Back to Journals » OncoTargets and Therapy » Volume 13
GOLPH3 Promotes Angiogenesis of Lung Adenocarcinoma by Regulating the Wnt/β-Catenin Signaling Pathway
Authors Zhao C, Zhang J, Ma L, Wu H, Zhang H, Su J, Geng B, Yao Q, Zheng J
Received 16 February 2020
Accepted for publication 16 May 2020
Published 30 June 2020 Volume 2020:13 Pages 6265—6277
DOI https://doi.org/10.2147/OTT.S249994
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Federico Perche
Canjun Zhao,1,* Jin Zhang,2,* Litian Ma,1,* Hao Wu,1 Hui Zhang,1 Jialin Su,1 Bizu Geng,1 Qinghua Yao,3 Jin Zheng1
1Department of Traditional Chinese Medicine, The Second Hospital Affiliated to Air Force Medical University, Xi’an, People’s Republic of China; 2First Clinical Medical College, Zhejiang Chinese Medical University, Hangzhou, People’s Republic of China; 3Department of Integrated Traditional Chinese and Western Medicine, Zhejiang Cancer Hospital, Hangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jin Zheng
Department of Traditional Chinese Medicine, The Second Hospital Affiliated to Air Force Medical University, Xi’an, Shaanxi Province 710038, People’s Republic of China
Tel +86-2984777441
Email [email protected]
Qinghua Yao
Department of Integrated Traditional Chinese and Western Medicine, Zhejiang Cancer Hospital, Gongshu District, Hangzhou, Zhejiang Province 310000, People’s Republic of China
Tel +86-57188122012
Email [email protected]
Purpose: We aim to investigate the role of Golgi phosphoprotein 3 (GOLPH3) and the possible regulation mechanism underlying lung adenocarcinoma (LADC).
Methods: The level of GOLPH3 was performed by quantitative real time (qRT)-PCR, Western blot and immunohistochemistry. Patient survival rate was analyzed by Kaplan–Meier method. MTT was used to detect cell viability. The levels of p-serine/threonine-protein kinase (Akt), Akt, p-p65, p65 and β-catenin were determined by Western blot. Cell apoptosis was tested using flow cytometry. Angiogenesis was determined by in vitro angiogenesis assay. qPCR and Western blot were performed to identify apoptotic protein B-cell lymphoma 2 (Bcl-2)/Bcl-2-associated X protein (Bax) and vascular endothelial growth factor (VEGF).
Results: GOLPH3 was highly expressed in LADC cell lines and tissues and was significantly correlated with poor overall survival among patients with LADC. Furthermore, GOLPH3 expression was reduced in A549 and H23 cells in a cisplatin-dependent manner. Silencing of GOLPH3 enhanced inhibition of A549 and H23 cells by cisplatin and suppressed the protein expression of p-Akt, while p-p65 expression remained stable. However, overexpression of GOLPH3 weakened the inhibition of A549 and H23 cells by cisplatin and improved the protein expression of p-Akt, while p-p65 expression remained stable. XAV939, an inhibitor of Wnt/β-catenin signaling pathway, decreased GOLPH3 overexpression-induced proliferation and enhanced cisplatin-induced angiogenesis inhibition and apoptosis, which was supported by the changes of VEGF, Bax and Bcl-2.
Conclusion: GOLPH3 promotes proliferation capacity in LADC through activating the Wnt/β-catenin signaling pathway.
Keywords: Golgi phosphoprotein 3, lung adenocarcinoma, Wnt/β-catenin, proliferation
Introduction
Lung cancer is a type of tumor with the highest morbidity and mortality in the world in recent years, and it is a serious threat to human life and health.1 Lung cancer is an abbreviation of primary bronchogenic carcinoma, which can be divided into two types: small cell lung cancer, accounting for about 15%, and non-small cell lung cancer, accounting for about 85%. Non-small cell lung cancer is mainly divided into three types according to pathological types as follows: lung adenocarcinoma (LADC), lung squamous cell carcinoma and large cell carcinoma.2 Traditional chemotherapy has limited survival time for patients with advanced non-small cell lung cancer, and this is mainly due to postoperative recurrence and metastasis.2 However, targeted therapy provides a new solution for breaking this bottleneck.
Golgi phosphoprotein 3 (GOLPH3), alternatively known as GMx33 or GPP34, is a highly conserved Golgi protein in eukaryotes.3 GOLPH3 is a recently discovered oncogene located on human chromosome 5p13, where many solid tumor oncogenes are highly amplified.4 A study found that GOLPH3 played an important role in promoting the differentiation and proliferation of tumor cells.5 GOLPH3 activates serine/threonine protein kinase (AKT),6 promotes cancer cell proliferation and inhibits apoptosis.7 GOLPH3 was high expressed in patients with non-small cell lung cancer.8 Lung adenocarcinoma progression was accelerated by activating GOLPH3-dependent vesicular release.9 In this paper, LADC was studied to investigate the function of Golgi phosphoprotein 3 (GOLPH3) in LADC and non-small cell lung cancer.
Wnt signaling pathway plays a key role in the regulation of cell proliferation, differentiation, apoptosis and migration. It has been observed that abnormal expression and activation of WNT signaling pathway components induce tumorigenesis.10 In Wnt signaling pathway, Wnt/β-catenin pathway is typical, with β-catenin serving as its major member. Wnt/β-catenin pathway activates the transcriptional activity of its target gene through nuclear translocation of β-catenin.11 Previous studies have shown that abnormal activation of the Wnt/β-catenin signaling pathway played a role in cell carcinogenesis, tumorigenesis and tumor invasion.12 It has been reported that tumor gene GOLPH3 promoted tumor metastasis and proliferation via activating the Wnt/β- catenin signaling pathway.13
The present study aims to study the role of GOLPH3 in LADC and investigate its association with clinical features for the purpose of providing insight for an early diagnosis of molecular-targeted therapy to LADC.
Patients and Methods
Patient Tissues
Tumor specimens used in the experimental procedure were taken from the Second Hospital Affiliated to Air Force Medical University, and between August 2016 and August 2017 to obtain fresh specimens of LADC, which surgically removed from patients. 85 patients with LADC consist of 35 cases of stage I LADC, 20 cases of stage II LADC, 17 cases of stage III LADC, 13 cases of stage IV LADC and 25 cases of adjacent mucosa of patients with stage I LADC were applied as Normal control. Tumor specimens were placed in a liquid nitrogen tank and frozen immediately after being taken. All patients were going to receive an operation firstly and the original disease would be diagnosed. No radiotherapy or chemotherapy was performed before surgery. Relevant protocols for experimental study have been approved by the Ethics Committee of the Second Hospital Affiliated to Air Force Medical University (No.:TDLL-201607-09), and the written informed consent was obtained from all subjects.
Reagents
GOLPH3-siRNA, GOLPH3-NC (negative control), GOLPH3 expression vector and GOLPH3-mock were all synthesized from GenePharma (Shanghai, China). Lipofectamine® 3000 Transfection Reagent (Life Technologies, Gaithersburg, MD) was used to conduct transfection of oligonucleotide sequences and plasmids. MTT (3-[4, 5-dimethylthiazol-2-yl]-2, 5,-diphenyltetrazolium bromide) was purchased from USB (Cleveland, OH). XAV939 was purchased from Sigma-Aldrich (St. Louis, MO, USA).
Cell Lines and Culture
Human LADC cell lines (A549, H23, H358, H1299, H1395, H1435, H1650 and PC-9), and Normal lung tissue cells (BEAS2B, 16HBE and HEK 293) were obtained from Shanghai Cell Biology Medical Research Institute, Chinese Academy of Sciences, and maintained as a monolayer at 37 °C with 5% CO2 in Dulbecco’s modified Eagle’s medium (DMEM) or in RPMI-1640 (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum (Gibco, Thermo Fisher Scientific).
Survival Analysis
Patients who were admitted to the First Affiliated Hospital of Zhejiang University between June 2011 and June 2016 were diagnosed with LADC. Extract basic information and data from the cases and related materials were obtained during a patient’s hospitalization period. Survival analysis was performed according to the expression of GOLPH3 in LADC patients. Kaplan-Meier method was used for survival analysis, and Kaplan-Meier survival curve was drawn.
MTT
Cell viability was measured by MTT method. The experimental group and the negative control group to be tested were inoculated into a 96-well plate. The cells were cultured under normal conditions, and cell fusion rate was determined to be about 80%. Cell density was adjusted to 5×103 cells/well and fresh medium containing MTT was added into the cells. After being incubated at 37 °C for 4 h, 150 μL of DMSO (Sigma) was added to each well and gently shaken for 10 min to fully dissolve the MTT. The absorbance of the sample at 570 nm was measured using an enzyme-linked immunosorbent assay.
Immunohistochemical Analysis of GOLPH3
Biopsy specimens of normal lung cancer tissues and clinically staged LADC tissues were routinely fixed in 4% paraformaldehyde and then dehydrated to obtain paraffin sections and cut to 5 μm thick. Tissue sections were dewaxed with xylene and classified. The ethanol series were hydrated with distilled water. After antigen retrieval, normal rabbit serum (ZSGB-Bio, China) was added and blocked at room temperature for 20 min. The samples were then stained at room temperature for 1 h using 1:200 rabbit anti-GOLPH3. Goat anti-rabbit secondary antibody (ZSGB-Bio) was then added at 37 °C for 15 min. Signal was displayed by 3, 3-DAB, and hematoxylin was used for counterstaining. Finally, the sections were dehydrated by fractionation of ethanol series and xylene, and their morphologies were observed by light microscopy and pictures were captured.
Quantitative Real-Time (qRT)-PCR
Cultured cells were collected to isolate RNA by using Trizol (Invitrogen) according to the manufacturer’s protocol. One microgram of RNA was used for reverse transcription into cDNA according to reverse transcription cDNA kit (Thermo Fisher Scientific, Waltham, USA). The reaction conditions were set as follows: at 42 °C for 60 min, at 70 °C for 5 min, at 4 °C preservation. SYBR Green PCR Master Mix (Roche, Basle, Switzerland) was used to conduct qPCR experiment using opticon RT-PCR Detection System (ABI 7500, Life technology, USA). The PCR cycle was set as follows: pretreatment at 95 °C for 10 min; followed by 40 cycles at 94 °C for 15 sec, at 60 °C for 1 min, finally at 60 °C for 1 min and at 4 °C for preservation. The expression levels of above genes were analyzed by the 2−ΔΔCT.14 Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression was used for normalization. The primer sequences were listed in Table 1.
 |
Table 1 Primers for RT-qPCR |
Western Blot
Total proteins were collected by RIPA (Cell Signaling Technology, Inc., Danvers, MA, USA). BCA Protein Assay Kit (Pierce) was used to measure the concentration of proteins, which was adjusted to a concentration of 6 μg/μL using 1 × loading and DEPC water. 10% SDS-PAGE gels were used to separate the samples, which were then transferred onto a piece of polyvinylidene fluoride membrane (PVDF, Millipore, and USA). After blocking in 5% non-fat milk in PBST (0.1% Tween 20 in phosphate-buffered saline (PBS)) for 1 h, the membrane was probed with the primary antibody overnight at 4 °C and then incubated with secondary antibody (horseradish peroxidase (HRP)-conjugated goat anti-mouse/rabbit IgG, 1:2000; sc-516102/sc-2357; Santa Cruz Biotechnology, Inc. Dallas, TX, USA) at room temperature for 2 h. Developer (EZ-ECL kit; Biological Industries BI) was used for development, and the gray value of the strips was analyzed and counted by imageJ (version 5.0; Bio-Rad, Hercules, CA, USA). Antibodies used were as follows: anti-GAPDH (mouse; 1:1000; LS-B1625; LifeSpan BioSciences, Inc.), anti-GOLPH3 (rabbit; 1:1000; ab91492; abcam), anti-E-cadherin (mouse; 1:1000; ab1416; abcam), anti- vascular endothelial growth factor (VEGF; rabbit; 1:1000; #2463; CST), anti- B-cell lymphoma 2 (Bcl-2; rabbit; 1:1000; ab32124; abcam), anti-Bcl-2-associated X protein (Bax; rabbit; 1:1000; ab32503; abcam), anti-AKT (rabbit; 1:1000; ab8805; abcam), anti- phosphorylated (p)- AKT (rabbit; 1:1000; ab38449; abcam), anti- nuclear factor kappa B (p-65; rabbit; 1:1000; ab16502; abcam), and anti-p-p-65 (mouse; 1:1000; ab6503; abcam).
Apoptosis
After the cells have been transfected for 48 h, 1 × 106 cells were collected and 1 mL of trypsin (trypsin) without ethylenediaminetetraacetic acid (EDTA) was gently shaken and used to digest the cells. Trypsin was taken when the wall was wet. After being stored at room temperature for 1 min, digestion was terminated by adding DMEM (Corning) containing 10% fetal bovine serum. The cells were centrifuged at 1000 × g for 3 min and the supernatant was removed. The cells were washed twice with pre-cooled PBS and resuspended in 1× Annexin V binding buffer. According to the Annexin-V-FITC (fluoresceine isothiocyanate) cell apoptosis detection kit (K201-100, BioVision, Milpitas, CA, USA), the cells was stained with 1. 25 μL Annexin V-FITC and 10 μL of propidium iodide (PI) and measured by flow cytometry (version 10.0, FlowJo, FACS CaliburTM, BD, Franklin Lakes, NJ, USA).
In vitro Angiogenesis Experiment
Matrigel plug angiogenesis assay (Corning, NY, USA) was used to determine in vivo angiogenic rate. Matrigel stock solution was thawed overnight at 4 °C. A gel solution was prepared using a Matrigel stock solution and serum-free DMEM, and the solution was placed in a 96-well plate and then allowed to be incubated for 2 h to cure. The cultured cells were collected and digested to prepare a single cell suspension under aseptic conditions, and the cell suspension was adjusted to a density of 1×105/mL. The cells were seeded in 96-well plates at 100 μL per well. The plates were incubated for 6–8 h in an incubator (5% CO2, 37 °C) and the cells were visualized using an inverted microscope (Thermo Fisher Scientific) and then photographed.
Statistical Analysis
The data were presented as Mean ± SD. GraphPad. Prism 5 was used to analyze the data. Comparison of data was analyzed by t-test. A P<0.05 was considered as a statistically significant.
Results
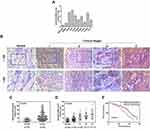
GOLPH3 Was Highly Expressed in LADC Cell Lines and Tissues, and Correlated with Poor Overall Survival in Patients with LADC
GOLPH3 expression in LADC cell lines and cancer tissues was examined using qPCR and Western blot analysis. GOLPH3 was obviously increased in all LADC cell lines including A549, H23, H358, H1299, H1395, H1435, H1650 and PC-9 (Figure 1A) at mRNA level. In addition, we performed immunohistochemical analysis on 85 tumor tissues and 25 normal lung tissues. The color of GLOPH3 was brighter and the positive area was larger in cancer tissues than those in normal tissues (Figure 1B). Consistent with the immunohistochemical analysis, GOLPH3 was highly expressed in tumor tissues in a clinical stages-dependent manner of lung cancer at mRNA level (Figure 1C and D). A survival analysis showed that an inverse correlation existed between a GOLPH3 level and an overall survival time of patients with LADC, that is, the median overall survival time of patients with GOLPH3 (high levels) was significantly lower than patients with GOLPH3 (low levels) (P=0.016, Figure 1E). A549 and H23 were used in later experiments.
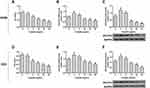
Cisplatin Inhibited the Cell Viability and the Level of GOLPH3 Was Decreased
We further explored the role of GOLPH3 in drug sensitivity. The viability of cell was examined by MTT and the expression level of GOLPH3 was detected by qPCR and Western blot. We found that the cell viability was inhibited by cisplatin with the increase of dose (Figure 2A) and the expression of GOLPH3 was decreased with increased concentration of cisplatin (10, 30 or 50 µg/mL) treatment at mRNA (Figure 2B) and protein levels (Figure 2C) in A549 cell. Similar results were discovered in H23 cell (Figure 2D–F). The increase of GOLPH3 levels caused by low concentrations of cisplatin (1 or 5 µg/mL) might be related to the stress response of cells.
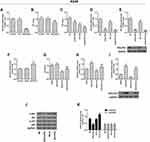
Inhibition of Cells by Cisplatin Was Enhanced and the Level of p-Akt Was Suppressed by Silencing GOLPH3 in A549 Cell, While Overexpression of GOLPH3 Had Contrast Effects
To elucidate the functional importance of GOLPH3, A549 cells were transfected with corresponding plasmids to stably knockdown or overexpression of GOLPH3. We found that GOLPH3 was successful knocked out (Figure 3A) and the A549 cell viability was inhibited by siGOLPH3 (Figure 3B). Meanwhile, inhibition of A549 cell by cisplatin was enhanced by siGOLPH3 (Figure 3C). We also discovered that the expression of GOLPH3 was lower in siGOLPH3+Cis group than that in Cis group at mRNA (Figure 3D) and protein levels (Figure 3E). However, overexpression of GOLPH3 had opposite effects. The GOLPH3 was successfully overexpressed (Figure 3F) and inhibition of A549 cell by cisplatin was weakened by overexpression of GOLPH3 (Figure 3G). The expression of GOLPH3 was higher in GOLPH3+Cis group than that in Cis group at mRNA (Figure 3H) and protein levels (Figure 3I). We also found that p-Akt expression was suppressed by siGOLPH3, while overexpression of GOLPH3 upregulated the expression of p-Akt (Figure 3J and K).
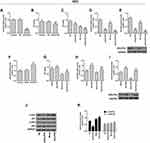
Inhibition of Cells by Cisplatin Was Enhanced and the Level of p-Akt Was Downregulated by Silencing GOLPH3 in H23 Cell, While Overexpression of GOLPH3 Led to the Opposite Results
We verified the role of GOLPH3 in H23 cells. Corresponding plasmids were used for knockout or overexpression of GOLPH3, and we found that GOLPH3 was successfully knocked out (Figure 4A) and H23 cell viability was inhibited by siGOLPH3 (Figure 4B). Meanwhile, downregulation of GOLPH3 increased the sensitivity of H23 cells to cisplatin, compared with the NC-Cis group (Figure 4C). We also discovered that the expression of GOLPH3 was lower in siGOLPH3+Cis group than that in Cis group at mRNA (Figure 4D) and protein levels (Figure 4E). However, overexpression of GOLPH3 had opposite effects. The GOLPH3 was successfully overexpressed (Figure 4F) and inhibition of H23 cell by cisplatin was weakened by overexpression of GOLPH3 (Figure 4G). The expression of GOLPH3 was higher in GOLPH3+Cis group than that in Cis group at mRNA (Figure 4H) and protein levels (Figure 4I). We also found that p-Akt expression was suppressed by siGOLPH3, while overexpression of GOLPH3 upregulated the expression of p-Akt (Figure 4J and K).
Effect of XAV939 on GOLPH3 and β-Catenin Protein Expressions in A549 and H23 Cells
To further identify the potential mechanism underlying the regulation of tumor cell cisplatin chemosensitivity by GOLPH3, XAV939, a small molecule inhibitor of the dysregulated Wnt signaling pathway, was used to treat A549 and H23 cells, and Western blot analysis was performed. The results showed that XAV939 changed little of the levels of GOLPH3, compared with GOLPH3 and Cis group in A549 and H23 cells. The protein level of β-catenin was obviously declined by XAV939 compared with GOLPH3 and Cis group (Figure 5).
XAV939 Decreased GOLPH3 Overexpression-Induced Proliferation and Promoted Cisplatin-Induced Growth Inhibition and Apoptosis in A549 Cells
The underlying mechanisms of GOLPH3 effect on chemosensitivity were further studied. A549 cells were treated with XAV939 and/or cisplatin, and MTT. Flow cytometry and in vitro angiogenesis experiment were used to respectively detect cell viability, apoptosis and angiogenesis rates. The results indicated that XAV939 decreased GOLPH3 overexpression-induced cell viability (Figure 6A), increased GOLPH3 overexpression-induced apoptosis (Figure 6B and D) and inhibited GOLPH3 overexpression-induced angiogenesis rates (Figure 6C and E). Meanwhile, we found that VEGF and Bcl-2 were lower and Bax was higher in XAV939 + GOLPH3 group than those in GOLPH3 group at mRNA (Figure 6F–H) and protein levels (Figure 6I and J). Our results also showed that XAV939 decreased cell viability (Figure 6A), enhanced apoptosis (Figure 6B and D) and suppressed angiogenesis rates (Figure 6C and E) induced by cisplatin. Meanwhile, we found that VEGF and Bcl-2 expressions were lower and Bax expression was higher in XAV939 + Cis group than those in Cis group at mRNA (Figure 6F–H) and protein levels (Figure 6I and J).
XAV939 Decreased GOLPH3 Overexpression-Induced Proliferation and Promoted Cisplatin-Induced Growth Inhibition and Apoptosis in H23 Cells
In order to further determine the involvement of Wnt/β-catenin in the effect of GOLPH3 on chemosensitivity, H23 cells were treated with XAV939 and/or cisplatin, and MTT assay, flow cytometry and in vitro angiogenesis experiment were used to detect cell viability, apoptosis and angiogenesis rates, respectively. The results indicated that XAV939 reversed the effects of GOLPH3 overexpression on increasing cell viability (Figure 7A), suppressing apoptosis (Figure 7B and D) and reducing angiogenesis rates (Figure 7C and E). Meanwhile, we found that VEGF and Bcl-2 expressions were lower and Bax expression was higher in XAV939 + GOLPH3 group than those in GOLPH3 group at mRNA (Figure 7F–H) and protein levels (Figure 7I and J). Our results also showed that XAV939 decreased cell viability (Figure 7A), promoted apoptosis (Figure 7B and D) and suppressed angiogenesis rates (Figure 7C and E) induced by cisplatin. Meanwhile, we found that VEGF and Bcl-2 expressions were lower and Bax expression was higher in XAV939 + Cis group than those in Cis group at mRNA (Figure 7F–H) and protein levels (Figure 7I and J).
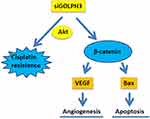
The Mechanism Schematic Figure of GOLPH3 on Lung Adenocarcinoma
The mechanism schematic figure of GOLPH3 on lung adenocarcinoma in this study was shown in Figure 8.
Discussion
Lung cancer is a malignant tumor that seriously threatens human life and health, and it has become a leading cause of cancer death to humans.15 Non-small cell lung cancer accounts for about 80% of lung cancer, and the 5-year survival rate is only about 30% due to postoperative recurrence and metastasis.2 The occurrence and infiltration of lung cancer is a complex process and is regulated by a variety of related genes. Researchers found that GOLPH3 played an important role in promoting the differentiation and proliferation of tumor cells.5 In this study, immunohistochemistry was used to detect the expression of GOLPH3 protein in human lung cancer tissues and normal tissues, and correlations among GOLPH3 expression, clinical pathological features and prognosis of LADC were explored. Our study provided a basis for clinical diagnosis, treatment and prognosis of patients with LADC.
Increasing evidence finds that GOLPH3 is highly expressed in non-small cell lung cancer tissues and cell lines,16 and that high expression of GOLPH3 protein is significantly associated with overall survival in patients with LADC.17 Consistent with previous studies, we found that GOLPH3 was highly expressed in LADC tissues and cell lines, and was positively correlated with lung cancer staging and negatively correlated with overall survival. These data suggested that GOLPH3 was involved in the development of LADC, and that targeting GOLPH3 may treat LADC. Therefore, we further studied the role of GOLPH3 in drug sensitivity.
Cisplatin is a platinum-containing chemotherapeutic drug with a wide range of clinical applications, and it plays an important role in cancer chemotherapy.18 Previous studies have shown that cisplatin could show efficacy in a variety of solid tumors, for example, ovarian cancer,19 prostate cancer, testicular cancer20 and lung cancer.21 In our study, cisplatin was found to reduce the activities of LADC, A549 and H23 cells, and the expression of GOLPH3 was also significantly reduced. To further investigate the role of GOLPH3 in anti-cancer effect of cisplatin, knocking out or overexpressing the GOLPH3 plasmid into A549 and H23 cells showed that silencing GOLPH3 could further inhibit cell viability, compared to cisplatin-treated cancer cells, and that the phosphorylation level of Akt protein was significantly reduced. However, overexpression of GOLPH3 had the opposite effect. These data suggested that GOLPH3 may be involved in the development of LADC by activating cisplatin resistance in LADC.
The Wnt/β-catenin signaling pathway is a complex and conserved signal transduction pathway that plays an important role in embryonic development, regulation of cell growth and differentiation, and tumorigenesis, development and metastasis.22 XAV939 is a small molecule inhibitor of dysregulated Wnt signaling pathway.23 Researchers found that XAV939 could inhibit the growth of non-small cell lung cancer cells by blocking the Wnt signaling pathway.23 Another study also discovered that XAV939 inhibited the proliferation and migration of A549 cells via WNT pathway.21 Western blot data demonstrated that β-catenin expression in XAV939 treatment groups (2μM) was decreased remarkably, compared with the GOLPH3 or Cis group. Meanwhile, XAV939 inhibited cell viability, angiogenesis rate and promoted apoptosis, which were all induced by overexpression of GOLPH3 or cisplatin, evidenced by the downregulated VEGF, Bcl-2 and upregulated Bax at mRNA and protein levels in A549 and H23 cells. Therefore, it was concluded that GOLPH3 activated cisplatin resistance in LADC may correlate to Wnt/β-catenin signaling pathway.
During this study, it might be difficult to clarify the effect of different levels of GOLPH3 on the effect of cisplatin because of sample size, which might be a limitation.
Conclusion
In summary, the present study reveals that GOLPH3 participates in the progression of LADC by activating cisplatin resistance in LADC and Wnt/β-catenin signaling pathway. The present study provides experimental evidence for the basis of novel therapeutic strategies for clinical treatment of LADC. Future studies are required to further elucidate this mechanism and its role in suppressing LADC progression in vivo.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Science and Technology Coordination Project of Shaanxi Province [2016KTCL03-16].
Disclosure
The authors declare no conflicts of interest in this work.
References
1. She J, Yang P, Hong Q, Bai C. Lung cancer in China: challenges and interventions. Chest. 2013;143(4):1117–1126. doi:10.1378/chest.11-2948
2. Hoffman PC, Mauer AM, Vokes EE. Lung cancer. Lancet. 2000;355(9202):479–485. doi:10.1016/S0140-6736(00)82038-3
3. Ren H, Tang X, Lee JJ, et al. Expression of hepatoma-derived growth factor is a strong prognostic predictor for patients with early-stage non-small-cell lung cancer. J Clin Oncol. 2004;22(16):3230–3237. doi:10.1200/JCO.2004.02.080
4. Brognard J, Clark AS, Ni Y, Dennis PA. Akt/protein kinase B is constitutively active in non-small cell lung cancer cells and promotes cellular survival and resistance to chemotherapy and radiation. Cancer Res. 2001;61(10):3986–3997.
5. Abraham RT. GOLPH3 links the golgi network to mTOR signaling and human cancer. Pigment Cell Melanoma Res. 2009;22(4):378–379. doi:10.1111/j.1755-148X.2009.00596.x
6. Snyder CM, Mardones GA, Ladinsky MS, Howell KE. GMx33 associates with the trans-golgi matrix in a dynamic manner and sorts within tubules exiting the golgi. Mol Biol Cell. 2006;17(1):511–524. doi:10.1091/mbc.e05-07-0682
7. Ciccia A, Elledge SJ. The DNA damage response: making it safe to play with knives. Mol Cell. 2010;40(2):179–204. doi:10.1016/j.molcel.2010.09.019
8. Shi W, Feng W, Wang J, et al. Clinicopathologic features and prognostic implications of golgi phosphoprotein 3 in non-small cell lung cancer: a meta-analysis. J Cancer. 2019;10(23):5754–5763. doi:10.7150/jca.30067
9. Tan X, Banerjee P, Pham EA, et al. PI4KIIIbeta is a therapeutic target in chromosome 1q-amplified lung adenocarcinoma. Sci Transl Med. 2009;459(7250):eaax3772. doi:10.1126/scitranslmed.aax3772
10. Gorringe KL, Boussioutas A, Bowtell DD. Novel regions of chromosomal amplification at 6p21, 5p13, and 12q14 in gastric cancer identified by array comparative genomic hybridization. Genes Chromosomes Cancer. 2005;42(3):247–259. doi:10.1002/gcc.20136
11. Bohm M, Wieland I, Schmidt C, Rubben H, Allhoff EP. Loss of heterozygosity on chromosome 5p13-12 predicts adverse prognosis in advanced bladder cancer independent of tumor stage and grade. J Urol. 2002;168(6):2655–2658. doi:10.1016/S0022-5347(05)64238-3
12. Yokoi S, Yasui K, Saito-Ohara F, et al. A novel target gene, SKP2, within the 5p13 amplicon that is frequently detected in small cell lung cancers. Am J Pathol. 2002;161(1):207–216. doi:10.1016/S0002-9440(10)64172-7
13. Qiu CZ, Wang MZ, Yu WS, Guo YT, Wang CX, Yang XF. Correlation of GOLPH3 gene with wnt signaling pathway in human colon cancer cells. J Cancer. 2016;7(8):928–934. doi:10.7150/jca.13968
14. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25(4):402–408. doi:10.1006/meth.2001.1262
15. Liang H, Liu X, Wang M. Immunotherapy combined with epidermal growth factor receptor-tyrosine kinase inhibitors in non-small-cell lung cancer treatment. Onco Targets Ther. 2018;11:6189–6196. doi:10.2147/OTT.S178497
16. Scott KL, Kabbarah O, Liang MC, et al. GOLPH3 modulates mTOR signalling and rapamycin sensitivity in cancer. Nature. 2009;459(7250):1085–1090. doi:10.1038/nature08109
17. Zeng Z, Lin H, Zhao X, et al. Overexpression of GOLPH3 promotes proliferation and tumorigenicity in breast cancer via suppression of the FOXO1 transcription factor. Clin Cancer Res. 2012;18(15):4059–4069. doi:10.1158/1078-0432.CCR-11-3156
18. Holland JD, Klaus A, Garratt AN, Birchmeier W. Wnt signaling in stem and cancer stem cells. Curr Opin Cell Biol. 2013;25(2):254–264. doi:10.1016/j.ceb.2013.01.004
19. Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer. 2013;13(1):11–26. doi:10.1038/nrc3419
20. Kasagi Y, Oki E, Ando K, et al. The expression of CCAT2, a novel long noncoding RNA transcript, and rs6983267 single-nucleotide polymorphism genotypes in colorectal cancers. Oncology. 2017;92(1):48–54. doi:10.1159/000452143
21. Li C, Zheng X, Han Y, Lv Y, Lan F, Zhao J. XAV939 inhibits the proliferation and migration of lung adenocarcinoma A549 cells through the WNT pathway. Oncol Lett. 2018;15(6):8973–8982. doi:10.3892/ol.2018.8491
22. Hodges SL, Lugo JN. Wnt/beta-catenin signaling as a potential target for novel epilepsy therapies. Epilepsy Res. 2018;146:9–16. doi:10.1016/j.eplepsyres.2018.07.002
23. Pan F, Shen F, Yang L, Zhang L, Guo W, Tian J. Inhibitory effects of XAV939 on the proliferation of small-cell lung cancer H446 cells and Wnt/beta-catenin signaling pathway in vitro. Oncol Lett. 2018;16(2):1953–1958. doi:10.3892/ol.2018.8790
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.