Back to Journals » Journal of Pain Research » Volume 14
Greater Pain Severity is Associated with Higher Glucocorticoid Levels in Hair Among a Cohort of People Living with HIV
Authors Zhang Q , Li X, Qiao S, Liu S, Shen Z, Zhou Y
Received 20 January 2021
Accepted for publication 19 February 2021
Published 9 March 2021 Volume 2021:14 Pages 645—652
DOI https://doi.org/10.2147/JPR.S301651
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Houman Danesh
Quan Zhang,1,2 Xiaoming Li,1 Shan Qiao,1 Shuaifeng Liu,3 Zhiyong Shen,3 Yuejiao Zhou3
1South Carolina SmartState Center for Healthcare Quality (CHQ), Arnold School of Public Health, University of South Carolina, Columbia, SC, USA; 2Institute of Pedagogy and Applied Psychology, School of Public Administration, Hohai University, Nanjing, Jiangsu, People’s Republic of China; 3Guangxi Zhuang Autonomous Region Center for Disease Control and Prevention, Nanning, Guangxi, People’s Republic of China
Correspondence: Quan Zhang
South Carolina SmartState Center for Healthcare Quality (CHQ), Arnold School of Public Health, University of South Carolina, Discovery I, Suite 408, 915 Greene Street, Columbia, SC, 29028, USA
Tel +1 803 777 8615
Fax +1 803 777 6290
Email [email protected]
Yuejiao Zhou
Guangxi Zhuang Autonomous Region Center for Disease Control and Prevention, No. 18 Jinzhou Road, Nanning, Guangxi, 530028, People’s Republic of China
Tel +86 771 251 8838
Email [email protected]
Background: Pain is a common occurrence and persistent symptom, which has an adverse impact on individual well-being and quality of life among people living with HIV (PLHIV). Alteration in the activity of the Hypothalamic-Pituitary-Adrenal (HPA) axis resulting in abnormal glucocorticoid levels had been proposed to play important roles in those associations.
Purpose: This study aimed to investigate whether pain severity was associated with hair glucocorticoid levels, a novel method of measuring long-term glucocorticoid exposure, among a large cohort of Chinese PLHIV.
Methods: A measure of pain severity and hair samples were collected from 431 adults PLHIV in Guangxi, China. Glucocorticoid (cortisol and cortisone) in hair were quantified by liquid chromatography-tandem mass spectrometry. The general linear model was used to test the associations of pain severity with hair glucocorticoid levels after adjusting for potential confounding factors.
Results: Of the 431 PLHIV, 273 reported none pain, 87 reported mild pain, and 71 reported moderate-severe pain. Hair cortisone, but not hair cortisol, was found to differ significantly among the three pain severity groups (F=3.90, p=0.021). PLHIV reported moderate-severe pain had higher hair cortisone than those reported mild (p=0.070) or none pain (p=0.014), with no differences between the latter two pain severity groups.
Conclusion: Greater pain severity is associated with higher hair cortisone levels among Chinese PLHIV. In order to reduce the long-term glucocorticoid levels, interventions managing pain should be considered for PLHIV with moderate-severe pain.
Keywords: pain, hair cortisol, hair cortisone, HIV
Introduction
Pain is one of the most commonly reported and persistent symptoms among people living with HIV (PLHIV), affecting 54% to 83% PLHIV, as indicated by a systematic review.1 The etiology of pain in PLHIV is varied and includes the direct effects of HIV on nervous systems, resultant opportunistic infections, side effects of combination antiretroviral therapy (cART), or other conditions unrelated to HIV.2 Regardless of etiology, pain in PLHIV is a significant source of function loss3 and decreased quality of life,4 and has been associated with elevated levels of psychological distress, including anxiety and depression,5–8 as well as higher adverse behaviors, including substance use9,10 and suboptimal ART adherence.11–13 While the increased attention has been paid to the relationship between pain and these adverse outcomes in PLHIV, limited data are available concerning the biological mechanisms underlying these associations in PLHIV.
The hypothalamic-pituitary-adrenal (HPA) axis is one of the main stress-sensitive systems regulating the body’s adaptation to stress by secretion of the glucocorticoid hormone.14,15 There is substantial evidence for a relation of hyper- or hypo-secretion of glucocorticoid with adverse health outcomes.16 Pain has been conceptualized as one type of stress that adds strain on the organism.17,18 Therefore, glucocorticoid is considered to play a crucial role in mediating the link between pain and the development of adverse health outcomes. While previous studies have investigated the relationship between pain and glucocorticoid levels in patients with chronic pain,19–24 critical gaps in the relationship between pain and glucocorticoid levels in PLHIV remain unaddressed.
As well known, cortisol is an active glucocorticoid, and cortisone is an inactive glucocorticoid originating from the local conversion of cortisol by the 11β hydroxysteroid dehydrogenase (11β-HSD) type 2 enzyme, and 11β-HSD type1 enzyme is responsible for the reversible conversion of cortisone to cortisol.25 Therefore, the interaction between cortisol and cortisone regulates stress-induced psychological and physiological responses together.26,27 In addition, cortisone levels are higher than those of cortisol levels in biomatrix (e.g., saliva and urine).25,28 Moreover, cortisone levels could more closely approximate unbound, biologically active cortisol levels than total cortisol levels.25 Therefore, the assessment of cortisone in parallel with cortisol could provide a more systematic evaluation of the glucocorticoid exposure.29
The conventional methods for cortisol and cortisone assessment in serum, saliva, or urine are susceptible to reflect mainly short-term cortisol and cortisone levels and affect by the circadian rhythm and other daily fluctuations.30 Recently, another method measures cortisol and cortisone levels in scalp hair, which circumvents many limitations of the previous methods and enables retrospective assessment of cortisol and cortisone levels in the past weeks to months, depending on the length of the collected sample.31 Comparing between hair cortisol and cortisone levels and cortisol and cortisone levels in repeated saliva sampling,29,32–34 testing the test-retest reliability of hair cortisol and cortisone levels across a period of several months to a year,28,35 and employing hair cortisol and cortisone levels in stress or chronic disease research,36–40 all suggest that hair cortisol and cortisone levels are novel retrospective indicators of long-term glucocorticoid exposure. To our knowledge, only two studies employed hair cortisol levels in HIV-related research.41,42 Limited data are available on employing hair cortisone levels in HIV-related research, and no study examined the association between pain severity and glucocorticoid levels in PLHIV by employing hair cortisol and cortisone levels.
Accordingly, we assessed pain severity and hair cortisol and cortisone levels in a large cohort of PLHIV in Guangxi, China, and aimed to examine whether pain severity was associated with hair cortisol and cortisone levels among Chinese PLHIV.
Methods
Participants
The participants of this study were recruited from an HIV disclosure study aiming to investigate the mechanism of the effects of HIV disclosure on clinical outcomes in Guangxi, China.43–45 With the assistance and collaboration of the Guangxi Center for Disease Prevention and Control (CDC), ten clinic sites with the largest number of HIV patients under care from 17 cities and 75 counties in Guangxi were selected as study sites.
The inclusion criteria were: (1) at least 18 years of age; (2) a confirmed diagnosis of HIV; (3) willing to provide hair samples; and (4) willing to consent the retrieval of their relevant clinical outcome data (eg, CD4+ T cell count) from their medical charts. The exclusion criteria were: (1) linguistic, mental or physical inability to respond to assessment questions; (2) opportunistic infections, coinfection, comorbidities, endocrine diseases, or reported any other diseases; (3) currently take hormonal drugs (eg, prednisolone); (4) known history of drug use; (5) chemical hair treated (eg, dyed, permed, or bleached) or scalp hair in the posterior vertex was less than 1 cm.
Medical staff or HIV case managers at the study sites referred potential participants to the research term members. Research team members screened PLHIV for eligibility, discussed the benefits and risks of the study, and invited them to participate. Research team members were local CDC staff or health care workers in the HIV clinics who had received intensive training on research ethics and interview skills with PLHIV before the field data and hair specimen collection. Finally, a total of 446 PLHIV participated in this study. This study followed the Declaration of Helsinki and was approved by the Institutional Review Boards of the University of South Carolina in the United States and the Guangxi CDC in China. The written informed consent also has been obtained from the participants.
Hair and Data Collection
Hair samples were cut from the posterior vertex region as close as possible to the scalp following a standard protocol46,47 in private rooms of local CDC or HIV clinics. The hair strands were cut with iron scissors that had been wiped with an alcohol pad. The hair thatch then was completely enclosed by a piece of foil, and a small label indicating the study ID number was placed over the distal end of the hair thatch. Then, the interviewer-administered questionnaire was used for data collection. After the hair sample collection, the interviewer-administered questionnaire was used for the collection of sociodemographic, lifestyle, and HIV-related information. Each participant received a gift with a value equal to the US $5.00 (≈ 35 Chinese Yuan) after finished the survey.
Pain Measure
To assess pain severity, we asked participants the following single-item question: “how much pain have you generally had during the past month”.48,49 Response options were on a 6-point scale ranging from “none” to “very severe.” Given that pain data are generally skewed, we divided our sample into three groups: those who reported none pain, those who reported mild pain, and those who reported moderate-severe pain.
Hair Cortisol and Cortisone
The proximal 1 cm of hair segments (approximately reflect the last month of accumulative cortisol and cortisone levels) was cut finely with scissors, and 20 mg were processed and analyzed using liquid chromatography-tandem mass spectrometry (LC/MS/MS) followed the protocol described by Gao and colleagues.50 The method showed good linearity (R2 > 0.99) in the range of 0.25–1250 pg/mg for both cortisol and cortisone, the limit of detection (LOD) for both cortisol and cortisone at 0.1 pg/mg, and the limit of quantitation (LOQ) for both cortisol and cortisone at 0.3 pg/mg. Intra-day and inter-day percentages coefficient of variation (CV) were less than 7% at standard concentrations of 1.25, 25, and 250 pg/mg, and recovery ranged between 95% and 107% for both cortisol and cortisone.
Sociodemographic and Clinical Characteristics
Participants provided information on their sociodemographic that might have a potential influence on hair cortisol and cortisone levels,30,51,52 including age (years), gender (male vs female), ethnicity (Han vs non-Han), marital status (married vs other), education level (> 9 years vs ≤ 9 years), employment status (employed vs unemployed), monthly household income level (≥ 3000 Yuan vs < 3000 Yuan).
Clinical characteristics were abstracted from the participants’ medical charts, including the date of HIV diagnosis, cART status, and CD4+ T cell count. Years of HIV diagnosis referred to the period from the initial date of confirmed HIV diagnosis to the time of the survey. CD4+ T cell count was dichotomized as >500 cells/mm3 vs ≤500 cells/mm3 because the low limit of normal CD4+ T cell count is 500 cells/mm3 in adults. The cART status was category into untreated and cART treated.
Data Analysis
Fifteen participants were excluded from the final analysis because of the insufficient weight of hair samples (less than 20mg) for assaying cortisol and cortisone (n=13) or unavailable data of pain measure (n=2). This left 431 participants for analysis.
Hair cortisol and cortisone levels were not normally distributed as indicated by the Kolmogorov–Smirnov test and were thus Winsorized (5th/95th percentile),53 and Box-Cox transformed54 for effectively reduced the skewness statistic. Univariate linear regression analysis was used to explore the associations of hair cortisol or hair cortisone with sociodemographic and clinical characteristics. The general linear model was used to examine differences in transformed glucocorticoid levels among the three pain studied groups. Findings of significant main effects were followed up by post hoc comparisons using LSD. Multivariate analysis of covariance was adjusted for sociodemographic and clinical characteristics. All data analyses were performed using SPSS 26.0 (SPSS Inc, Chicago, IL).
Results
Of the 431participants with a mean (SD) age of 41 (8) years (Table 1), 68% were male, 61.9% were Han ethnicity, 78.9% were married, 77.7% were employed, 91.2% were receiving cART. Most of the sample had low levels of education and income, with 89.6% reporting not complete junior high school and 76.1% reporting a monthly household income of less than 3000 Chinese Yuan (or approximately US$460 during the time of the survey). The median CD4+ T cell count was 359 cells/mm3, and the median duration of HIV diagnosis was 1.50 years. Among the 431 PLHIV, 273 (63.3%) reported none pain, 87 (20.2%) reported very mild or maid pain, and 71 (16.5%) reported moderate, severe, or very severe pain. The median cortisol and cortisone levels were 7.60 pg/mg and 44.02 pg/mg, respectively. Hair cortisol and hair cortisone levels were positively correlated (r = 0.26, p < 0.001).
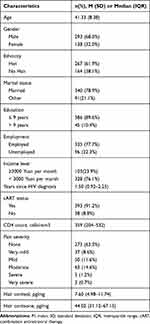 |
Table 1 Characteristics of the 431 Participants |
Table 2 shows the univariate regression of hair cortisol and cortisone levels with sociodemographic and clinical characteristics, respectively. Age, no-Han ethnicity, and be married were positively associated with hair cortisol levels. Female sex was positively associated with both hair cortisol and cortisone levels. Education levels and income levels were negatively associated with hair cortisol levels. Clinical characteristics were not associated with either hair cortisol levels or hair cortisone levels.
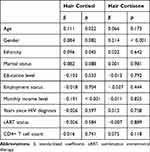 |
Table 2 Univariate Regressions of Hair Cortisol and Cortisone Levels with Sociodemographic and Clinical Characteristics |
The median hair cortisol levels were 7.09 pg/mg, 8.07 pg/mg, and 7.93 pg/mg for the none, maid, and moderate-severe pain groups, respectively (see Figure 1). No significant difference was found in hair cortisol levels among the three pain groups (F=0.14, p=0.868).
 |
Figure 1 Hair cortisol levels (pg/mg) in none, mild, and moderate-severe pain groups. Horizontal lines represent the group median. |
The median hair cortisone levels were 41.78 pg/mg, 47.71 pg/mg, and 51.62 pg/mg for the none, maid, and moderate-severe pain groups, respectively (see Figure 2). A significant difference was detected in hair cortisone levels (F=3.90, p=0.021). Post hoc analyses for transformed data revealed that PLHIV with moderate-severe pain had higher hair cortisone levels than those with none pain (p=0.014) and had marginally higher cortisone levels than those with maid pain (p=0.070), with no difference between the latter two groups (p>0.10). Adjusting for the covariates on these measures also did not alter the result for hair cortisol levels (F=0.02, p=0.983) or hair cortisone levels (F=4.03, p=0.018).
 |
Figure 2 Hair cortisone levels (pg/mg) in none, mild, and moderate-severe pain groups. Horizontal lines represent the group median. |
Discussion
The present study investigated whether pain severity was associated with hair glucocorticoid levels, a novel method of measuring long-term glucocorticoid exposure, among a large cohort of Chinese PLHIV. We found nonsignificant difference in hair cortisol levels among the three pain groups. However, PLHIV with moderate-severe pain had higher hair cortisone levels than those with mild pain or none pain, with no difference between PLHIV with mild pain and none pain.
To the best of our knowledge, only two previous studies investigated the relationship between pain and hair cortisol levels in patients with chronic pain, and no study has directly examined the relationship between pian and hair cortisone levels. Our finding regarding the lack of association of pain severity with hair cortisol levels in PLHIV is inconsistent with the two previous studies that reported higher hair cortisol levels in patients with chronic pain than controls.21,24 However, we found that PLHIV with moderate-severe pain had higher hair cortisone levels than those with none pain or mild pain. There are two potential explanations for why significant associations were demonstrated for hair cortisone levels, but not hair cortisol levels in our study. Firstly, hair cortisone levels could indicate more systematic glucocorticoid levels than hair cortisol levels. In line with the previous evidence,39,55,56 our data also found that hair cortisone levels were higher than hair cortisol levels (Table 1) and positively correlated. Previous studies also found that salivary cortisone levels demonstrated a stronger correlation with free serum cortisol levels than total serum cortisol levels and salivary cortisol levels25 and salivary cortisone levels showed a considerable correlation with hair cortisone levels.29 Therefore, hair cortisone levels have been employed as an additional measure of systematic glucocorticoid levels.26,27 Secondly, hair cortisone levels also appear- to hold certain benefits to hair cortisol levels. For instance, both our study (see Table 2) and previous studies found hair cortisol levels were influenced by additional factors.51,52 Other studies have also found stronger associations between hair cortisone levels than hair cortisol levels and variables studied, including Parkinson’s disease,40 cardiometabolic variables,37,56 and stress-related variables.36,40 One study found diagnostic accuracy for Cushing’s syndrome was significantly better for hair cortisone levels than hair cortisol levels.57 Therefore, along with previous studies, our study also provides implications for future research to consider both hair cortisol levels and hair cortisone levels, rather than hair cortisol levels alone to represent long-term glucocorticoid levels. Regardless of the relatively little is known about cortisone’s physiological significance, our findings regarding the association between pain severity and hair cortisone levels indicated that health providers in caring for PLHIV should consider interventions (eg, mindfulness) to reduce the long-term glucocorticoid levels and to manage pain.58,59
We found that clinical characteristics were not associated with hair cortisol or cortisone levels. Two recent studies also reported no association between hair cortisol levels and CD4+ T cell counts42 or cART status.41 In addition, our results contribute to HIV research by exploring the association of several other sociodemographic variables with hair glucocorticoid levels in a large cohort study and other fields by confirming the association of those variables with hair glucocorticoid levels previously observed in a different population.51,52
Several study limitations need to be acknowledged. First, the current study was based on cross-sectional data, which prevents making causal inferences. Future research should benefit from using longitudinal designs to investigate whether the change in glucocorticoid levels is consistent in PLHIV who experienced pain and whether the possible cause of pain in PLHIV is abnormalities of the glucocorticoid levels. Second, we only employed single-item questions to assess pain severity. Several other essential pain components were not evaluated in our study, including the pain site, chronicity and frequency of pain, pain coping, and pain medical history. Future research should benefit from employing multiple validity pain scales (eg, brief pain inventory and verbal numerical rating scale) that can include those components of pain. Third, because all participants are from Guangxi, China, these findings may not be generalizable to other PLHIV settings. Fourth, while data were not available in the current study on some other potential factors (eg, physical exercise) that might influence hair glucocorticoid levels,30,60 those factors should be considered in future research.
Conclusion
In summary, this study is the first to report that greater pain severity is associated with higher hair cortisol levels among Chinese PLHIV. In order to reduce the long-term glucocorticoid levels, interventions managing pain should be considered for PLHIV with moderate-severe pain. Future work will focus on the longitudinal relationship between these variables and further explore hair cortisone’s utility as a neuroendocrine biomarker in PLHIV.
Acknowledgments
The authors thank the study participants and data collectors for their collaboration during the data collection.
Funding
This study was supported by the National Institutes of Health (NIH) Research Grant [Grant numbers R01HD074221, R21AI122919].
Disclosure
All authors declare that they have no conflict of interest.
References
1. Parker R, Stein DJ, Jelsma J. Pain in people living with HIV/AIDS: a systematic review. J Int AIDS Soc. 2014;17(1):18719. doi:10.7448/IAS.17.1.18719
2. Hewitt DJ, McDonald M, Portenoy RK, Rosenfeld B, Passik S, Breitbart W. Pain syndromes and etiologies in ambulatory AIDS patients. Pain. 1997;70(2–3):117–123. doi:10.1016/S0304-3959(96)03281-2
3. Merlin JS, Westfall AO, Chamot E, et al. Pain is independently associated with impaired physical function in HIV-infected patients. Pain Med. 2013;14(12):1985–1993. doi:10.1111/pme.12255
4. da Silva JG, da Rocha Morgan DA, Melo FCM, et al. Level of pain and quality of life of people living with HIV/AIDS pain and quality of life in HIV/AIDS. AIDS Care. 2017;29(8):1041–1048.
5. Tsao JC, Dobalian A, Naliboff BD. Panic disorder and pain in a national sample of persons living with HIV. Pain. 2004;109(1–2):172–180.
6. Evans S, Ferrando S, Sewell M, Goggin K, Fishman B, Rabkin J. Pain and Depression in HIV Illness. Psychosomatics. 1998;39(6):528–535.
7. Uebelacker LA, Weisberg RB, Herman DS, Bailey GL, Pinkston-Camp MM, Stein MD. Chronic Pain in HIV-Infected Patients: relationship to Depression, Substance Use, and Mental Health and Pain Treatment. Pain Med. 2015;16(10):1870–1881.
8. Smith MY, Egert J, Winkel G, Jacobson J. The impact of PTSD on pain experience in persons with HIV/AIDS. Pain. 2002;98(1):9–17.
9. Krashin DL, Merrill JO, Trescot AM. Opioids in the management of HIV-related pain. Pain Physician. 2012;15(3 Suppl):ES157–ES168.
10. Tsao JC, Stein JA, Dobalian A. Pain, problem drug use history, and aberrant analgesic use behaviors in persons living with HIV. Pain. 2007;133(1–3):128–137. doi:10.1016/j.pain.2007.03.016
11. Merlin JS, Westfall AO, Raper JL, et al. Pain, mood, and substance abuse in HIV: implications for clinic visit utilization, antiretroviral therapy adherence, and virologic failure. J Acquir Immune Defic Syndr. 2012;61(2):164–170. doi:10.1097/QAI.0b013e3182662215
12. Surratt HL, Kurtz SP, Levi-Minzi MA, Cicero TJ, Tsuyuki K, O’Grady CL. Pain treatment and antiretroviral medication adherence among vulnerable HIV-positive patients. AIDS Patient Care STDS. 2015;29(4):186–192. doi:10.1089/apc.2014.0104
13. Berg KM, Cooperman NA, Newville H, Arnsten JH. Self-efficacy and depression as mediators of the relationship between pain and antiretroviral adherence. AIDS Care. 2009;21(2):244–248. doi:10.1080/09540120802001697
14. Papadimitriou A, Priftis KN. Regulation of the hypothalamic-pituitary-adrenal axis. Neuroimmunomodulation. 2009;16(5):265–271. doi:10.1159/000216184
15. McEwen BS. Neurobiological and Systemic Effects of Chronic Stress. Chronic Stress. 2017;1:1. doi:10.1177/2470547017692328
16. Nicolaides NC, Kyratzi E, Lamprokostopoulou A, Chrousos GP, Charmandari E. Stress, the stress system and the role of glucocorticoids. Neuroimmunomodulation. 2015;22(1–2):6–19. doi:10.1159/000362736
17. Abdallah CG, Geha P. Chronic pain and chronic stress: two sides of the same coin? Chronic Stress. 2017;1.
18. Chapman CR, Tuckett RP, Song CW. Pain and stress in a systems perspective: reciprocal neural, endocrine, and immune interactions. J Pain. 2008;9(2):122–145. doi:10.1016/j.jpain.2007.09.006
19. Muhtz C, Rodriguez-Raecke R, Hinkelmann K, et al. Cortisol response to experimental pain in patients with chronic low back pain and patients with major depression. Pain Med. 2013;14(4):498–503. doi:10.1111/j.1526-4637.2012.01514.x
20. Petrelluzzi KF, Garcia MC, Petta CA, Grassi-Kassisse DM, Spadari-Bratfisch RC. Salivary cortisol concentrations, stress and quality of life in women with endometriosis and chronic pelvic pain. Stress. 2008;11(5):390–397. doi:10.1080/10253890701840610
21. van Aken M, Oosterman J, van Rijn T, et al. Hair cortisol and the relationship with chronic pain and quality of life in endometriosis patients. Psychoneuroendocrinology. 2018;89:216–222. doi:10.1016/j.psyneuen.2018.01.001
22. Turner-Cobb JM, Osborn M, da Silva L, Keogh E, Jessop DS. Sex differences in hypothalamic-pituitary-adrenal axis function in patients with chronic pain syndrome. Stress. 2010;13(4):292–300. doi:10.3109/10253890903524785
23. Vachon-Presseau E, Roy M, Martel MO, et al. The stress model of chronic pain: evidence from basal cortisol and hippocampal structure and function in humans. Brain. 2013;136(Pt 3):815–827. doi:10.1093/brain/aws371
24. Van Uum SH, Sauve B, Fraser LA, Morley-Forster P, Paul TL, Koren G. Elevated content of cortisol in hair of patients with severe chronic pain: a novel biomarker for stress. Stress. 2008;11(6):483–488. doi:10.1080/10253890801887388
25. Perogamvros I, Keevil BG, Ray DW, Trainer PJ. Salivary cortisone is a potential biomarker for serum free cortisol. J Clin Endocrinol Metab. 2010;95(11):4951–4958. doi:10.1210/jc.2010-1215
26. Vanaelst B, Michels N, De Vriendt T, et al. Cortisone in hair of elementary school girls and its relationship with childhood stress. Eur J Pediatr. 2013;172(6):843–846. doi:10.1007/s00431-013-1955-1
27. Wang W, Deng H, Wang L, Cao C, Xu H, Zhang J. Hair cortisone level is associated with PTSDs dysphoric arousal symptoms in highly traumatized Chinese females. J Affect Disord. 2015;182:18–22. doi:10.1016/j.jad.2015.04.036
28. Zhang Q, Chen Z, Chen S, Xu Y, Deng H. Intraindividual stability of cortisol and cortisone and the ratio of cortisol to cortisone in saliva, urine and hair. Steroids. 2017;118:61–67. doi:10.1016/j.steroids.2016.12.008
29. Zhang Q, Chen Z, Chen S, et al. Correlations of hair level with salivary level in cortisol and cortisone. Life Sci. 2018;193:57–63. doi:10.1016/j.lfs.2017.11.037
30. Wosu AC, Valdimarsdottir U, Shields AE, Williams DR, Williams MA. Correlates of cortisol in human hair: implications for epidemiologic studies on health effects of chronic stress. Ann Epidemiol. 2013;23(12):797–811 e792. doi:10.1016/j.annepidem.2013.09.006
31. Stalder T, Kirschbaum C. Analysis of cortisol in hair–state of the art and future directions. Brain Behav Immun. 2012;26(7):1019–1029. doi:10.1016/j.bbi.2012.02.002
32. Short SJ, Stalder T, Marceau K, et al. Correspondence between hair cortisol concentrations and 30-day integrated daily salivary and weekly urinary cortisol measures. Psychoneuroendocrinology. 2016;71:12–18. doi:10.1016/j.psyneuen.2016.05.007
33. Sugaya N, Izawa S, Ogawa N, Shirotsuki K, Nomura S. Association between hair cortisol and diurnal basal cortisol levels: a 30-day validation study. Psychoneuroendocrinology. 2020;116:104650. doi:10.1016/j.psyneuen.2020.104650
34. Chen Z, Zhang Q, Chen S, Wang W, Liu G, Deng H. Determination, intercorrelation and intraindividual stability of five steroids in hair, saliva and urine among chinese college students. Steroids. 2019;149:108418. doi:10.1016/j.steroids.2019.05.010
35. Stalder T, Steudte S, Miller R, Skoluda N, Dettenborn L, Kirschbaum C. Intraindividual stability of hair cortisol concentrations. Psychoneuroendocrinology. 2012;37(5):602–610. doi:10.1016/j.psyneuen.2011.08.007
36. Davison B, Singh GR, McFarlane J. Hair cortisol and cortisone as markers of stress in Indigenous and non-Indigenous young adults. Stress. 2019;22:1–11.
37. Feeney JC, O’Halloran AM, Kenny RA. The association between hair cortisol, hair cortisone, and cognitive function in a population-based cohort of older adults: results from the irish longitudinal study on ageing. J Gerontology. 2018.
38. Musana JW, Cohen CR, Kuppermann M, et al. Association of differential symptoms of stress to hair cortisol and cortisone concentrations among pregnant women in Kenya. Stress. 2019;23:1–11.
39. Scharlau F, Pietzner D, Vogel M, et al. Evaluation of hair cortisol and cortisone change during pregnancy and the association with self-reported depression, somatization, and stress symptoms. Stress. 2018;21(1):43–50.
40. van den Heuvel LL. du Plessis S, Stalder T, et al. Hair glucocorticoid levels in Parkinson’s disease. Psychoneuroendocrinology. 2020;117:104704.
41. Qiao S, Li X, Zilioli S, et al. Hair measurements of cortisol, DHEA, and DHEA to cortisol ratio as biomarkers of chronic stress among people living with HIV in china: known-group validation. PLoS One. 2017;12(1):e0169827.
42. Langerak T, van den Dries LW, Wester VL, et al. The relation between long-term cortisol levels and the metabolic syndrome in HIV-infected patients. Clin Endocrinol (Oxf). 2015;83(2):167–172.
43. Yang X, Li X, Qiao S, Zhang Q, Shen Z, Zhou Y. Immunological and virologic outcomes of people living with HIV in Guangxi, China: 2012–2017. PLoS One. 2019;14(3):e0213205.
44. Zhang Q, Li X, Qiao S, Shen Z, Zhou Y. Comparing self-reported medication adherence measures with hair antiretroviral concentration among people living with HIV in Guangxi, China. AIDS Res Ther. 2020;17(1):8.
45. Zhang Q, Li X, Qiao S, Shen Z, Zhou Y. Factors influencing hair lamivudine concentration among people living with HIV in Guangxi, China. Antivir Ther. 2020;25(3):143–149.
46. Cooper GA, Kronstrand R, Kintz P. Society of Hair T. society of hair testing guidelines for drug testing in hair. Forensic Sci Int. 2012;218(1–3):20–24.
47. Sachs H. Quality control by the Society of Hair Testing. Forensic Sci Int. 1997;84(1–3):145–150.
48. Lau JT, Tsui HY, Patrick LC, Rita CW, Molassiotis A. Validation of a Chinese version of the Medical Outcomes Study HIV Health Survey (MOS-HIV) among Chinese people living with HIV/AIDS in Hong Kong. Qual Life Res. 2006;15(6):1079–1089.
49. Wu AW, Revicki DA, Jacobson D, Malitz FE. Evidence for reliability, validity and usefulness of the Medical Outcomes Study HIV Health Survey (MOS-HIV). Qual Life Res. 1997;6(6):481–493.
50. Gao W, Stalder T, Foley P, Rauh M, Deng H, Kirschbaum C. Quantitative analysis of steroid hormones in human hair using a column-switching LC-APCI-MS/MS assay. J Chromatogr B Analyt Technol Biomed Life Sci. 2013;928:1–8.
51. Rippe RC, Noppe G, Windhorst DA, et al. Splitting hair for cortisol? Associations of socio-economic status, ethnicity, hair color, gender and other child characteristics with hair cortisol and cortisone. Psychoneuroendocrinology. 2016;66:56–64.
52. Staufenbiel SM, Penninx BW, de Rijke YB, van den Akker EL, van Rossum EF. Determinants of hair cortisol and hair cortisone concentrations in adults. Psychoneuroendocrinology. 2015;60:182–194.
53. Dixon WJ, Yuen KK. Trimming and winsorization: a review. Statistische Hefte. 1974;15(2–3):157–170.
54. Clark JE, Osborne JW, Gallagher P, Watson S. A simple method for optimising transformation of non-parametric data: an illustration by reference to cortisol assays. Hum Psychopharmacol. 2016;31(4):259–267.
55. Kuehl LK, Hinkelmann K, Muhtz C, et al. Hair cortisol and cortisol awakening response are associated with criteria of the metabolic syndrome in opposite directions. Psychoneuroendocrinology. 2015;51:365–370.
56. Stalder T, Kirschbaum C, Alexander N, et al. Cortisol in hair and the metabolic syndrome. J Clin Endocrinol Metab. 2013;98(6):2573–2580.
57. Savas M, Wester VL, de Rijke YB, et al. Hair glucocorticoids as a biomarker for endogenous cushing’s syndrome: validation in two independent cohorts. Neuroendocrinology. 2019;109(2):171–178.
58. Hilton L, Hempel S, Ewing BA, et al. Mindfulness meditation for chronic pain: systematic review and meta-analysis. Ann Behav Med. 2017;51(2):199–213.
59. Goldberg SB, Manley AR, Smith SS, et al. Hair cortisol as a biomarker of stress in mindfulness training for smokers. J Altern Complement Med. 2014;20(8):630–634.
60. Gray NA, Dhana A, Van Der Vyver L, Van Wyk J, Khumalo NP, Stein DJ. Determinants of hair cortisol concentration in children: a systematic review. Psychoneuroendocrinology. 2018;87:204–214.
 © 2021 The Author(s). This work is published by Dove Medical Press Limited, and licensed under a Creative Commons Attribution License.
The full terms of the License are available at http://creativecommons.org/licenses/by/4.0/.
The license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
© 2021 The Author(s). This work is published by Dove Medical Press Limited, and licensed under a Creative Commons Attribution License.
The full terms of the License are available at http://creativecommons.org/licenses/by/4.0/.
The license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
