Back to Journals » International Journal of Nanomedicine » Volume 10 » Issue 1
Gold nanoparticles induce heme oxygenase-1 expression through Nrf2 activation and Bach1 export in human vascular endothelial cells
Authors Lai T, Shieh J, Tsou C, Wu W
Received 13 May 2015
Accepted for publication 8 July 2015
Published 21 September 2015 Volume 2015:10(1) Pages 5925—5939
DOI https://doi.org/10.2147/IJN.S88514
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Thomas Webster
Tsung-Hsuan Lai,1–3 Jiunn-Min Shieh,4,5 Chih-Jen Tsou,1 Wen-Bin Wu1
1School of Medicine, Fu-Jen Catholic University, New Taipei City, Taiwan; 2Department of Obstetrics and Gynecology, Cathay General Hospital, Taipei, Taiwan; 3Institute of Systems Biology and Bioinformatics, National Central University, Jhongli City, Taiwan; 4Department of Internal Medicine, Chi-Mei Medical Center, Tainan, Taiwan; 5Department of Recreation and Healthcare Management, Chia Nan University of Pharmacy and Science, Tainan, Taiwan
Abstract: It has been reported that increased levels and activity of the heme oxygenase-1 (HO-1) protein ameliorate tissue injuries. In the present study, we investigated the effects and mechanisms of action of gold nanoparticles (AuNPs) on HO-1 protein expression in human vascular endothelial cells (ECs). The AuNPs induced HO-1 protein and mRNA expression in a concentration- and time-dependent manner. The induction was reduced by the thiol-containing antioxidants, including N-acetylcysteine and glutathione, but not by the non-thiol-containing antioxidants and inhibitors that block the enzymes for intracellular reactive oxygen species generation. The AuNPs enhanced Nrf2 protein levels but did not affect Nrf2 mRNA expression. In response to the AuNP treatment, the cytosolic Nrf2 translocated to the nucleus, and, concomitantly, Bach1 exited the nucleus and its tyrosine phosphorylation increased. The chromatin immunoprecipitation assay revealed that the translocated Nrf2 bound to the antioxidant-response element located in the E2 enhancer region of the HO-1 gene promoter and acted as a transcription factor. Although N-acetylcysteine inhibited the AuNP-induced Nrf2 nuclear translocation, the AuNPs did not promote intracellular reactive oxygen species production or endoplasmic reticulum stress in the ECs. Knockdown of Nrf2 expression by RNA interference significantly inhibited AuNP-induced HO-1 expression at the protein and mRNA levels. In summary, AuNPs enhance the levels and nuclear translocation of the Nrf2 protein and Bach1 export/tyrosine phosphorylation, leading to Nrf2 binding to the HO-1 E2 enhancer promoter region to drive HO-1 expression in ECs. This study, together with our parallel findings, demonstrates that AuNPs can act as an HO-1 inducer, which may partially contribute to their anti-inflammatory bioactivity in human vascular ECs.
Keywords: endothelium, inflammation, Keap1, nuclear export, translocation, tyrosine phosphorylation
Introduction
The endothelium plays a pivotal role in vasoregulation.1 Vascular dysfunction or injury induced by aging, smoking, inflammation, trauma, hyperlipidemia, and hyperglycemia are among a myriad of risk factors that may contribute to the pathogenesis of many cardiovascular diseases, such as hypertension, diabetes, and atherosclerosis.2 These factors also produce reactive oxygen species (ROS), which regulate fundamental cellular functions such as growth (hyperplastic or hypertrophic), endothelial dysfunction, migration, and inflammation, which have been demonstrated to play a role in atherogenesis.3 Therefore, cells have evolved endogenous defense mechanisms to counteract oxidative stress. Among these, the redox-sensitive transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2) serves as a “master regulator” of cell survival through the coordinated induction of the phase II and antioxidant defense enzymes via a cis-acting element, designated the antioxidant-response element (ARE), in the promoter of the target genes.4 These include a myriad of genes encoding chemopreventive proteins, including detoxifying enzymes NAD(P)H:quinone oxidoreductases (NQO1 and NQO2), the glutathione (GSH) S-transferase Ya subunit, γ-glutamylcysteine synthetase, and heme oxygenase (HO)-1.5
HO is the rate-limiting enzyme in the catabolism of heme, a process that leads to formation of equimolar amounts of the bile pigment biliverdin, free iron, and carbon monoxide (CO).6 There are two isoenzymes of HO: the original enzyme, designated HO-1, and the second isoenzyme, designated HO-2. These two enzymes share approximately 40% amino acid sequence homology.7,8 It is apparent that HO-2 is constitutively expressed, whereas HO-1 is inducible by a large number of structurally unrelated pharmacological and natural agents as well as by a variety of environmental influences, such as heat shock, and other forms of cellular stress.6 Heme degradation is now considered critical for cellular defense, because the prooxidant heme is removed and the increased production of bilirubin and CO is beneficial and critical to the cellular defense mechanisms.6 Major pathological alterations of the endothelium have been observed in HO-1 knockout mice, in which the endothelial cells (ECs) were more susceptible to apoptotic cell death and denudation from the extracellular matrix.9 In addition, anti-inflammatory endothelial protection via HO-1 has been shown to be mediated by its ability to downregulate TNF-α-induced expression of various adhesion molecules.10,11
The common pathway for inducing HO-1 expression depends on the activation of redox-dependent transcriptional activators, such as Nrf2, NF-κB, and AP-1, along with the transcription repressor BTB and CNC homolog 1 (Bach1).12 Activation of Nrf2 by oxidative stress is primarily controlled by the cytosolic inhibitor Kelch-like ECH-associated protein 1 (Keap1).13 Numerous prooxidant stimuli and electrophiles cause the dissociation of Nrf2 from Keap1, which permits the subsequent nuclear translocation of Nrf2 and its interaction with AREs of HO-1 promoter.12,14 However, it was recently reported that Nrf2 can also be regulated and activated independent of Keap1, including the phosphorylation of Nrf2 by several signal transduction pathways, the involvement of epigenetic factors such as microRNAs, or the interaction of Nrf2 with other proteins.12 Interestingly, an antioxidant, tert-butylhydroquinone, also induces tyrosine486 phosphorylation of Bach1 and leads to rapid nuclear export of Bach1, which allows Nrf2 to bind to the ARE and activate defensive gene expression.15
Gold-based compounds have been paid much attention and have a long history of being used for therapeutic purposes in arthritis and cancer. Most commercially available colloidal gold solutions are obtained either by chemical reduction or physical synthesis. Previous studies have shown that gold nanoparticles (AuNPs) can affect vascular endothelial growth factor (VEGF)- and basic fibroblast growth factor (bFGF)-induced cell proliferation in vitro and angiogenesis in vivo.16,17 In addition, AuNPs bind to VEGF in arthritic synovial fluid, exert antiangiogenic activities, and reduce inflammation and arthritis.18 However, some studies have also shown that nanoparticles, particularly high concentrations of silver nanoparticles, exhibit cell cytotoxicity and, therefore, induce HO-1 expression.19,20
In our parallel study, we observed that AuNPs are able to inhibit cell adhesion molecule (CAM) expression and have anti-inflammatory effects on human vascular ECs (unpublished data). Given that HO-1 expression has been associated with anti-inflammation and injury in some tissues,6 we hypothesized that AuNPs can affect HO-1 expression. Therefore, the ability of AuNPs to induce HO-1 expression was evaluated and their mechanism of action in human vascular ECs was investigated in this study.
Materials and methods
AuNPs
AuNPs were obtained from Gold NanoTech Inc. (Taipei, Taiwan), and their preparation has been previously described.21,22 Briefly, the gold was cut into the target material and evaporated to the atomic level by an electrical gasification method. The evaporated gold was slowly deposited as AuNPs in distilled water under high vacuum (10−8 Pa). The sizes of the AuNPs were controlled by the evaporation time and electric current used. The AuNPs, ranging from 3 to 5 nm, were dissolved in distilled water and gently agitated before being applied to the ECs. Their basic physical properties have been characterized, including their size distribution and average size. The average size was estimated to be 3.38±0.974 nm by transmission electronic microscopy (TEM) (unpublished data).
Materials
N-acetylcysteine (NAC), reduced GSH, vitamin C (vit C), Trolox, dithiothreitol (DTT), and the MAPK and PI-3K inhibitors were purchased from Sigma-Aldrich Co. (St Louis, MO, USA). Rotenone was obtained from Tocris Cookson Ltd. (Bristol, UK). Leptomycin B (LMB), apocynin, and thapsigargin were from Cayman Chemicals (Ann Arbor, MI, USA). CM-H2DCFDA was purchased from Thermo Fisher Scientific (Waltham, MA, USA). The antibody (Ab) for HO-1 was from Abcam (Cambridge, MA, USA). The endoplasmic reticulum (ER) stress sampler kit with Abs raised against stress proteins was purchased from Cell Signaling, Inc. (Danvers, MA, USA). The Abs for Bach1 and lamin B were obtained from Santa Cruz Biotechnology Inc. (Dallas, TX, USA). The Ab for α-tubulin was purchased from EMD Millipore (Billerica, MA, USA). The Ab raised against phosphotyrosine (4G10) was from EMD Milllipore (Billerica, MA, USA).
Cell cultures
The use of human umbilical cord veins for preparing human vascular ECs was approved by the Institutional Review Board of Cathay General Hospital (Taipei, Taiwan). The ECs were prepared and characterized by a previously described method.23,24 Isolated ECs were maintained in M199 containing 20% fetal bovine serum, 30 μg/mL endothelial cell growth supplement, 4 mM L-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, and 250 ng/mL Fungizone (Thermo Fisher Scientific). The cells were used between the second and fourth passages in this study.
Cell lysate preparation, Western blot analysis, and immunoprecipitation
The cell lysates were prepared as previously described.23 The total proteins were separated by electrophoresis through sodium dodecyl sulfate polyacrylamide gels, electroblotted onto polyvinylidene fluoride membranes, and then probed using a primary Ab. The immunoblots were developed using Immobilon Western Chemiluminescent HRP Substrate (EMD Millipore, Billerica, MA, USA). To perform the immunoprecipitations (IPs), equal amounts of the total proteins were incubated with anti-Bach1 or phosphotyrosine Abs (1 μg Ab/200 μg of the total proteins) at 4°C for 24 hours, followed by incubation with Protein A/G PLUS-agarose (Santa Cruz Biotechnology Inc.) at 4°C for 2 hours. The beads were sedimented by brief centrifugation after the IP and washed extensively with RIPA lysis buffer. The proteins were released by boiling in Laemmli sample buffer and analyzed by Western blotting.
Enzyme-linked immunosorbent assay measurement of the HO-1 levels
The absolute intracellular levels of HO-1 were determined using a human HO-1 enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Inc., Minneapolis, MN, USA), according to the manufacturer’s protocol. Briefly, the ECs were treated with vehicle (ddH2O) or the AuNPs. The cell lysates were prepared, and the level of HO-1 was measured by ELISA at 450 nm. The absolute HO-1 concentrations were calculated from a standard curve.
Reverse transcription polymerase chain reaction analysis of HO-1, Nrf2, and β-actin mRNA expression
Oligonucleotide polymerase chain reaction (PCR) primers targeting human HO-1, Nrf2, and β-actin were synthesized and are listed in Table 1. Briefly, the total RNA from the ECs was extracted with TRIzol (Thermo Fisher Scientific) and the reverse transcription (RT) reaction was performed by using the iScript cDNA Synthesis Kit (Bio-Rad Laboratories Inc., Hercules, CA, USA). The cDNAs were synthesized from 1 μg of total RNA and the iScript reaction mix according to the manufacturer’s protocol. Aliquots of transcribed cDNAs were subjected to PCR in a 25 μL reaction mixture containing the GoTaq green master mix (Promega Corporation, Fitchburg, WI, USA). PCR was performed with a hot start at 94°C for 5 minutes and then with 30 cycles of denaturation at 94°C for 1 minute, annealing at 51°C for 1 minute, and elongation at 72°C for 1.5 minutes on the ABI 7200 Thermal Cycler (Thermo Fisher Scientific). The amplification products were then analyzed by 2% agarose gel electrophoresis.
  | Table 1 Primers for the reverse transcription polymerase chain reaction analysis |
Western blot analysis of Nrf2 translocation and Bach1 export
The cytosolic and nuclear fractions of the ECs were prepared using the NE-PER™ nuclear and cytoplasmic extraction reagents (Thermo Fisher Scientific) according to the manufacturer’s protocol. The Nrf2 translocation and Bach1 export were determined by Western blot analysis of their distributions in the cytosolic and nuclear fractions.
Immunofluorescence microscopy of the subcellular distribution of Nrf2 and Bach1
The cells were seeded on chamber slides and treated with the AuNPs. The cells were then washed, fixed with 1% paraformaldehyde for 20 minutes, and then permeabilized with 0.1% Triton X-100 for 10 minutes. After blocking with 3% BSA, the cells were incubated with control and specific Abs for Nrf2 (1:150; Abcam) or Bach1 (1:175; Santa Cruz Biotechnology Inc.) at 4°C overnight. Next, the chamber slides were incubated with DAPI (1:3,000; Thermo Fisher Scientific), analyzed under a Nikon Eclipse Ti-S fluorescence microscope (Nikon Corporation, Tokyo, Japan), and photographed using a digital camera. The images were merged using Adobe Photoshop 7.0.
Chromatin IP assay
The chromatin IP (ChIP) assay was performed using the EZ-Magna ChIP kit (EMD Milllipore) according to the manufacturer’s protocol. Briefly, the fixed cells were sonicated and centrifuged, and the supernatant fraction was used as the cell lysate in the ChIP analysis. Anti-Nrf2 Ab (Abcam) and normal rabbit IgG (EMD Milllipore), as a negative control, were used. These Abs were immobilized to magnetic protein G beads and used for the IP reactions. The precipitated DNA fragments were detected using RT-PCR and real-time PCR with the following previously described primers:25 HO-1 E2 enhancer region: 5′-CCCTGCTGAGTAATCCTTTCC-3′ and 5′-GGCGGTGACTTAGCGAAAAT-3′; and HO-1 gene promoter region: 5′-GCCAGAAAGTGGGCATCAG-3′ and 5′-CTGAGGACGCTCGAGGGAG-3′. The real-time PCR was performed using a 7500 StepOne Real-Time PCR System (Thermo Fisher Scientific). The relative gene expression was determined by the ΔΔCt method, where Ct was the threshold cycle. All experiments were performed in duplicate or triplicate.
Measurement of intracellular ROS production
The ECs were seeded in 96-well plates and cultured until the cell density reached 90% confluency. The cells were incubated with M199 containing CM-H2DCFDA (5 μM) at 37°C for 30 minutes, followed by a brief wash. H2O (control), AuNPs, or H2O2 (100 μM) was added to ECs for the indicated time intervals, and the fluorescence intensity was determined by fluorometry at 485 nm (excitation) and 525 nm (emission).
Nrf2 siRNA transfection
ON-TARGET plus SMARTpool control and Nrf2 siRNAs were purchased from Thermo Fisher Scientific. The transfection was performed according to the manufacturer’s protocol, with some modifications. The ECs were seeded in six-well plates and incubated overnight in complete medium. The cell cultures were transfected with control or Nrf2 siRNAs (150 nM, 2 mL) for 3 days using the DharmaFECT transfection reagent. After being treated with the AuNPs, the cells were collected and prepared for Western blotting and RT-PCR analysis.
Statistical analysis
The data are expressed as the means ± standard error of the means. A comparison of the means of two groups of data was performed using the unpaired, two-tailed Student’s t-test. *P<0.05, **P<0.01, and ***P<0.001 are considered significant.
Results
AuNPs induce HO-1 protein and mRNA expression in ECs
Western blotting was performed to determine whether the AuNPs induced HO-1 expression in the ECs. As shown in Figure 1A, HO-1 was expressed at a low level in unstimulated cells, but was apparently increased by the AuNP treatment. A quantitative analysis indicated that the AuNPs induced HO-1 expression in a concentration- and time-dependent manner. A similar concentration-dependent result was obtained by ELISA, which showed that the HO-1 levels were significantly induced by the AuNPs at 1 and 2 ppm (Figure 1B). The AuNPs were dissolved in ddH2O. Therefore, to exclude the possibility that the HO-1 induction resulted from endotoxin contamination, a similar experiment was performed in the presence of polymyxin B, an antibiotic that can bind to the lipid A portion of lipopolysaccharide (LPS) and interferes with LPS function.26 As shown in Figure 1C, LPS alone did not cause HO-1 expression, but induced intercellular adhesion molecule-1 (ICAM-1) expression. Polymyxin B did not interfere with the AuNP-induced HO-1 expression; however, it inhibited LPS-induced ICAM-1 expression.
Next, we examined whether the AuNPs affected the HO-1 mRNA level in the ECs. As shown in Figure 1D, the AuNPs induced HO-1 mRNA expression in a concentration- and time-dependent manner. In contrast, the β-actin level remained unchanged. The HO-1 mRNA induction could be observed at 1 ppm of AuNPs and after 2 hours of AuNP treatment.
Effect of signaling inhibitors on AuNP-induced HO-1 expression
In an attempt to investigate the possible signaling pathways involved in the induction of HO-1 expression by the AuNPs, the effects of several pharmacological inhibitors targeting intracellular signaling pathways were determined. The inhibitors included SP600125 for JNK, SB202190 for p38 MAPK, PD98059 for MAPKK, LY294002 for PI-3K, H-89 for protein kinase A (PKA), GF109203X for PKC, NAC for scavenging ROS or destroying disulfide linkages,27 Bay117085 for NF-κB, diphenyleneiodonium for NADPH oxidase inhibitor, rapamycin for the mTOR pathway, tanshinone IIA for AP-1, and steroidal dexamethasone. In Figure 2A, the Western blot analysis shows that only NAC could abolish the AuNP-induced HO-1 expression. As shown in Figure 2B and C, the effects of different NAC doses and other ROS inhibitors on the ECs were further determined. NAC inhibited the AuNP-induced HO-1 expression in a concentration-dependent manner, as assayed by Western blotting and ELISA (Figure 2B). However, the AuNP-induced HO-1 expression was not affected by the other ROS-related inhibitors, including rotenone (a mitochondrial ROS inhibitor) and apocynin (a NADPH oxidase inhibitor) (Figure 2C). Moreover, the RT-PCR analysis indicated that NAC could suppress the AuNP-induced HO-1 mRNA expression (Figure 2D). As NAC have ROS scavenging and reducing activity, next, two ROS scavengers (vit C and Trolox) and two reducing agents (GSH and DTT) were used to clarify which types of antioxidants could affect the AuNP-induced HO-1 expression. As shown in Figure 2E, only GSH and DTT could abolish the AuNP-induced HO-1 expression at 4- and 16-hour incubation, suggesting that the AuNPs affect HO-1 expression via a redox reaction.
Effect of the AuNPs on the Nrf2-mediated signaling pathway
We have shown that NF-κB and AP-1 did not participate in the AuNP-induced HO-1 expression in the ECs (Figure 2A). Next, we tested whether the AuNPs influenced the Nrf2 signaling pathway. As shown in Figure 3A, treatment of ECs with the AuNPs led to a significant increase of the Nrf2 protein but not mRNA expression in a time- and concentration-dependent manner, suggesting the involvement of posttranslational modifications of Nrf2 in this induction.
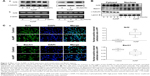
Next, we investigated whether the AuNPs affected subcellular distribution of Nrf2. As shown in Figure 3B, Nrf2 was preferentially expressed in the cytosol of the unstimulated ECs; however, Nrf2 expression in the nucleus was markedly increased in the AuNP-treated ECs. This indicates that Nrf2 translocated from the cytosol to nucleus. Interestingly, Bach1, a nuclear protein shown to associate with small MAF proteins and serve as a repressor of HO-1 gene expression28 was simultaneously increased in the cytosol of the ECs in response to the AuNP treatment, indicating that Bach1 was exported from the nucleus to cytosol (Figure 3B). This bidirectional transport of Nrf2 and Bach1 was further confirmed by immunofluorescence microscopy (Figure 3C). The location of nucleus was identified by DAPI (blue color), and the Nrf2 or Bach1 expression was identified by anti-Nrf2 and anti-Bach1 Abs (green color). The merger of these two images revealed more Nrf2-positive nuclear staining in the AuNP-treated cells and less Bach1-positive nuclear staining in the control group (indigo color). Taken together, the AuNP treatment significantly increased Nrf2 nuclear translocation and Bach1 cytosolic export in the ECs.
The phosphorylation of tyrosine486 can lead to rapid nuclear export of Bach1 and allows Nrf2 to bind to the ARE.15 To examine the role of Bach1, LMB, a specific nuclear export inhibitor,29 was used to determine its effect on the AuNP-induced HO-1 expression. Figure 4A shows that HO-1 protein and mRNA expression was notably reduced by LMB. Next, the alterations in Bach1 phosphorylation were examined in the presence of the AuNPs. Figure 4B shows that the tyrosine phosphorylation of Bach1 increased upon the AuNP treatment, as determined by IP. The internal controls indicated an equivalent loading and preparation of the samples.
Effect of the AuNPs on Nrf2 binding to the HO-1 promoter region
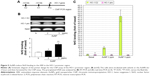
In the human HO-1 gene locus, the regions at −4 and −9 kb upstream of the transcription start site contain multiple ARE sites.30 To investigate whether the translocated Nrf2 interacted with the HO-1 promoter region, the primer sets for the enhancer region (E2) and promoter region near transcription start site were synthesized for ChIP assays (Figure 5A, upper panel schematic). The ChIP analyses demonstrated that AuNP treatment significantly induced Nrf2 binding to the human HO-1 E2 enhancer region, but not to the promoter region near the transcription start site. The binding to the enhancer region was concentration dependent, as determined by RT-PCR and real-time PCR (Figure 5B and C).
Effect of the AuNPs on intracellular ROS production and ER stress
It has been reported that modification of one or more critical cysteine residues in Keap1 represents a likely chemico-biological trigger for the activation of Nrf2.12 To determine whether AuNPs are a stressor, we examined ROS production and ER stress in the ECs upon the AuNP treatment. As shown in Figure 6A, the AuNPs did not cause intracellular ROS production, which was in contrast to the exogenous H2O2. The AuNPs also did not induce ER stress in the ECs. However, thapsigargin, a compound known to cause ER stress by emptying the ER of calcium ions, decreased calnexin and increased the BiP and PDI proteins after 4 hours of treatment (Figure 6B).
NAC diminishes Nrf2 translocation and Nrf2 knockdown reduces AuNP-induced HO-1 expression
We have shown that the AuNPs induced Nrf2 expression, translocation, and binding to the E2 enhancer region of HO-1 (Figures 2, 3, and 5). We next examined whether NAC can affect the AuNP-induced Nrf2 translocation. Figure 7A shows that NAC significantly inhibited Nrf2 translocation to the nucleus. Then, to verify the importance of Nrf2 in promoting HO-1 expression following AuNP treatment in the ECs, siRNA knockdown (KD) of Nrf2 was performed and its effect on the HO-1 mRNA and protein levels was determined. As shown in Figure 7B and C, siRNA KD of Nrf2 reduced the Nrf2 mRNA and protein levels by approximately 50~70%, and, concomitantly, the AuNP-induced HO-1 mRNA and protein expression was suppressed by approximately 40% and 30%, respectively.
Discussion
HO-1 is the inducible isoform of the first and rate-limiting enzyme in the heme degradation pathway. HO-1 induction generally protects the cell against cytotoxicity from oxidative stress and apoptotic cell death. However, it was recently reported that HO-1 can also have immunomodulatory and anti-inflammatory properties.6 In the present study, we showed that the AuNPs were able to induce HO-1 expression in human vascular ECs (Figure 1). The AuNPs at 2 ppm were sufficient to promote HO-1 expression, and this induction could be observed at 4 hours of AuNP treatment. Based on the radius and atomic weight of the Au atom, the molarity of the 2 ppm AuNPs (average size: 3.38 nm) was estimated to be 1.08 nM, and it is possible that this concentration could be achieved in vivo.
Regarding the action mechanism of the AuNPs in promoting HO-1 expression in the ECs, we suggest that the Nrf2- and Bach1-mediated transcriptional pathways are major targets. A previous study has shown that Bach1 is a specific repressor of HO-1 and can be inactivated by arsenite, a potent inducer of oxidative stress.30 However, an antioxidant (tert-butylhydroquinone) can also induce Bach1 phosphorylation at tyrosine486 and lead to rapid nuclear export of Bach1 that allows Nrf2 to bind to the ARE and activate defensive gene expression.15 In the present study, there are several lines of evidence supporting the hypothesis that AuNPs target the Nrf2/Bach1 signaling pathways. First, the AuNPs increased the Nrf2 protein level (Figure 3A). Second, the AuNPs induced Nrf2 translocation to the nucleus and Bach1 export from the nucleus (Figure 3B and C). Third, the AuNP-mediated HO-1 expression could be attenuated by LMB, and the AuNPs induced Bach1 tyrosine phosphorylation (Figure 4). Fourth, the AuNP treatment augmented Nrf2 binding to the E2 enhancer region of the HO-1 promoter (Figure 5). Fifth, siRNA KD of Nrf2 reduced the cellular Nrf2 level and compromised the AuNP-induced HO-1 mRNA and protein expression (Figure 7B and C). Several natural and synthetic compounds are reported to be able to induce Nrf2.12 Some of them are plant-derived compounds with chemopreventive properties, such as sulforaphane (broccoli),31 curcumin (turmeric),32,33 and resveratrol (grapes).34,35 Others include trivalent arsenite and heavy metals.
Nrf2 has been shown to associate with Keap1, which is sensitive to redox reactions. Generally, in the absence of oxidative stress, Nrf2 is sequestered in the cytosol by the Keap1 homodimer, which acts as a substrate adaptor for the ubiquitination of Nrf2 in a cullin-3 (Cul3)-dependent manner.36 It is proposed that subsets of the cysteine residues in Keap1 are modified in cells in response to chemical/oxidative stimuli. These modifications potentially result in the release of Nrf2 from Keap1, disturbing the transfer of ubiquitin. As a result, Nrf2 is no longer targeted for degradation, and the newly synthesized, free Nrf2 accumulates in the cytosol.37 Interestingly, it has also been reported that DNA binding sites (ARE/xenobiotic response element) within the promoter region of Nrf2 are present, suggesting the ability of Nrf2 to regulate its own transcription, ie, autoregulation.38 In this study, the AuNPs were able to increase Nrf2 protein, but not mRNA, expression in the ECs, implying that the AuNP-mediated induction resulted from posttranslational modifications of Nrf2 protein or Keap1. Because the AuNP-induced HO-1 expression and Nrf2 translocation could be abolished by the ROS scavenger/disulfide linkage reductant NAC (Figures 2A–D and 7A), we hypothesized the involvement of a redox reaction/oxidative stress on Keap1 in the ECs in response to the AuNP treatment. However, the AuNPs did not cause intracellular ROS production or ER stress in the ECs (Figure 6). Therefore, we speculate that NAC may not act as a ROS scavenger to inhibit the AuNP-induced HO-1 expression. It has been reported that NAC possesses a reducing property through its thiol-disulfide exchange activity39 and can exert its action by directly reacting with some small molecules such as GSH and thiol-containing components, which then have the ability to interact with other thiol-containing proteins.40,41 In fact, NAC is also known for breaking or reducing disulfide linkages (-S-S-) and is largely used in the clinic as an antitussive/mucolytic agent.27 Moreover, NAC is an antioxidant and possesses the ability to enhance cellular GSH synthesis.27 From this viewpoint, we suggest that AuNPs can affect the cysteinyl thiol-group status of Keap1 because the oxidation, disulfide linkage formation, S-alkylation, and modification of the thiol-containing cysteines have been reported to change the conformation of Keap1, leading to Nrf2 dissociation from Keap1.42 This may be the reason for the diminished AuNP-induced Nrf2 activation and/or HO-1 expression only observed in the presence of NAC, GSH, and DTT (thiol-containing antioxidants) rather than vit C and Trolox (non-thiol-containing antioxidants) (Figures 2B–E and 7A). Some polyenes, such as chlorophylls and porphyrins, are Nrf2 inducers, and the polyphenols curcumin and quercetin have antioxidant activity and can also be HO-1 inducers.32,43 However, AuNPs do not possess these polyenic structures. Therefore, it remains to be investigated how AuNPs directly or indirectly affect Keap1 status and/or Nrf2 activation.
A previous study has shown that AuNPs from the same source as this study, but with larger sizes, elicit cytotoxicity in human CML K562 cells. Clusters of the AuNPs are detected in the plasma membrane or are endocytosed by cells, as determined by TEM.22 Several toxicological reports show that many AuNPs have cytotoxicity in vitro and in vivo.44–46 Furthermore, some AuNPs and silver nanoparticles are able to induce HO-1 expression by causing cytotoxicity and oxidative stress.19,20,47 However, the AuNPs in this study caused Nrf2 activation and HO-1 expression in a short incubation. The TEM analysis revealed that some AuNPs were located in the cytoplasm at 2 hours of treatment (data not shown), suggesting that the uptake of AuNPs by ECs can be an early event. Nevertheless, they did not cause cytotoxicity in the ECs, even with treatments up to 16 hours (unpublished data). Therefore, the induction of HO-1 by AuNPs does not appear to promote cell death. A report has shown that AuNPs do not increase cytotoxicity in the ECs, but increase their susceptibility to cell death.48 In this regard, the long-term effects of AuNPs on ECs need to be further investigated.
Increased levels and activity of HO-1 have been reported to ameliorate injuries to several tissues induced by a number of diseases, including inflammatory diseases, diabetes, heart disease, hypertension, and others.6 For example, HO-1 has been reported to inhibit the expression of proinflammatory CAM genes associated with EC activation.11 Our preliminary result showed that HO-1 induction was partially involved in regulating CAM expression in the ECs, suggesting HO-1 induction and anti-EC inflammation.
Conclusion
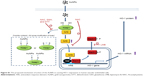
We provide here the first evidence that AuNPs can induce HO-1 expression in human vascular ECs by enhancing Nrf2 translocation and Bach1 tyrosine phosphorylation and export, leading to Nrf2 binding to the ARE promoter region of the HO-1 gene to drive HO-1 mRNA and protein expression. The induction does not appear to be mediated by inducing ROS and ER stress, but can be blocked by a ROS scavenger (NAC) and an inhibitor of nucleocytoplasmic export (LMB). The possible mechanism for the induction is summarized in Figure S1. Therefore, this study elucidates the mechanism of AuNP-induced HO-1 expression and may also explain the anti-inflammatory activity of AuNPs toward vascular ECs.
Acknowledgments
This work was supported by research grants (MOST 101-2632-B-030-001-MY3 and 101CGH-FJU-08) from the National Science Council and Cathay General Hospital (Taipei, Taiwan), respectively.
Disclosure
The authors report no conflicts of interest in this work.
References
Cines DB, Pollak ES, Buck CA, et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91(10):3527–3561. | ||
Zhang H, Park Y, Wu J, et al. Role of TNF-alpha in vascular dysfunction. Clin Sci (Lond). 2009;116(3):219–230. | ||
Sorescu D, Szöcs K, Griendling KK. NAD(P)H oxidases and their relevance to atherosclerosis. Trends Cardiovasc Med. 2001;11(3–4):124–131. | ||
Niture SK, Khatri R, Jaiswal AK. Regulation of Nrf2-an update. Free Radic Biol Med. 2014;66:36–44. | ||
Jaiswal AK. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic Biol Med. 2004;36(10):1199–1207. | ||
Abraham NG, Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol Rev. 2008;60(1):79–127. | ||
Cruse I, Maines MD. Evidence suggesting that the two forms of heme oxygenase are products of different genes. J Biol Chem. 1988;263(7):3348–3353. | ||
Maines MD, Trakshel GM, Kutty RK. Characterization of two constitutive forms of rat liver microsomal heme oxygenase. Only one molecular species of the enzyme is inducible. J Biol Chem. 1986;261(1):411–419. | ||
True AL, Olive M, Boehm M, et al. Heme oxygenase-1 deficiency accelerates formation of arterial thrombosis through oxidative damage to the endothelium, which is rescued by inhaled carbon monoxide. Circ Res. 2007;101(9):893–901. | ||
Liu X, Spolarics Z. Methemoglobin is a potent activator of endothelial cells by stimulating IL-6 and IL-8 production and E-selectin membrane expression. Am J Physiol Cell Physiol. 2003;285(5):C1036–C1046. | ||
Soares MP, Seldon MP, Gregoire IP, et al. Heme oxygenase-1 modulates the expression of adhesion molecules associated with endothelial cell activation. J Immunol. 2004;172(6):3553–3563. | ||
Paine A, Eiz-Vesper B, Blasczyk R, Immenschuh S. Signaling to heme oxygenase-1 and its anti-inflammatory therapeutic potential. Biochem Pharmacol. 2010;80(12):1895–1903. | ||
Itoh K, Wakabayashi N, Katoh Y, et al. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13(1):76–86. | ||
Kaspar JW, Niture SK, Jaiswal AK. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic Biol Med. 2009;47(9):1304–1309. | ||
Kaspar JW, Jaiswal AK. Antioxidant-induced phosphorylation of tyrosine 486 leads to rapid nuclear export of Bach1 that allows Nrf2 to bind to the antioxidant response element and activate defensive gene expression. J Biol Chem. 2010;285(1):153–162. | ||
Mukherjee P, Bhattacharya R, Wang P, et al. Antiangiogenic properties of gold nanoparticles. Clin Cancer Res. 2005;11(9):3530–3534. | ||
Kemp MM, Kumar A, Mousa S, et al. Gold and silver nanoparticles conjugated with heparin derivative possess anti-angiogenesis properties. Nanotechnology. 2009;20(45):455104. | ||
Tsai CY, Shiau AL, Chen SY, et al. Amelioration of collagen-induced arthritis in rats by nanogold. Arthritis Rheum. 2007;56(2):544–554. | ||
Kang SJ, Ryoo IG, Lee YJ, Kwak MK. Role of the Nrf2-heme oxygenase-1 pathway in silver nanoparticle-mediated cytotoxicity. Toxicol Appl Pharmacol. 2012;258(1):89–98. | ||
Aueviriyavit S, Phummiratch D, Maniratanachote R. Mechanistic study on the biological effects of silver and gold nanoparticles in Caco-2 cells – induction of the Nrf2/HO-1 pathway by high concentrations of silver nanoparticles. Toxicol Lett. 2014;224(1):73–83. | ||
Yen HJ, Hsu SH, Tsai CL. Cytotoxicity and immunological response of gold and silver nanoparticles of different sizes. Small. 2009;5(13):1553–1561. | ||
Tsai YY, Huang YH, Chao YL, et al. Identification of the nanogold particle-induced endoplasmic reticulum stress by omic techniques and systems biology analysis. ACS Nano. 2011;5(12):9354–9369. | ||
Wu WB, Hung DK, Chang FW, Ong ET, Chen BH. Anti-inflammatory and anti-angiogenic effects of flavonoids isolated from Lycium barbarum Linnaeus on human umbilical vein endothelial cells. Food Funct. 2012;3(10):1068–1081. | ||
Lo HM, Lai TH, Li CH, Wu WB. TNF-α induces CXCL1 chemokine expression and release in human vascular endothelial cells in vitro via two distinct signaling pathways. Acta Pharmacol Sin. 2014;35(3):339–350. | ||
Maruyama A, Mimura J, Harada N, Itoh K. Nrf2 activation is associated with Z-DNA formation in the human HO-1 promoter. Nucleic Acids Res. 2013;41(10):5223–5234. | ||
Morrison DC, Jacobs DM. Binding of polymyxin B to the lipid A portion of bacterial lipopolysaccharides. Immunochemistry. 1976;13(10):813–818. | ||
Samuni Y, Goldstein S, Dean OM, Berk M. The chemistry and biological activities of N-acetylcysteine. Biochim Biophys Acta. 2013;1830(8):4117–4129. | ||
Shan Y, Lambrecht RW, Donohue SE, Bonkovsky HL. Role of Bach1 and Nrf2 in up-regulation of the heme oxygenase-1 gene by cobalt protoporphyrin. FASEB J. 2006;20(14):2651–2653. | ||
Wolff B, Sanglier JJ, Wang Y. Leptomycin B is an inhibitor of nuclear export: inhibition of nucleo-cytoplasmic translocation of the human immunodeficiency virus type 1 (HIV-1) Rev protein and Rev-dependent mRNA. Chem Biol. 1997;4(2):139–147. | ||
Reichard JF, Motz GT, Puga A. Heme oxygenase-1 induction by NRF2 requires inactivation of the transcriptional repressor BACH1. Nucleic Acids Res. 2007;35(21):7074–7086. | ||
Kensler TW, Curphey TJ, Maxiutenko Y, Roebuck BD. Chemoprotection by organosulfur inducers of phase 2 enzymes: dithiolethiones and dithiins. Drug Metabol Drug Interact. 2000;17(1–4):3–22. | ||
Balogun E, Hoque M, Gong P, et al. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem J. 2003;371(Pt 3):887–895. | ||
Garg R, Gupta S, Maru GB. Dietary curcumin modulates transcriptional regulators of phase I and phase II enzymes in benzo[a]pyrene-treated mice: mechanism of its anti-initiating action. Carcinogenesis. 2008;29(5):1022–1032. | ||
Kode A, Rajendrasozhan S, Caito S, Yang SR, Megson IL, Rahman I. Resveratrol induces glutathione synthesis by activation of Nrf2 and protects against cigarette smoke-mediated oxidative stress in human lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2008;294(3):L478–L488. | ||
Chen CY, Jang JH, Li MH, Surh YJ. Resveratrol upregulates heme oxygenase-1 expression via activation of NF-E2-related factor 2 in PC12 cells. Biochem Biophys Res Commun. 2005;331(4):993–1000. | ||
Kobayashi A, Kang MI, Okawa H, et al. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol Cell Biol. 2004;24(16):7130–7139. | ||
Bryan HK, Olayanju A, Goldring CE, Park BK. The Nrf2 cell defence pathway: Keap1-dependent and -independent mechanisms of regulation. Biochem Pharmacol. 2013;85(6):705–717. | ||
Kwak MK, Itoh K, Yamamoto M, Kensler TW. Enhanced expression of the transcription factor Nrf2 by cancer chemopreventive agents: role of antioxidant response element-like sequences in the nrf2 promoter. Mol Cell Biol. 2002;22(9):2883–2892. | ||
Zafarullah M, Li WQ, Sylvester J, Ahmad M. Molecular mechanisms of N-acetylcysteine actions. Cell Mol Life Sci. 2003;60(1):6–20. | ||
Sun SY. N-acetylcysteine, reactive oxygen species and beyond. Cancer Biol Ther. 2010;9(2):109–110. | ||
Yue P, Zhou Z, Khuri FR, Sun SY. Depletion of intracellular glutathione contributes to JNK-mediated death receptor 5 upregulation and apoptosis induction by the novel synthetic triterpenoid methyl-2-cyano-3, 12-dioxooleana-1, 9-dien-28-oate (CDDO-Me). Cancer Biol Ther. 2006;5(5):492–497. | ||
Dinkova-Kostova AT, Holtzclaw WD, Cole RN, et al. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc Natl Acad Sci U S A. 2002;99(18):11908–11913. | ||
Shen Y, Ward NC, Hodgson JM, et al. Dietary quercetin attenuates oxidant-induced endothelial dysfunction and atherosclerosis in apolipoprotein E knockout mice fed a high-fat diet: a critical role for heme oxygenase-1. Free Radic Biol Med. 2013;65:908–915. | ||
Sasidharan A, Monteiro-Riviere NA. Biomedical applications of gold nanomaterials: opportunities and challenges. Wiley Interdiscip Rev Nanomed Nanobiotechnol. Epub 2015 Mar 24. | ||
Zhang XD, Wu HY, Wu D, et al. Toxicologic effects of gold nanoparticles in vivo by different administration routes. Int J Nanomedicine. 2010;5:771–781. | ||
Coulter JA, Jain S, Butterworth KT, et al. Cell type-dependent uptake, localization, and cytotoxicity of 1.9 nm gold nanoparticles. Int J Nanomedicine. 2012;7:2673–2685. | ||
Fan Z, Yang X, Li Y, et al. Deciphering an underlying mechanism of differential cellular effects of nanoparticles: an example of Bach-1 dependent induction of HO-1 expression by gold nanorod. Biointerphases. 2012;7(1–4):10. | ||
Leite PE, Pereira MR, Santos CA, Campos AP, Esteves TM, Granjeiro JM. Gold nanoparticles do not induce myotube cytotoxicity but increase the susceptibility to cell death. Toxicol In Vitro. 2015;29(5):819–827. |
Supplementary material
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.