Back to Journals » OncoTargets and Therapy » Volume 12
Grape seed procyanidin B2 promotes the autophagy and apoptosis in colorectal cancer cells via regulating PI3K/Akt signaling pathway
Authors Zhang R, Yu Q, Lu W, Shen J, Zhou D, Wang Y, Gao S, Wang Z
Received 22 November 2018
Accepted for publication 7 March 2019
Published 24 May 2019 Volume 2019:12 Pages 4109—4118
DOI https://doi.org/10.2147/OTT.S195615
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Prof. Dr. Takuya Aoki
Ruijuan Zhang,1,* Qianyun Yu,2,* Wenqiang Lu,1 Jun Shen,1 Dongqing Zhou,1 Yingjue Wang,1 Shurong Gao,1 Zhijun Wang1
1Department of TCM, Shanghai Putuo District People’s Hospital, Shanghai 200060, People’s Republic of China; 2Department of TCM, Shanghai Huangpu District Wuliqiao Community Health Center, Shanghai, 200023, People’s Republic of China
*These authors contributed equally to this work
Aim: Colorectal cancer (CRC) is a major malignancy in China, which is the critical risk of people health. Many natural herbs extracts have been found to exhibit good therapeutic effect on CRC. Our previous study found that grape seed procyanidins B2 (PB2) would induce CRC cell death. However, the molecular mechanism underlying its anti-tumor effect on CRC remains unclear. Thereby, this study aimed to investigate the anti-tumor mechanism of PB2 on CRC.
Methods: CCK-8, western blotting, flow cytometry, qRT-PCR and animal study were used in the current study.
Results: The in vitro and in vivo data demonstrated that PB2 could promote the apoptosis of CRC cells in a dose-dependent manner, which was significantly reversed by caspase 3 inhibitor. Meanwhile, PB2 dose-dependently induced autophagy in CRC cells, which was markedly attenuated by autophagy inhibitor 3-MA. In addition, PB2 dose-dependently inhibited the expressions of p-PI3K, p-Akt and p-mTOR in the cells.
Conclusion: PB2 dose-dependently induced apoptosis and autophagy in CRC cells via downregulation of PI3K/Akt pathway. This study provided the experimental basis for further development of PB2 as a new effective anticancer drug for the patients with CRC.
Keywords: colorectal cancer, grape seed procyanidin extract, autophagy, apoptosis, PI3K/Akt/mTOR signaling pathway
Corrigendum for this paper has been published
Introduction
Colorectal cancer (CRC) is one of the high incident malignant tumors in digestive system.1,2 In recent years, with the life improvement and dietary changes, the incidence of CRC increased significantly, accompanying with serious threats to people health. The real cause of CRC remains unclear, but the environment, diet, lifestyle and diseases such as chronic intestinal inflammation, polyps, adenoma, Crohn, disease are the risk factors. According to the Cancer Data from the United States in 2015, the incidence and mortality of CRC ranked third in the top ten cancers in the United States.1 The number of global CRC cases increased by nearly 1 million cases, and the number of deaths increased by about 500,000 cases.2 In addition, the incidence of CRC in China is 28.08/10 million, and the mortality rate is 13.41/10 million.3
Apoptosis and autophagy are common in numerous cancer cells and relative to cell death, growth, differentiation and survival.4,5 Previous report found that induction of autophagy and apoptosis could prevent tumor growth in CRC cells.6 It has been found that phosphatidylinositol 3-kinase (PI3K)/Akt/mTOR signaling pathway is one of the major signaling pathways regulating autophagy and apoptosis in CRC.7,8 Apatinib, a small molecule tyrosine kinase inhibitor, demonstrated encouraging efficiency in human cancers.9,10 Previous studies indicated that apatinib inhibited human CRC progression via inhibiting PI3K/Akt pathways.11,12 Autophagy and apoptosis are the important effect pathways of this drug.13 In addition, some natural bioactive compounds such as black raspberries and dietary phytochemicals play important roles in combating CRC progression.14,15 Moreover, many natural herbs' extracts have been found to exhibit good therapeutic effect on CRC.3,16 Fan et al found that salidroside induced apoptosis and autophagy of CRC cells via suppressing PI3K/Akt/mTOR pathway.17 However, CRC is still associated with the high recurrence and mortality rates nowadays;18 it is urgent to discover novel therapeutics for CRC. Grape seed procyanidin B2 (PB2) is a natural herbal medicine with a variety of pharmacological effects. As a natural drug, PB2 exhibits well anti-cancer activity with non-toxic effect on the normal tissue and cells.19 In addition, PB2 attenuated pathological changes in diabetic nephropathy mice via downregulation of Akt signaling pathway.20,21 Moreover, our previous study has found that PB2 could induce CRC cell death.3 However, the molecular mechanism underlying its anti-tumor effect on CRC remains unclear. Thereby, the aim of this study was to investigate the effect of PB2 on autophagy and apoptosis in CRC, in order to provide the experimental basis for the further development of PB2 as a new effective anticancer drug for the patients with CRC.
Materials and methods
Cell culture and PB2 preparation
HT29 and LoVo cell lines were purchased from ATCC (American Type Culture Collection, Manassas, VA, US). These cell lines were cultured in RPMI 1640 or F12K (Kaighn’s Modification of Ham’s F-12) medium (Sigma Aldrich, St. Louis, MO, USA). All mediums were supplemented with 10% fetal bovine serum (Thermo Fisher Scientific, Waltham, MA, USA) and maintained in a 5% CO2 humidified atmosphere at 37°C. PB2 was purchased from (Sigma Aldrich, St. Louis, MO, USA) and dissolved at a concentration of 20 mg/mL as a stock solution in RPMI 1640 medium or F12K medium containing 10% fetal bovine serum, followed by ultrasonic mixing overnight and filtration with 0.22 μm filter.
CCK-8 (Cell Counting Kit-8) assay
HT29 or LoVo cell lines were firstly seeded in 96-well plates at 1×104 cells/well. When the cells reached 60% confluence, the medium was replaced with fresh medium containing different concentrations of PB2. When the cells were incubated with PB2 for 24, 48 and 72 hrs, the mediums were added with 10 μL CCK-8 reagent (Dojindo, Kumamoto, Japan). After incubating for another 4 hrs, the optical density values of wells were detected using a microplate reader (Bio-Rad Laboratories, Hercules, CA, USA) at 450 nm.
Flow cytometry
LoVo cells were plated in six-well plates at a density of 2×105 cells/well overnight, and then cells were treated with different concentrations of PB2 (range 6–24 μM) for 48 hrs. Subsequently, the cells were harvested by trypsinization and washed once with cold PBS. The cells were subsequently treated with PI and anti-annexin-V antibody (Becton Dickinson, USA) at 4°C for 1 hr. Subsequently, cells were washed once with PBS and analyzed by flow cytometer.
Acridine orange staining
LoVo cells were treated with different concentrations of PB2 for indicated times, stained with acridine orange (AO; 5 μg/mL) and ethidium bromide (EB; 5 μg/mL) for 40 s, and washed with PBS three times. After that, the acidic vacuoles were detected under a fluorescence microscope (Nikon TE2000-U, Shizvoka, Japan).
Transmission electron microscopy assay
Cells were fixed in Karnovsky’s fixative (2% paraformaldehyde and 5% glutaraldehyde in 0.1 Mcacodylate, pH 7.4) followed by osmium tetroxide. Then, the cells were dehydrated in ethanol, infiltrated and embedded with TAAB Low Viscosity Resin mixture at 60°C for 24 hrs and sectioned to 80 nm in thickness on 300 mesh copper slot grids. The observation was performed by transmission electron microscopy.
Fluorescence microscopy assay
Cells were seeded onto coverslip and transient transfected with mRFP-eGFP-LC3 (ptfLC3) expressing plasmid with Lipofectamine 2000 transfection reagent (Thermo Fisher Scientific, Waltham, MA, USA). Then, the cells were fixed with 4% paraformaldehyde and the localization of LC-3 puncta was observed by fluorescence microscopy (Olympus).
Western blotting
The cells or tissues lysates were centrifuged at 4°C at 12,000 g for 15 mins, and the supernatant was used for western blotting. The protein concentration was measured with the BCA kit (Promega, Madison, WI, USA). Forty micrograms of protein were mixed with 5× SDS sample buffer and denatured by boiling for 10 mins. Denatured proteins were separated with 10% polyacrylamide SDS gels and transferred onto PVDF membranes (Millipore, Billerica, MA, USA). Later on, membranes were blocked in 5% BSA for 2 hrs followed by incubation with primary antibodies overnight at 4°C. Then, the membranes were incubated with the HRP-conjugated secondary antibody for 2 hrs at room temperature. Subsequently, the membranes were washed four times for 10 mins in TBST and visualized using ECL reagents (Millipore).
Xenograft tumor model
For in vivo xenograft tumor growth assay, male nude mice (BALB/c nu/nu, 5-week-old, purchased from SLAC (Shanghai laboratory animal center, China)) were weighed and randomly divided into five groups: (1) Negative control; (2) PB2_25 mg/kg/day (PB2-L); (3) PB2_50 mg/kg/day (PB2-M); (4) PB2_100 mg/kg/day (PB2-H); (5) 5-FU_10 mg/kg/day. 2×106 LoVo cells in 0.1 mL of PBS were injected into subcutaneous tissues of each mouse. After two weeks, the mice were randomized into five groups, and administrated with indicated drugs. Four weeks after administration, the tumor-bearing mice were sacrificed and the tumors were excised and weighed. Part of the tumor tissues was used for protein detection by western blotting. All animal experiments were performed in accordance with institutional guidelines, following a protocol approved by the Ethics Committees of Shanghai Putuo District People’s Hospital.
Statistical analysis
Each group was executed at least three independent experiments and data were analyzed with single factor analysis of variance and a Student’s t-test using SPSS 20.0 software. The comparisons among multiple groups were made with one-way analysis of variance (ANOVA) followed by Dunnett’s test. P<0.05 or P<0.01 was considered to indicate a statistically significant difference (*P<0.05, **P<0.01).
Results
Effects of PB2 on the viability of CRC cells
First, CCK-8 method was used to observe the effect of PB2 on the viability of CRC cells. The results showed that PB2 inhibited the proliferation of CRC cells HT29 and LoVo in a dose-and time-dependent manners, and the half inhibitory concentrations at 48 hrs were 15 μM and 12 μM, respectively (Figure 1A and B). Since LoVo cells were more sensitive to PB2 compared with HT-29, it was chosen for use in the subsequent experiments.
PB2 induced autophagy in CRC cells
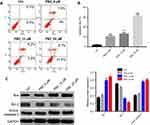
In order to examine if PB2 could induce autophagy in CRC cells, LoVo cells were treated with 6, 12 or 24 μM PB2 for 48 hrs. Then, Transmission Electron Microscope (TEM) was applied to observe the formation of autophagosomes. As shown in Figure 2A, the numbers of the autophagosomes were gradually increased with the elevation of the PB2 concentration. Meanwhile, the results of acridine orange (AO, Amresco, Inc) staining indicated that PB2 dose-dependently promoted the formation of autophagosomes in LoVo cells (Figure 2B). Since protein LC3 was a specific marker of autophagy, the GPF-LC3 expression vector was used to observe the autophagy. As demonstrated in Figure 2C, the expression of the GPF-LC3 was increased by PB2 in a dose-dependent manner. In addition, autophagy-related proteins were detected by Western blot. The results indicated that PB2 increased the expression of Beclin1, LC3 II and Atg5 in a dose-dependent manner and inhibited the expression of LC3 I (Figure 2D). All these results suggested that PB2 dose-dependently induced autophagy in CRC cells.
PB2 induced apoptosis in CRC cells
We next investigated if PB2 could induce apoptosis in LoVo cells. The result of flow cytometry suggested that PB2 significantly promoted the apoptosis of LoVo cells in a dose-dependent manner (Figure 3A and B). Moreover, western blot analysis indicated that PB2 could up-regulate the expressions of pro-apoptotic proteins Bax and Cleaved Caspase-3 and down-regulate the level of anti-apoptotic protein Bcl-2 in a dose-dependent manner (Figure 3C).
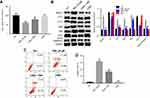
PB2-induced apoptosis in CRC cells was reversed by autophagy inhibitor 3-MA
In order to explore the interaction between apoptosis and autophagy induced by PB2 in CRC cell, autophagy inhibitor 3-MA was applied. The CCK-8 results suggested that 24 μM PB2 significantly inhibited the proliferation of LoVo cells, while this anti-proliferation effect was notably reversed by 3-MA. Meanwhile, 3-MA alone had no effect on the viability of CRC cells (Figure 4A). In addition, western blot results confirmed that the up-regulation of Beclin1, LC3 II and Atg5 induced by PB2 was alleviated by 3-MA in LoVo cells (Figure 4B). Moreover, PB-2-induced apoptosis in LoVo cells was significantly inhibited by 3-MA (Figure 4C and D). All these data suggested inhibition of autophagy attenuated the anti-proliferation effect of PB2 on CRC cells. That meant autophagy played a pro-apoptosis role in PB2-treated CRC cells.
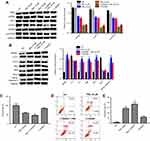
PB2 induced autophagy and apoptosis in CRC cells via regulation of PI3K/Akt signaling pathway
Next, we investigated whether PB2 induced autophagy and apoptosis in CRC cells via regulation of PI3K/Akt signaling pathway. Western blot analysis showed that PB2 inhibited the expressions of p-PI3K, p-Akt and p-mTOR in LoVo cells in a dose-dependent manner (Figure 5A). In addition, PI3K selective inhibitor LY294002 was applied to explore the interaction between PB2-inducedautophagy and PI3K/Akt pathway in CRC cells. We found that the addition of LY294002 significantly inhibited PB2-induced autophagy in LoVo cells, while LY294002 alone showed little impact on autophagy. In consistent to previous experiment, PB2 could increase the expression of pro-apoptotic proteins Bax and Cleaved Caspase-3, and decrease the expression of Bcl-2 in CRC cells (Figure 5B). In contrast, these effects were attenuated in the presence of LY294002 (Figure 5B). In addition, CCK8 results indicated the anti-proliferation effect of PB2 on LoVo cells were significantly enhanced by LY294002 (Figure 5C). Moreover, the data of flow cytometry confirmed that LY294002 notably increased PB2-induce apoptosis in LoVo cells (Figure 5D and E). All these results suggested PB2 induced autophagy and apoptosis in CRC cells via regulation of PI3K/Akt signaling pathway
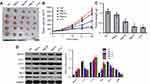
Anti-tumor effect of PB2 on CRC xenograft in vivo
Finally, anti-tumor effect of PB2 on CRC subcutaneous xenograft was investigated. The results indicated that PB2 inhibited tumor growth in nude mice in a dose-dependent manner, and the inhibitory effect of 100 mg/kg PB2 was very close to that of positive control 5-FU (Figure 6A–C). Additionally, PB2 significant upregulated apoptosis and autophagy-related proteins including Bax, Beclin 1 and LC II, and downregulated Bcl-2, LC I and p-Akt in tumor tissues (Figure 6D). These data suggested PB2 exhibited notably anti-tumor effect on CRC in vivo, which was consistent with in vitro data.
Discussion
Apoptosis is called type I programmed cell death. In recent years, autophagy is known as type II programmed cell death, which is a highly conserved behavior in eukaryotic cells.22 As we know, apoptosis and autophagy are involved in cell growth, differentiation, death, invasion and metastasis.22,23 In addition, cell apoptosis and autophagy are closely related to the development of tumor. Therefore, studying the relationship between autophagy and apoptosis is of great significance to elucidate the mechanism of antitumor drugs. In the present study, we investigated the anti-tumor effect of PB2 on CRC in vitro and in vivo. We found PB2 induced apoptosis and autophagy in CRC cells through regulation of PI3K/Akt/mTOR signaling pathway. In addition, we found that PB2 increased CRC cells autophagy via increasing the level of LC3II, beclin1 and Atg5, decreasing the level of LC3I. Moreover, PB2 increased apoptosis in CRC cells via increasing the level of Bax, cleaved-caspase-3 and decreasing the level of Bcl-2. Ohsumi et al found that activation of autophagy may be an effective treatment on CRC.24 Zhang et al indicated that PB2 induced granulosa cell via increasing the expression of LC3II.25 Our data was in accordance with previous findings. Meanwhile, Yin et al found that PB2 induced endothelial cell apoptosis via increasing the levels of caspase-3 activity, Bax/Bcl-2 ratio.21 These results suggested that PB2 could induce apoptosis and autophagy in CRC cells.
Previous study indicated that tetrandrine could induce CRC cell death by activating caspase-dependent apoptosis and LC3-I, LC3-II-dependent activation of autophagy, while autophagy inhibitor could reduce tetrandrine-induced apoptosis.26 Therefore, we further investigated the role of autophagy in PB2-treated CRC cells. The results indicated that PB2-induced apoptosis was reversed by autophagy inhibitor 3MA. These data suggested that inhibition of autophagy decreased the pro-apoptotic effects of PB2 in CRC cells, which was consistent with the result of tetrandrine.
Cell autophagy and apoptosis are a complex regulatory process that involves a large number of upstream regulatory signaling pathways. Numerous evidences have suggested that PI3K-Akt-mTOR is one of the most classical signaling pathways. Activation of PI3K/Akt pathway promotes the depolymerize of Bcl-2 and Bcl-xl, allowing the free Bcl-2 to exert its anti-apoptotic effect and directly catalyze the inactivation of Caspase-3 and Caspase-9 at Ser196, and finally promote the proliferation and growth of tumor cells by inhibiting the pro-apoptotic function of Caspase.27,28 In addition, the process of autophagy is regulated by three PI3K (Class I–III PI3K), in which Class I PI3K activates Akt, kinase B (PKB) and its downstream pathway of signal pathway mTOR, which results in inhibition of autophagy, while inhibition of PKB and mTOR activity will induce cell autophagy.29,30 Derry et al indicated that grape seed extract inhibited CRC cells apoptosis via suppressing PI3k-Akt-mTOR pathway.31 This study found that PB2 inhibited the expressions of p-PI3K, p-Akt and p-mTOR in CRC cells dose-dependently. All these results demonstrated that PB2 could inhibit the activation of PI3K-Akt-mTOR signaling pathway and promote the apoptosis and autophagy formation in CRC cells.
In the present study, we showed that PB2 promoted the death of CRC cells by regulating the autophagy and apoptosis through PI3K/Akt/mTOR signaling pathway. This study provided the experimental basis for further development of PB2 as a new effective anticancer drug for the patients with CRC.
Acknowledgments
This work was supported by Project supported by independent innovation research fund of Shanghai Putuo District health system (No. KW15204).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi:10.3322/caac.21387
2. Saif MW, Chu E. Biology of colorectal cancer. Cancer J. 2010;16(3):196–201. doi:10.1097/PPO.0b013e3181e076af
3. Miura K, Satoh M, Kinouchi M, et al. The use of natural products in colorectal cancer drug discovery. Expert Opin Drug Discov. 2015;10(4):411–426. doi:10.1517/17460441.2015.1018174
4. Ouyang L, Shi Z, Zhao S, et al. Programmed cell death pathways in cancer: a review of apoptosis, autophagy and programmed necrosis. Cell Prolif. 2012;45(6):487–498. doi:10.1111/j.1365-2184.2012.00845.x
5. Qian H-R, Shi Z-Q, Zhu H-P, et al. Interplay between apoptosis and autophagy in colorectal cancer. Oncotarget. 2017;8(37):62759–62768. doi:10.18632/oncotarget.v8i37
6. Chang T-C, Wei P-L, Makondi PT, et al. Bromelain inhibits the ability of colorectal cancer cells to proliferate via activation of ROS production and autophagy. PLoS One. 2019;14(1):e0210274. doi:10.1371/journal.pone.0210274
7. Tsai D-H, Chung C-H, Lee K-T. Antrodia cinnamomea induces autophagic cell death via the CHOP/TRB3/Akt/mTOR pathway in colorectal cancer cells. Sci Rep. 2018;8(1):17424. doi:10.1038/s41598-018-35780-y
8. Yang L, Liu Y, Wang M, et al. Celastrus orbiculatus extract triggers apoptosis and autophagy via PI3K/Akt/mTOR inhibition in human colorectal cancer cells. Oncol Lett. 2016;12(5):3771–3778. doi:10.3892/ol.2016.5213
9. Fang S-C, Huang W, Zhang Y-M, Zhang H-T, Xie W-P. Hypertension as a predictive biomarker in patients with advanced non-small-cell lung cancer treated with apatinib. Onco Targets Ther. 2019;12:985–992. doi:10.2147/OTT.S189984
10. Li T, Wang S-B, Lei K-J, Jiang M-Q, Jia Y-M. Significant response of low-dose apatinib monotherapy in brain metastases of triple-negative breast cancer: a case report. Medicine (Baltimore). 2019;98(4):e14182. doi:10.1097/MD.0000000000014182
11. Cheng X, Feng H, Wu H, et al. Targeting autophagy enhancesapatinib-induced apoptosis via endoplasmic reticulum stress for human colorectal cancer. Cancer Lett. 2018;431:105–114. doi:10.1016/j.canlet.2018.05.046
12. Chen X, Guan Z, Lu J, et al. Synergistic antitumor effects of cMet inhibitor in combination with anti-VEGF in colorectal cancer patient-derived xenograft models. J Cancer. 2018;9(7):1207–1217. doi:10.7150/jca.20964
13. Feng H, Cheng X, Kuang J, et al. Apatinib-induced protective autophagy and apoptosis through the AKT-mTOR pathway in anaplastic thyroid cancer. Cell Death Dis. 2018;9(10):1030. doi:10.1038/s41419-018-1054-3
14. Pan P, Kang S, Wang Y, et al. Black raspberries enhance natural killer cell infiltration into the colon and suppress the progression of colorectal cancer. Front Immunol. 2017;8:997. doi:10.3389/fimmu.2017.00997
15. Afrin S, Giampieri F, Gasparrini M, et al. Dietary phytochemicals in colorectal cancer prevention and treatment: a focus on the molecular mechanisms involved. Biotechnol Adv. 2018. doi:10.1016/j.biotechadv.2018.11.011
16. Zhu J, Zhao B, Xiong P, et al. Curcumin induces autophagy via inhibition of Yes-Associated Protein (YAP) in human colon cancer cells. Med Sci Monit. 2018;24:7035–7042. doi:10.12659/MSM.910650
17. Fan X-J, Wang Y, Wang L, Zhu M. Salidroside induces apoptosis and autophagy in human colorectal cancer cells through inhibition of PI3K/Akt/mTOR pathway. Oncol Rep. 2016;36(6):3559–3567. doi:10.3892/or.2016.5138
18. Lu M, Wang C, Wang J. Tanshinone I induces human colorectal cancer cell apoptosis: the potential roles of Aurora A-p53 and survivin-mediated signaling pathways. Int J Oncol. 2016;49(2):603–610. doi:10.3892/ijo.2016.3565
19. Rossi M, Negri E, Parpinel M, et al. Proanthocyanidins and the risk of colorectal cancer in Italy. Cancer Causes Control. 2010;21(2):243–250. doi:10.1007/s10552-009-9455-3
20. Zhang Z, Li B-Y, Li X-L, et al. Proteomic analysis of kidney and protective effects of grape seed procyanidin B2 in db/db mice indicate MFG-E8 as a key molecule in the development of diabetic nephropathy. Biochim Biophys Acta. 2013;1832(6):805–816. doi:10.1016/j.bbadis.2013.02.022
21. Yin W, Li B, Li X, et al. Critical role of prohibitin in endothelial cell apoptosis caused by glycated low-density lipoproteins and protective effects of grape seed procyanidin B2. J Cardiovasc Pharmacol. 2015;65(1):13–21. doi:10.1097/FJC.0000000000000157
22. Fiandalo MV, Kyprianou N. Caspase control: protagonists of cancer cell apoptosis. Exp Oncol. 2012;34(3):165–175.
23. Hussey S, Terebiznik MR, Jones NL. Autophagy: healthy eating and self-digestion for gastroenterologists. J Pediatr Gastroenterol Nutr. 2008;46(5):496–506. doi:10.1097/MPG.0b013e3181617895
24. Ohsumi Y. Historical landmarks of autophagy research. Cell Res. 2014;24(1):9–23. doi:10.1038/cr.2013.169
25. Zhang J-Q, Gao B-W, Wang J, et al. Critical role of FoxO1 in granulosa cell apoptosis caused by oxidative stress and protective effects of grape seed procyanidin B2. Oxid Med Cell Longev. 2016;2016:1–16. doi:10.1155/2016/6147345
26. Chen Z, Li Y, Zhang C, et al. Downregulation of Beclin 1 and impairment of autophagy in a small population of colorectal cancer. Dig Dis Sci. 2013;58(10):2887–2894. doi:10.1007/s10620-013-2732-8
27. Pan S-T, Qin Y, Zhou Z-W, et al. Plumbagin induces G2/M arrest, apoptosis, and autophagy via p38 MAPK- and PI3K/Akt/mTOR-mediated pathways in human tongue squamous cell carcinoma cells. Drug Des Devel Ther. 2015;9:1601–1626. doi:10.2147/DDDT.S76057
28. Choi AM, Ryter SW, Levine B. Autophagy in human health and disease. N Engl J Med. 2013;368(19):1845–1846. doi:10.1056/NEJMc1303158
29. Kumar D, Shankar S, Srivastava RK. Rottlerin induces autophagy and apoptosis in prostate cancer stem cells via PI3K/Akt/mTOR signaling pathway. Cancer Lett. 2014;343(2):179–189. doi:10.1016/j.canlet.2013.10.003
30. Roy B, Pattanaik AK, Das J, et al. Role of PI3K/Akt/mTOR and MEK/ERK pathway in Concanavalin A induced autophagy in HeLa cells. Chem Biol Interact. 2014;210:96–102. doi:10.1016/j.cbi.2014.01.003
31. Derry MM, Somasagara RR, Raina K, et al. Target identification of grape seed extract in colorectal cancer using drug affinity responsive target stability (DARTS) technique: role of endoplasmic reticulum stress response proteins. Curr Cancer Drug Targets. 2014;14(4):323–336.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.