW, Willcox ML, Gilbert B. Whole plant extracts versus single compounds for the treatment of malaria: synergy and positive interactions. Malar J. 2011;10(Suppl 1):S4. doi:10.1186/1475-2875-10-S1-S4
21. Caesar LK, Cech NB. Synergy and antagonism in natural product extracts: when 1 + 1 does not equal 2. Nat Prod Rep. 2019;36:869–888.
22. Mshelia EH, Adamu HM, Abayeh OJ, Maigari AU, Watirahyel EM. The quantitative phytochemicals content of the root bark of Grewia mollis, the antioxidant and cytotoxicity activities of its extracts. Eur J Pure Appl Sci. 2016;3(2).
23. Edeoga HO, Okwu DE, Mbaebie BO. Phytochemical constituents of some Nigerian medicinal plants. Afr J Biotechnol. 2005;4(7):685–688. doi:10.5897/AJB2005.000-3127
24. Obidah W, Godwin IJ, Fate JZ, Madusolumuo MA. Toxic effects of Grewia mollis stem bark in experimental rats. J Am Sci. 2010;6(12):1544–1548.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.