Back to Journals » OncoTargets and Therapy » Volume 12
Great efficacy of afatinib on a patient with lung adenocarcinoma harboring uncommon EGFR delE709_T710insD mutations: a case report
Authors An N, Wang H, Zhu H, Yan W, Jing W, Kong L, Zhang Y , Yu J
Received 2 July 2019
Accepted for publication 25 August 2019
Published 10 September 2019 Volume 2019:12 Pages 7399—7404
DOI https://doi.org/10.2147/OTT.S221638
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Leo Jen-Liang Su
Ning An,1,* Haoyi Wang,2,* Hui Zhu,3 Weiwei Yan,3 Wang Jing,3 Li Kong,3 Yan Zhang,3 Jinming Yu3
1Department of Radiation Oncology, Shandong Cancer Hospital and Institute, Shandong University, Jinan, People’s Republic of China; 2Department of Hematology, Qilu Hospital, Shandong University, Jinan, People’s Republic of China; 3Department of Radiation Oncology, Shandong Cancer Hospital and Institute, Shandong First Medical University and Shandong Academy of Medical Sciences, Jinan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yan Zhang; Jinming Yu
Department of Radiation Oncology, Shandong Cancer Hospital and Institute, Shandong First Medical University and Shandong Academy of Medical Sciences, Jinan 250117, People’s Republic of China
Tel +86 5 316 762 6782; +86 5 316 762 6947
Fax +86 53 167 62 6782; +86 5 316 762 6947
Email [email protected]; [email protected]
Abstract: EGFR)-targeted drugs have been the first-line treatment for patients with EGFR-mutant non-small cell lung cancer (NSCLC), especially exon 19 deletions and L858R mutation in exon 21. However, there is insufficient evidence for other less common types of EGFR mutations, such as delE709_T710insD (del 18). Recent studies have revealed that these rare genotypes could be targetable if appropriate mutations, such as delE709_T710insD (del 18). Recent studies have revealed that these rare genotypes could be targetable if appropriate EGFR tyrosine kinase inhibitors are selected. Here we reported a stage Ⅳ NSCLC patient with delE709_T710insD mutation who responded well to afatinib, a second-generation TKI. Afatinib had taken good control of the patient’s brain metastasis with a progression-free survival of 11 months and an overall survival exceeded 21 months, although he had received multi-line therapy. This case demonstrates EGFR delE709_T710insD is a rare but potentially afatinib responsive mutation in NSCLC, which may contribute to changes in clinical practice and further research into the precise detection and treatment of rare mutations in EGFR.
Keywords: non-small-cell lung cancer, epidermal growth factor receptor, molecular targeted therapy, tyrosine kinase inhibitor, afatinib, EGFR rare mutation
Introduction
It has been reported that the rate of EGFR positive mutation is 39.6% among NSCLC in Asian Pacific countries.1 For patients with common EGFR-mutant NSCLC (i.e. exon 19 deletions [del19] and L858R), EGFR-targeted drugs (EGFR tyrosine kinase inhibitors, TKIs) are used in the first-line treatment.2 The clinical efficacy of TKIs has been confirmed in several phase III clinical trials.2 In recent years, with the development of gene sequencing technology, EGFR mutations other than del19 and L858R which may potentially benefit from TKIs have received more and more attention.
 |
Table 1 Summary of clinical information of patients harboring delE709_T710insD treated with EGFR-TKIs |
Afatinib, a second-generation TKI, significantly improves progression-free survival (PFS) in patients with exon 19 deletions and L858R.3,4 It has also been reported that NSCLC patients which harbor certain rare EGFR mutation types had an objective response to afatinib, especially Gly719Xaa, Leu861Gln, and Ser768Ile, but afatinib was less active in other mutation types.5 Recently, some studies found that afatinib may be effective for other uncommon mutations such as del 18 (delE709_T710insD).6 However, few clinical data have been reported.
Case report
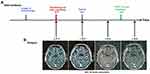
A 71-year-old Asian male presented with right lower lobe peripheral lung cancer and ipsilateral mediastinal lymph nodes metastases. He was initially diagnosed with cT2N2M0 (AJCC 8th Edition), stage ⅢA lung adenocarcinoma according to PET-CT and needle biopsy. Therefore, the treatment of right lobectomy and complete mediastinal lymph node dissection followed by four cycles of adjuvant chemotherapy consisting of Pemetrexed and cisplatin was given. The high-input sequencing for postoperative tissue samples indicated the presence of EGFR delE709_T710insD (del 18) mutation, but wild-type KRAS and ALK (Figure 1), however, there were no recommended targeted drugs for this mutation. After close surveillance for 15 months, the brain magnetic resonance imaging (MRI) showed a lesion suspected for metastasis in the left temporal lobe of the brain (Figure 2B, a) which was subsequently verified by PET-CT. Radiotherapy of 44 Gray, 11 fractions was carried out on the oligometastasis. The efficacy evaluation of radiotherapy is partial response (PR) by MRI, as determined by Response Evaluation Criteria in Solid Tumors version 1.1. However, the brain metastasis progressed again after 1 year, validated by MRI (Figure 2B, b). In consideration of the rare mutation of EGFR delE709_T710insD, afatinib 40 mg/day was administered in combination with bevacizumab 400 mg intravenously monthly. The brain metastasis shrunk significantly after 3 months and almost disappeared after 8 months, confirmed by MRI (Figure 2B, c). There were no significant adverse events during the treatment. Nevertheless, a PET-CT showed a recurrence in the right lung after treatment with afatinib for 11 months. To control the metastasis of the right lung, stereotactic radiotherapy of 60 Gy/10 f was given. At the same time, afatinib continued to be used. The patient is alive 21 months from initially taking afatinib, now with stable disease (Figures 2B, d and 3).
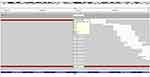 |
Figure 1 Identification of EGFR delE709_T710insD (del 18) mutation in exon 18 from lung cancer tumor biopsy. |
 |
Figure 3 A metastasis appeared in the right lung on the chest computed tomography image obtained at 38 months and achieved stable disease after the use of SBRT and afatinib until 48 months. |
Discussion
This case reported a NSCLC patient with metastasis carrying the delE709_T710insD mutation which was sensitivity to afatinib. From the initial treatment of afatinib, the PFS has exceeded 11 months and the overall survival has exceeded 21 months until now.
EGFR Del19 and the Leu858Arg point mutation in exon 21 are the most common EGFR mutations, which account for around 90% of all mutation-positive NSCLC.5,7 Most of the existing TKIs have been targeted for those two mutations. The remaining 10% of mutations involve exons 18 and 20, which has not been fully characterized in detail.5,8 Mutations in exon 18 present in 3.6%9 of all the EGFR mutations, including three representative mutations, G719X, E709K, and exon 18 deletion (del18: delE709_T710insD).6 Although DelE709_T710insD exon 18 mutation accounts for a considerable parts of EGFR mutations in NSCLC, as previously reported,10,11 it has not received sufficient attention compared with other types of mutations. According to the COSMIC database, delE709_T710insD mutation accounts for 0.11% among all EGFR mutations.12 It is the most common deletion, with E709K and G719A being the most common point mutations in exon 18 mutations.13 Wu et al14 reported that, in 3,146 patients with NSCLC, there were 25 cases with exon 18 deletion mutations, accounting for 0.79% of all patients, and 1.48% carrying EGFR mutations. Nevertheless, the DelE709_T710insD mutation has rarely been reported in the literature, and its response to TKI treatment is unclear.
To the best of our knowledge, six patients with delE709_T710insD mutation treated with TKIs have been reported (Table 1). However, the response rate of first generation TKIs such as gefitinib or erlotinib is only 25%.10,11 And the PFS of them was only about 4 months, as reported.11 A few cases reported that the treatment of afatinib to patients with adenocarcinoma harboring delE709_T710insD successfully shrank the tumor, but the data on prognosis are incomplete.6,12,13 It has also been reported that, compared to first generation or third generation-TKIs, afatinib appeared to have high sensitivity to del 18 in the in vitro study.6 Although afatinib has been known for its effectiveness on certain types of uncommon EGFR mutations, such as Gly719X,5 there are not many relevant clinical data about the response of it on delE709_T710insD.
In our case, delE709_T710insD was the only detected mutant oncogene. The brain metastasis of NSCLC with delE709_T710insD mutation had an effective response to afatinib. Although the patient had received multiline treatments and the brain metastasis was still progressing, afatinib still reached a good therapeutic effect, almost eliminated brain metastasis. Patients with brain metastasis usually have a low quality-of-life and poor prognosis, and the treatment strategy is limited.15,16 It has been reported that the first generation TKI, named erlotinib, had an intracranial response in patients with common EGFR mutant NSCLC17 because it could achieve therapeutic cerebrospinal fluid concentrations.18 However, the efficacy of afatinib in central nervous system (CNS) metastasis is unclear.19 In this case, afatinib not only achieved good local control (11 months), but also had a good CNS response. It is indicated that afatinib may also have good effects on the brain metastasis with del 18 mutation. Combining TKIs with bevacizumab may improve the therapeutic efficacy in the treatment of brain metastasis of lung adenocarcinoma.20,21 Several prospective studies have reported bevacizumab plus TKIs therapy improves PFS compared with TKIs alone in patients with EGFR-positive NSCLC.22,23 Recently, it has also been reported that the combination of afatinib and bevacizumab was also well tolerated and shows evidence of favorable disease control.24,25 In this case, the combination of afatinib and bevacizumab has achieved a good effect. However, at present we cannot get a reliable conclusion because there is no comparison.
Our case provides evidence that EGFR delE709_T710insD may lead to enhanced sensitivity to afatinib. And the PFS of this case exceeded 11 months, which was comparable to that of patients with tumors harboring del 19 or L858R.2
Conclusion
In summary, delE709_T710insD(del18) is a rare but potentially afatinib responsive mutation in NSCLC. Further study needs to be taken into consideration to let more patients with del18 mutation benefit from it. And for the further development of targeted therapy in all EGFR mutations, it is important to precisely detect targetable mutations, to select the most appropriate TKIs for each mutation, and to continue investigating in vitro and collecting clinical data on even rare mutations.
Ethics approval and consent for publication
This research was approved by the research ethics committee of Shandong Cancer Hospital, and written informed consent for publication of the clinical details and images was obtained from the patient.
Abbreviations
EGFR, epidermal growth factor receptor; NSCLC, non-small cell lung cancer; Del19, exon 19 deletions; Del 18, E709_T710insD; TKI, tyrosine kinase inhibitors; PFS, progression-free survival; MRI, magnetic resonance imaging; PR, partial response; CNS, central nervous system.
Acknowledgment
The authors would like to express their great thanks to the Innovation Project of the Shandong Academy of Medical Science.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Yatabe Y, Kerr KM, Utomo A, et al. EGFR mutation testing practices within the Asia Pacific region: results of a multicenter diagnostic survey. J Thorac Oncol. 2015;10(3):438–445. doi:10.1097/JTO.0000000000000422
2. Greenhalgh J, Dwan K, Boland A, et al. First-line treatment of advanced epidermal growth factor receptor (EGFR) mutation positive non-squamous non-small cell lung cancer. Cochrane Database Syst Rev. 2016;5:Cd010383.
3. Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-lung 6): an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15(2):213–222. doi:10.1016/S1470-2045(13)70604-1
4. Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31(27):3327–3334. doi:10.1200/JCO.2012.44.2806
5. Yang JC, Sequist LV, Geater SL, et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-lung 2, LUX-lung 3, and LUX-lung 6. Lancet Oncol. 2015;16(7):830–838. doi:10.1016/S1470-2045(15)00026-1
6. Kobayashi Y, Togashi Y, Yatabe Y, et al. EGFR exon 18 mutations in lung cancer: molecular predictors of augmented sensitivity to afatinib or neratinib as compared with first- or third-generation TKIs. Clin Cancer Res. 2015;21(23):5305–5313. doi:10.1158/1078-0432.CCR-15-1046
7. Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007;7(3):169–181. doi:10.1038/nrc2088
8. Beau-Faller M, Prim N, Ruppert AM, et al. Rare EGFR exon 18 and exon 20 mutations in non-small-cell lung cancer on 10 117 patients: a multicentre observational study by the French ERMETIC-IFCT network. Ann Oncol. 2014;25(1):126–131. doi:10.1093/annonc/mdt418
9. Shi Y, Au JS, Thongprasert S, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol. 2014;9(2):154–162. doi:10.1097/JTO.0000000000000033
10. Kobayashi Y, Mitsudomi T. Not all epidermal growth factor receptor mutations in lung cancer are created equal: perspectives for individualized treatment strategy. Cancer Sci. 2016;107(9):1179–1186. doi:10.1111/cas.12996
11. Ackerman A, Goldstein MA, Kobayashi S, Costa DB. EGFR delE709_T710insD: a rare but potentially EGFR inhibitor responsive mutation in non-small-cell lung cancer. J Thorac Oncol. 2012;7(10):e19–e20. doi:10.1097/JTO.0b013e3182635ab4
12. Iwamoto Y, Ichihara E, Hara N, et al. Efficacy of afatinib treatment for lung adenocarcinoma harboring exon 18 delE709_T710insD mutation. Jpn J Clin Oncol. 2019. doi:10.1093/jjco/hyz086
13. Ibrahim U, Saqib A, Atallah JP. EGFR exon 18 delE709_T710insD mutated stage IV lung adenocarcinoma with response to afatinib. Lung Cancer. 2017;108:45–47. doi:10.1016/j.lungcan.2017.02.023
14. Wu J-Y, Shih J-Y. Effectiveness of tyrosine kinase inhibitors on uncommon E709X epidermal growth factor receptor mutations in non-small-cell lung cancer. Onco Targets Ther. 2016;9:6137. doi:10.2147/OTT.S118071
15. Burel-Vandenbos F, Ambrosetti D, Coutts M, Pedeutour F. EGFR mutation status in brain metastases of non-small cell lung carcinoma. J Neurooncol. 2013;111(1):1–10. doi:10.1007/s11060-012-0990-5
16. Chen AM, Jahan TM, Jablons DM, Garcia J, Larson DA. Risk of cerebral metastases and neurological death after pathological complete response to neoadjuvant therapy for locally advanced nonsmall-cell lung cancer: clinical implications for the subsequent management of the brain. Cancer. 2007;109(8):1668–1675. doi:10.1002/cncr.22565
17. Porta R, Sanchez-Torres JM, Paz-Ares L, et al. Brain metastases from lung cancer responding to erlotinib: the importance of EGFR mutation. Eur Respir J. 2011;37(3):624–631. doi:10.1183/09031936.00195609
18. Togashi Y, Masago K, Fukudo M, et al. Cerebrospinal fluid concentration of erlotinib and its active metabolite OSI-420 in patients with central nervous system metastases of non-small cell lung cancer. J Thorac Oncol. 2010;5(7):950–955. doi:10.1097/JTO.0b013e3181e2138b
19. Baik CS, Chamberlain MC, Chow LQ. Targeted therapy for brain metastases in EGFR-mutated and ALK-rearranged non-small-cell lung cancer. J Thorac Oncol. 2015;10(9):1268–1278. doi:10.1097/JTO.0000000000000615
20. Feng PH, Chen KY, Huang YC, et al. Bevacizumab reduces S100A9-positive MDSCs linked to intracranial control in patients with EGFR-mutant lung adenocarcinoma. J Thorac Oncol. 2018. doi:10.1016/j.jtho.2018.03.032
21. EGFR-mutant lung cancers. Journal of Clinical Oncology. 2019;37(15):9086–9086. doi:10.1200/JCO.2019.37.15_suppl.9086
22. Saito H, Fukuhara T, Furuya N, et al. Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR-positive advanced non-squamous non-small-cell lung cancer (NEJ026): interim analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Oncol. 2019;20(5):625–635. doi:10.1016/S1470-2045(19)30035-X
23. Seto T, Kato T, Nishio M, et al. Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): an open-label, randomised, multicentre, phase 2 study. Lancet Oncol. 2014;15(11):1236–1244. doi:10.1016/S1470-2045(14)70381-X
24. Hata A, Katakami N, Kaji R, et al. Afatinib plus bevacizumab combination after acquired resistance to EGFR tyrosine kinase inhibitors in EGFR-mutant non-small cell lung cancer: multicenter, single-arm, phase 2 trial (ABC study). Cancer. 2018;124(19):3830–3838. doi:10.1002/cncr.31678
25. Ninomiya T, Nogami N, Kozuki T, et al. A phase I trial of afatinib and bevacizumab in chemo-naive patients with advanced non-small-cell lung cancer harboring EGFR mutations: okayama lung cancer study group trial 1404. Lung Cancer. 2018;115:103–108. doi:10.1016/j.lungcan.2017.11.025
26. Wu J-Y, Yu C-J, Chang Y-C, Yang C-H, Shih J-Y, Yang P-C. Effectiveness of tyrosine kinase inhibitors on “uncommon” epidermal growth factor receptor mutations of unknown clinical significance in non–small cell lung cancer. Clin Cancer Res. 2011;17(11):3812–3821. doi:10.1158/1078-0432.CCR-10-3408
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.