Back to Journals » International Journal of Nanomedicine » Volume 15
Gold Nanoparticles as Radiosensitizers in Cancer Radiotherapy
Authors Chen Y, Yang J, Fu S , Wu J
Received 20 July 2020
Accepted for publication 22 October 2020
Published 24 November 2020 Volume 2020:15 Pages 9407—9430
DOI https://doi.org/10.2147/IJN.S272902
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Ebrahim Mostafavi
Yao Chen,1,* Juan Yang,1,* Shaozhi Fu,1 Jingbo Wu1,2
1Department of Oncology, The Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan Province, People’s Republic of China; 2Nuclear Medicine and Molecular Imaging Key Laboratory of Sichuan Province, Luzhou, Sichuan Province, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Shaozhi Fu; Jingbo Wu
Department of Oncology, The Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan 646000, People’s Republic of China
Tel/Fax +86 8303165696
Email [email protected]; [email protected]
Abstract: The rapid development of nanotechnology offers a variety of potential therapeutic strategies for cancer treatment. High atomic element nanomaterials are often utilized as radiosensitizers due to their unique photoelectric decay characteristics. Among them, gold nanoparticles (GNPs) are one of the most widely investigated and are considered to be an ideal radiosensitizers for radiotherapy due to their high X-ray absorption and unique physicochemical properties. Over the last few decades, multi-disciplinary studies have focused on the design and optimization of GNPs to achieve greater dosing capability and higher therapeutic effects and highlight potential mechanisms for radiosensitization of GNPs. Although the radiosensitizing potential of GNPs has been widely recognized, its clinical translation still faces many challenges. This review analyses the different roles of GNPs as radiosensitizers in cancer radiotherapy and summarizes recent advances. In addition, the underlying mechanisms of GNP radiosensitization, including physical, chemical and biological mechanisms are discussed, which may provide new directions for the optimization and clinical transformation of next-generation GNPs.
Keywords: gold nanoparticles, cancer radiotherapy, radiosensitization, mechanisms
Introduction
Cancers are a major threat to human health and quality of life. Radiotherapy, including external beam radiotherapy and internal radioisotope therapy, plays a significant role in the treatment of early and terminal solid tumors, as well as metastatic tumors and regional lymph nodes. This type of treatment relies on cellular damage caused when biological tissues are exposed to ionizing radiation.1–3 The high energy radiation beam (external or internal implanted source of radiation) is delivered to tumors for the destruction of intracellular components or stem cells that induces tumor death. This course of treatment is effective in more than half of cancer patients.1,4–7 However, this treatment still has some limitations due to toxic side effects such as dose heterogeneity, local discomfort, and long-term exposure to healthy tissues.4,8 There is strong evidence to suggest that with the development of tumor cell radio-resistance, higher radiation doses may be required for treatment, which may cause damage or even death of normal cells and tissues.8
Numerous innovative therapeutic methods have emerged in radiotherapy during the last few decades, such as intensity-modulated radiation therapy, improved computer-assisted inverse treatment planning, image guidance, stereotactic radiation therapy, and particle therapy, that aim to achieve more efficient and accurate dose delivery to the targeted organs and tissues.4,7,9–11 However, increasing the maximum dose accumulation in tumor tissues while also looking to reduce the damage to normal tissues has always been a great challenge in radiotherapy. Different strategies have been proposed to balance treatment outcomes and side effects, such as reversing the radiation resistance of tumor tissues, enhancing the radiation tolerance of normal tissues, increasing the radio-sensitization of tumor tissues and limiting the deposition of radiation dose in tumor volume.2 Hence, radiosensitizers, defined as chemical or biological compounds that enhance the effective dose of radiotherapy in cancer cells, have gained widespread attention.11–13 In particular, high atomic element (Z) nanomaterials, such as bismuth (Z=83),14 gold (Z=79),15–17 tungsten (Z=74),18,19 tantalum (Z=73),20 hafnium (Z=72),21 and silver (Z=47),22 are commonly used as dose enhancers for radiotherapy, due to their strong attenuation of photons, and ability to increase the deposition of radiation. These elements are also called “nanoenhancers” and they have much higher mass-energy absorption coefficients than soft tissues.4,23 For keV photon energies, the absorption advantage can even increase ~100 times.11 Among the various nanomaterials investigated for radiotherapeutic applications, gold nanoparticles (GNPs) have long been considered as a potential tool for the diagnosis and treatment of multiple cancers.1–3,5,13,24–28
As emerging tumor radiosensitizers, GNPs are widely studied based on the following advantages:
- They have strong photoelectric absorption coefficients because of the high Z number;
- They are inert materials with excellent biocompatibility and low biological toxicity when compared with traditional agents (such as cisplatin, nitroimidazole, cyclooxygenase-2 inhibitor and iodinated DNA-targeting agents);
- They have a high surface area to volume ratio that allows drugs and other therapeutic agents (eg, peptides, proteins, antibodies, small molecules) to attach to their surface for targeted treatment and combination therapy of tumors;11
- Owing to their enhanced permeability and retention (EPR) effect and low systemic clearance, GNPs preferentially deposit at tumor sites. They have low permeability to normal capillaries and blood vessels in different tissues such as heart, lung, and skin;8,11,16
- As an imaging contrast agent, they can be used in disease diagnosis as well as biological imaging;9,24,29,30
- They have a well-controlled size distribution and GNPs have unique chemical, electrical, and optical properties in the range of 1–150 nm;3,26,31
A number of unique GNP formats are currently undergoing preclinical development for various therapeutic and diagnostic applications – including radiotherapy, disease diagnosis, bioimaging (eg, computed tomography, photoacoustic imaging), therapeutic agent delivery (eg, drugs, genes, RNAs), biosensing and other therapies (eg, photothermal therapy, photodynamic therapy).24,32–40 In addition, many different parameters of GNPs such as particle size, shape, surface chemistry, concentration, biological distribution and localization may influence their effectiveness in radiotherapy.39,41–46 Therefore, several efforts have been made in the past few decades to continuously improve the different impact parameters of GNPs and achieve high radiation efficiency.
This review summarizes the role and applications of GNPs under various types of ionizing radiation including γ-rays, X-rays and proton therapy. We also discuss the potential mechanisms of radiosensitization to provide a theoretical basis for the future development of nanoparticle-assisted radiotherapy.
The Forms of GNPs as Radiosensitizers
Numerous reports have demonstrated that the use of nanoplatforms to selectively deliver therapeutic agents to tumor tissues not only enhances the bioavailability of cytotoxic agents but also minimizes potential toxicity to healthy tissues. This strategy is also applicable to tumor radiotherapy, limiting treatment radiation doses to tumor tissues as much as possible. The radiosensitization potential of GNPs has been supported and confirmed by various experimental data (Tables 1 and 2). Additionally, studies have suggested that the biodistribution of GNPs is one of the most important factors affecting radiation efficiency. Assuming that GNPs have preferentially distributed in tumor tissue and when it follows the same energy absorption mode as that of surrounding healthy tissues, they produce high local ionization in tumor tissues that shortens treatment time and reduces radiation doses. As such, the radiation doses absorbed by healthy tissues are lowered and adverse reactions and side effects caused by radiotherapy are also decreased. However, the size, shape, surface chemistry, and surface modification of GNPs have different effects on its biodistribution, explaining the different radiotherapy outcomes reported.2,5,11,24,47–50 Based on these potential advantages, various strategies have been modeled in simulations or demonstrated in experiments to explore the interaction of GNPs with radiation. Researchers are continuously working on these properties of GNPs to improve radiation effects.
 |
Table 1 In vitro Studies on Radiosensitization of GNPs |
 |
Table 2 In vivo Studies on Radiosensitization of GNPs |
Undecorated GNPs
The unique advantages of GNPs have motivated researchers to apply them to tumor radiotherapy. A pioneering study by Hainfeld et al51 found that EMT-6 mammary xenograft tumors in mice were completely eliminated in 30 days after intravenous injection of 1.9 nm GNPs and exposure to 250 kVp X-rays, while a high radiosensitization effect of GNPs was observed. This study provided the first strong experimental evidence for in vivo radiosensitization effects of GNPs. The team further verified the radiosensitization effects of GNPs at different radiation doses and in different tumor models.52,53 As radiosensitizers, GNPs have been applied to various tumors – including brain tumors,54 breast cancer,55 melanoma,25,56 colon cancer,57,58 cervix carcinoma59 and lymphoma.60
Due to the increased permeability of tumor blood vessels, nanomaterials can be preferentially delivered to tumor tissue through the EPR effect. Recent intracorporal studies have shown that small-sized GNPs (< 6 nm) are cleared through renal excretion within minutes, and that larger nanoparticles are captured by the reticuloendothelial system (RES).9,42,61 However, GNPs (< 100 nm) effectively accumulate in tumor tissues by taking advantage of the EPR effect; for instance, GNPs < 50 nm can easily pass through the cell membrane, and particles < 20 nm can pass through the vascular endothelium.16,62,63 Chithrani et al64 verified the irradiation enhancement effect of 50 nm GNPs in lower- (~100–220kVp) and higher- (6 MVp) energy photons by utilizing nanoparticles ranging in size from 14–74nm. They showed that GNPs up to 50 nm in diameter have the highest radiosensitization enhancement factor (REF) (1.43 at 220kVp) when compared with GNPs of 14 and 74 nm sizes (1.20 and 1.26, respectively). In addition, they pointed out that the kinetics and saturation concentrations also rely on the physical shape of the nanoparticles. It has been reported that spherical GNPs are more easily internalized by HeLa cells than rod-like particles of similar size.42,43 For spherical GNPs, the cell uptake rate is significantly increased by ~375% - 500%. The in vitro cell uptake heterogeneity may be due to differences in particle curvature, which affects their interaction with cell membranes.9,45,65
It is now widely accepted that small-sized spherical GNPs show significant advantages in tumor localization and penetration. They are more likely to penetrate into the tumor stroma and tumor microenvironment, and thus are more effectively internalized by nonphagocytic cells.41,66 However, it is well known that citrate-coated GNPs have a high zeta potential at physiological pH and that the particles easily aggregate.67 Additionally, an insufficient blood circulation time of GNPs and unavoidable phagocytosis by the RES will cause limited bioavailability. Therefore, surface modification or functionalization should be kept in mind in the design of GNPs to expand their range of applications and reduce side effects.
Multi-Functional GNPs
Given the limitations of the first generation of nanoparticles, such as the challenges of in vivo delivery,9 the design of second- and third-generation nanomaterials primarily focuses on surface modifications. The modifications could increase the absorption of nanoparticles, enable stealth and targeting effects, allow greater accumulation in the target volume and achieve a more precise controlled biodistribution.44,68 Overall, functionalized GNPs exhibit a wider range of application advantages (Figure 1):8
- Increase blood circulation time and uptake into tumor cells, as well as modulate clearance, aimed at inhibiting tumor invasion and metastasis;
- Selective targeting of sites for drug delivery, reduce toxicity, evade surveillance and clearance by the immune system, and inhibit multi-drug resistance (MDR);
- Offer coupling sites for biomarkers for disease diagnosis and efficacy prediction;
- Provide controlled release sites for drugs and reduce the side effects.
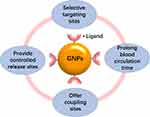 |
Figure 1 The application advantages of functionalized GNPs. |
Based on the available data and after optimizing key features, GNPs will be more appropriate for improving the efficacy of radiotherapy. Two types of targeting strategies are employed to ensure adequate concentrations of GNPs in tumor cells: passive targeting and active targeting. In passive targeting, one utilizes the higher endocytic uptake of cancer cells and vascular leakage around the tumors, which allows for the higher accumulation of nanoparticles in tumor tissues. For active targeting, GNPs are coated with specific molecules which then target receptors on cancer cells, similar to antibodies.
Continuous improvements to surface functions of GNPs were made to achieve better biocompatibility, longer circulation time, lower cytotoxicity, and higher tumor uptake. However, due to the lack of tumor-targeting ligands, whether GNPs reach tumor tissues mainly depends on the EPR effect (Figure 2). One could design different surface chemistry and surface functions on GNPs, so they could exert more significant biological functions in vitro and in vivo, and achieve superior radiosensitization effects in radiotherapy. Commonly used surface modifiers include polyethylene glycol, chitosan, cytotoxic drugs, proteins, and radioactive elements.
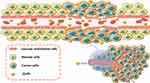 |
Figure 2 Passive targeting for GNPs. |
Polyethylene glycol (PEG) is a widely used surface modifier. PEGylation can be defined as the “hide” of nanoparticles by modifying their surface with a PEG layer. This may reduce particle surveillance and identification by the RES, effectively extending the blood circulation time.16,43,69–74 PEGylation can be accomplished by covalent linking; the covalent bond encapsulates or adsorbs the PEG chain on to the surface of the nanoparticles.75
The use of non-immunogenic and biocompatible hetero-bifunctional PEG molecules stabilizes the core size of the GNPs and also regulates the surface charge of the nanoparticles which affects their biodistribution. Kumar et al44 demonstrated the low cytotoxicity and biocompatibility of PEGylated GNPs (pGNPs) in Hela cells. When coupled with in vitro X-ray irradiation, cell death after treatment with different concentrations of pGNPs increased by 1.3–2.8 times compared to controlled group cells (without pGNPs). In another study, Yasui et al69 reported that PEGylated nanogels containing GNPs combined with 220 kVp X-ray irradiation radiosensitized murine squamous cell carcinoma SCCVII and Chinese hamster lung fibroblasts V79. The mechanism of radiosensitization may be due to the increased apoptosis and impaired DNA repair capacity by overexpressing endoplasmic reticulum stress-related proteins. Another in vitro study showed that PEGylated GNPs enhanced cellular uptake in B16F10 murine melanoma cells and caused radiosensitization under irradiation with 6MeV.76 By analyzing the cell survival curves plotted by fitting the linear-quadratic model, PEG-GNPs could achieve a maximum dose enhancement factor (DEF) of 1.22 and a maximum sensitization enhancement ratio (SER) value of 1.21 at a concentration of 30uM.
These results provide evidence for the clinical application of GNPs as theranostic agents in radiotherapy. In vivo, a majority of PEGylated nanoparticles aggregate around the blood vessels, but cellular uptake is limited. Hence, it is necessary to improve the surface of GNPs to preferentially sensitize tumors to irradiation.
Chitosan is a pseudonatural cationic polymer with unique properties, used in wide-ranging applications such as flocculants for protein recovery, and depollution. It also has favorable biocompatibility, biodegradability, sensitivity to chemical modifications, and modulates drug release; these pharmaceutical characteristics are applicable to certain areas of medicine.77–80 Fathy et al79 explored a novel treatment based on the chitosan structure, to effectively achieve the simultaneous loading of chemotherapy drugs (doxorubicin, DOX) and GNPs as radiosensitizers. In combination with external irradiation, DOX acted as a broad-spectrum anticancer drug, while chitosan-capped GNPs (CS-GNPs) increased the sensitivity of tumor cells to radiation, thus achieving combination chemotherapy and radiotherapy (chemo-radiotherapy). In chemo-radiotherapy, intravenous chemotherapy drugs regulate systemic metastasis and exert synergistic anti-tumor effects with localized radiotherapy.81 The study was supported by a large number of randomized clinical trials; this combination of the two methods may solve the issue of sublethal damage repair and hypoxia-related radioactive resistance.82,83 Although this study confirmed that CS-GNPs-DOX combined with radiotherapy was a promising strategy, additional in vivo experiments have not revealed more complex biological mechanisms that can be translated into clinical trials.
The advantages of bovine serum albumin (BSA) include biocompatibility, biosafety, excellent biodegradability, and flexible surface modification; all these make BSA a common surface modifier.84–86 BSA-modified nanoparticles can be temporarily “invisible” in the blood circulation, which prolongs the blood circulation time and allows nanoparticles to exert a better therapeutic effect. In addition, BSA-modified GNPs have been extensively studied in various fields such as biosensing, bioimaging, drug carriers, gene carriers, and cancer therapy.85–88
To achieve better targeting, Liu et al89 used BSA as a biological template to synthesize different sized particles of BSA-protected GNPs (8, 50 and 187 nm). They used these nanomaterials as radiosensitizers to examine their radiosensitization effects on H22 hepatocarcinoma-bearing mice. Their results have shown that BSA-GNPs efficiently aggregate in the cytoplasm and that there is no obvious cytotoxicity to HeLa, HepG2, and HeCat cells. In vivo experiments indicate that there is no apparent physiological injury to tumor-bearing mice after intravenous injection of BSA-GNPs. In the case of combined radiotherapy, 8 and 50 nm BSA-GNPs were found to induce apoptosis and inhibit tumor growth by up-regulating the expression of caspase-3 and Bax protein and down-regulating the expression of Bcl-2 protein, to obtain enhancement factors of 1.93 and 2.02, respectively. Therefore, BSA-templated GNPs are potential radiosensitizers for the radiotherapy of hepatocarcinoma.
The narrow therapeutic range of many chemotherapeutic drugs is mainly due to their systemic toxicity. Cisplatin, for example, plays an important role in tumor chemotherapy.90,91 It has the ability to cross-link DNA, change the structure, and activate a variety of signal transduction pathways to induce apoptosis. However, its clinical application is limited by its significant systemic toxicity (ototoxicity, nephrotoxicity, and neurotoxicity). Therefore, different methods have been tried to reduce systemic exposure and side effects. Setua et al92 demonstrated that GNP+ RT-mediated monotherapy was not effective for all patients. To overcome this obstacle, they grafted cisplatin on to the surface of gold nanospheres (GNP-Pt) and evaluated the multimodal chemical-radiotherapy potential of the nanospheres in three glioblastoma multiforme (GBM) cell lines. Their results showed that similar inhibition effects were observed in the growth curves of all three patient-derived cell lines under brachytherapy (137Cs as irradiation source), indicating that GNP-Pt-mediated synergistic chemical-radiotherapy may effectively eliminate the therapeutic resistance in GBM cells. Recent studies have confirmed the potential of this therapeutic modality.93,94
In addition, as a multi-functional nanoplatform, hollow GNPs (HGNPs) loaded with doxorubicin (DOX) has been used in combination chemotherapy, radiotherapy, thermotherapy, and CT imaging. Park et al95 evaluated the anti-tumor effects of DOX-HGNPs when combined with NIR laser irradiation and radiation. Compared to the control tumors, the combination strategy caused a 4.3-fold increase in tumor-growth delay and a 6.8-fold reduction in tumor weight.
Although surface modifications can improve the stability of GNPs and decrease the problem of aggregation and flocculation, the GNPs lack preferential concentration in the tumor areas that effect the efficacy of radiotherapy. This type of passive approach does not utilize specific units to target cancer cells. The passive-targeting ability of surface coatings depends on several factors such as the size of the nanoparticle core or the length and surface density of the capping molecules, which are major limitations of these methods.2 Radiation damage caused by the non-specific biodistribution of radiosensitizers or radioisotopes are additional reasons that limit its application. Thus, increasing specific tumor accumulation of GNPs and enhancing the therapeutic efficiency of radiotherapy is still a challenge.
It is well known that the radiosensitization of GNPs depends on their cellular uptake and localization. Studies have shown that areas highly radiosensitive to GNPs are located within or near the nucleus. Such nuclear-localized GNPs can be achieved by active targeting. In general, active targeting works primarily through the interaction of targeting ligands with receptors overexpressed on the surface of cancer cells, rather than simply relying on EPR effects.96,97 Active-targeting agents can be divided into three main types based on their target substrates: cell surface carbohydrates (carbohydrate targeting), cellular antigens for antibody (antibody targeting), and cell surface receptors (receptor targeting) (Figure 3). Currently, the targeting ligands used include proteins, aptamers, and small molecules such as peptides, monoclonal antibodies, hormones, glucose molecules, and carbohydrates (Table 3). Based on molecular recognition processes, these ligands deliver therapeutic agents to pathological sites or through biological barriers.
 |
Table 3 Summary of Active Targeting and Targeting Approaches of Surface-Modified GNPs |
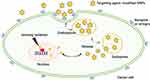 |
Figure 3 Active targeting for GNPs. |
Folate (folic acid, FA) is essential for the biosynthesis of purines and pyrimidines in DNA synthesis pathways, acting as one of the promising targeting ligands for cancer treatments. Folate is suitable as a targeting agent due to its stability, non-immunogenicity, specificity for cancer cells, and simple conjugation chemistry.98,99 Some rapidly dividing cancer cells upregulate levels of folate receptors to satisfy the increased demand of folate for DNA synthesis and rapid growth, which is the basis of folate targeting strategies. Previous attempts have shown that head and neck, cervix and ovarian tumors are positive models for folate targeting studies. Conversely, bladder, pancreas and liver tumors can be considered as negative models.99 Overall, one can take advantage of the active-targeting property of folate and combine it with nanotechnology platforms to discover novel treatment strategies for cancer.
Khoshgard et al98 compared the internalization of folate-conjugated GNPs and non-folate-conjugated (PEGylated) GNPs in Hela cells, and also focused on enhanced cell damage following orthovoltage X-rays (120–250 kVp) and megavoltage γ-rays (Co-60) exposure. They found that folate-conjugated GNPs had higher internalization ability in Hela cells, as well as higher cancer cell death rates and DEF under different irradiation energies when compared to PEGlated-GNPs. Therefore, they highlighted that GNPs could effectively enhance cell lethality under orthovoltage X-ray photons and that folate nanoconjugates increase the selectivity of this lethal effect. In another study, Kefayat et al100 prepared folate and BSA decorated gold nanoclusters (FA-AuNCs) and investigated their radiosensitivity effects on C6 glioma tumors in radiotherapy. ICP-OES was used to assess FA-AuNCs targeting efficiency and they showed that its accumulation in C6 cells was 2.5 times higher than that in normal cells, demonstrating excellent targeting ability. Furthermore, in tumor-bearing mice, the concentration of FA-GNPs localized in brain tumors was significantly higher than in surrounding normal tissues, and the mice had increased total survival time compared to the control group. The DEF of FA-AuNCs was 1.6 when irradiated with a single dose of 6 Gy. These studies indicate that folate-coated GNPs may improve radiotherapy efficacy.
Human epidermis growth factor receptor 2 (HER2), an important predictor of breast cancer, is overexpressed in 20–30% of breast cancers and is a predictor of poor prognosis. As the first targeted drug in breast cancer treatment, trastuzumab may selectively bind to the extracellular structure of HER2 and destroy the dimer of the receptor via down-regulating HER2/nue. This induces endocytosis and degrades cell membranes through the signal transduction of downstream PI3K pathway to play a therapeutic role.101,102 In order to improve the therapeutic effects of locally advanced breast cancer, Cai et al103 developed a novel radiation treatment termed “radiation nanomedicine”. This treatment consisted of trastuzumab-conjugated GNPs and then labelled it with 177Lu (Trastuzumab-GNPs-177Lu), a radionuclide emitting β-particles [Eβmax=0.5MeV; t1/2=6.7days] with a maximum range of 2 mm. The binding and internalization of this polymer have been observed in multiple HER2-positive breast cancer cell lines (SK-BR-3, BT474, MDA-MB-361). It was found that the internalization of GNPs increased in these three cell lines after incubation with trastuzumab-GNP-177Lu and that there was negligible uptake of GNPs after incubation with non-targeted GNPs (GNP-177Lu). The radioligand binding assay revealed that trastuzumab-GNP-177Lu may be multivalently bound to HER2. Increased internalization of trastuzumab-GNP-177Lu in the cytoplasm was supposed to deposit greater radiation doses, which showed increased apoptosis and more DNA double-strand breaks (DSBs). Further, the antitumor properties and normal tissue toxicity of trastuzumab-GNP-177Lu and GNP-177Lu were compared in MDA-MB-361 mice. Compared to treatment with GNP-177Lu or untreated mice, trastuzumab-GNP-177Lu significantly inhibited tumor growth and no toxic reactions were observed in normal tissues over a 16-day observation period. These results are similar to the results of previous reports which demonstrated that panitumumab-GNP-177Lu deposited more radiation dose in epidermal growth factor receptor (EGFR) positive breast cancer cells when compared to GNP-177Lu.104,105 The radiosensitizing effect of HER2 antibody-decorated GNPs was also confirmed in an ovarian cancer cell line (SK-OV3).106
Similarly, Popovtzer et al107 used cetuximab to target EGFR, allowing GNPs to selectively target head and neck tumors and significantly increase the radiation dose absorbed in tumor tissues. When combined with radiotherapy (25 Gy,6 MV,1.4 Gy/min), a significant tumor-growth delay in tumor-bearing mice was observed. Further analysis indicated that there was no significant toxicity or side effects in any of the experimental mice. Inspired by GNPs and108 In (a radionuclide commonly used for SPECT imaging), Song et al109 used epidermal growth factor (EGF) as a target ligand, resulting in the effective nuclear localization of108 In-radiolabelling of GNPs in breast cancer cells with high expression of EGFR. Such a distribution would increase the radiation toxicity of108 In-EGF-GNPs when combined with external irradiation, as the electron pair emitted around perinuclear and the DNA radiation dose are both increased. They expect the108 In-labelled EGF-GNPs nano-system to be a new tool for the treatment of EGFR-positive cancers.
Glucose is the main source of cellular metabolic energy. The rapid growth and proliferation of cancer cells depend on specific proteins and glucose. As such, glucose transporter (GLUT) receptors on the surface of cancer cells enable the internalization of a greater number of glucose molecules than normal cells108,110,111 to meet the increased energy demands of cancer cells. GNPs can covalently conjugate to various biomolecules via thiol groups, that allows effective coupling with glucose molecules to achieve better internalization of GNPs. The targeting ability of glucose-coated GNPs has been demonstrated in various solid tumors (eg, breast, lung, and ovarian cancers).112–115
In a study by Hu et al,116 THP-1 cancer cells were suspended to simulate cancer stem cells and cancer metastasis and the adherent MCF-7 cancer cells mimicked solid tumors. In this system, the Glu-PEG-GNPs could successfully target cancer cells. The use of starvation media for an appropriate time could aid Glu-PEG-GNPs uptake in cancer cells. Based on the radiosensitization of GNPs under megavoltage energy irradiation, they also assessed the therapeutic effects of Glu-PEG-GNPs combined with radiotherapy on both MCF-7 and THP-1 cells. The results showed that Glu-PEG-GNPs exhibit enhanced tumor-killing ability at 6 or 9Gy irradiation when compared to that by X-ray irradiation alone or GNPs treatment alone. This finding may provide a new treatment strategy for cancer stem cells and cancer metastasis. Similarly, as a hepatocyte-specific receptor, asialoglycoprotein receptor (ASGPR) is overexpressed on the sinusoidal surface of hepatocytes and mediates the uptake and endocytosis of galactose- or N-acetylgalactosamine-terminating glycoproteins by hepatocytes.117,118 Zhu et al118 used β-d-galactose (GAL) as a homing agent to design ASGPR-targeted GNPs to enhance the cytotoxicity of radiation therapy. This composite (GAL-PEG-GNPs) could be more rapidly and efficiently taken up by ASGPR over-expressing HepG2 cells and could avoid the rapid phagocytosis of RES. Compared to bare GNPs and radiation alone, HepG2 cells treated with GAL-PEG-GNPs showed significant DNA DSBs and apoptosis after exposure to 6MeV X-rays irradiation and achieved better radiosensitization effects.
Polypeptides are one of the most commonly used targeted modifiers. RGD (Arg-Gly-Asp) is a polypeptide polymer composed of arginine, glycine, and aspartate and constitutes the core structure for cell surface receptor recognition. The oligopeptide RGD has a high affinity for the transmembrane heterodimeric αvβ3 integrin receptor, which is highly overexpressed on the activated neonatal endothelium and has been used as a tumor vascular targeting ligand since the 1980s.119–122 Additionally, tumor neovascularization has been widely investigated as one of the important targets for both chemotherapy and radiotherapy. Clonal cell dysfunction triggered by radiation may be due to microvascular endothelial injury.123,124 In a study by Kunjachan et al,123 GNPs were modified by the addition of RGD to enhance intracellular retention of NPs and served to deposit more radiation dose in tumor tissues. Under the radiation exposure by image guidance, more vascular damage could be directly induced to improve the radiation effect, thereby enhancing the tumor-killing effect indirectly. Another study by Yang and colleagues125 confirmed the clinical potential of this innovative therapy. They used RGD-modified GNPs in combination with the chemotherapeutic drug cisplatin (also with? Radiosensitizers) and demonstrated the therapeutic effect of this combination therapy on MDA-MB-231 cells in the context of MV radiation. Even at low concentrations, the survival rate of cells treated with GNP-RGD:Cis and radiation decreased significantly, at levels much lower than the cells treated with cisplatin and radiation alone. Hence, they predicted that this GNP-mediated chemoradiation would be integrated into cancer treatment in the near future.
Cell-penetrating peptides (CPPs) are another type of short peptides that can cross cell membranes and deliver various biologically active molecules, usually consisting of ~7–30 amino acids.126–129 It is widely believed that macrophagocytosis, clathrin-mediated endocytosis and caveolae/lipid raft mediated-endocytosis are the main routes for most CPPs and CPP-cargo complexes to enter cells. They have been used to transport various therapeutic agents into cells such as plasmid DNA, siRNA, proteins, and other nanoparticles.128,129 Octaarginine peptide (R8), an arginine-dependent cationic CPP, has been shown to promote drug release. Zhang et al130 utilized R8 and PEG to functionalize the surfaces of GNPs, increase cellular uptake, improve the stability of the NPs, and prevent particle aggregation. Subsequently, they then used the colorectal cancer cell line LS180 as a model system to verify the radiosensitization effect of R8-modified GNPs on in vitro megavoltage radiotherapy. In this study, R8 as a transmembrane vector could result in efficient internalization and cellular uptake of GNP-PEG-R8. When combined with 6 MV X-rays, the cells incubated with GNP-PEG-R8 showed a significant decrease in surviving fraction, increased apoptosis, and ROS levels.
As a vital target of cancer radiotherapy, the nucleus has attracted wide interest. Recent studies demonstrated that therapeutic effects enhanced when nanoparticles targeted the nucleus.122,131–133 However, previous studies have shown that NPs enter cells through the endo-lyso pathway. In this pathway, nanoparticles are trapped either in endosomes or lysosomes and are unable to enter the cytoplasm and nucleus of cells. Nuclear localization sequences (NLSs) are specific amino acid sequences that can transport the macromolecules across the nuclear pore complex (NPC). NLS modification on the surface of nanoparticles results in the binding of NPs to nuclear transport molecules (such as importin) through NLS peptides and enter via the NPC into the nucleus. One study published in 2014131 showed that GNPs modified with nuclear targeting peptides can target cell membranes and enter nuclei of cancer cells to significantly enhance the killing effect of cancer cells in radiotherapy. The authors functionalized GNPs with multiple nuclear targeting and non-targeting peptides. Cancer cells were exposed to 2 Gy of radiation dose (radiation energy: 220 kVp) after incubation with different GNPs. It was observed that cell deaths (when incubated with targeted GNPs) increased 4-fold when compared to control cells (without GNPs), and increased 3.5-fold when compared to cells cultured with non-targeted GNPs. An increase in intracellular GNPs led to more DNA DSBs and cell death.
Nucleolin aptamer is a G-quadruplex oligonucleotide with a high affinity for nucleolin and low immunogenicity. It can transfer molecules between the nucleus and the cytoplasm in cells and cytoplasm of nuclide overexpression, and it can also act as a receptor for targeting tumor cells.134–137 Additionally, STAT3 (a key regulator of the EGFR-STAT3-BclXL signalling pathway), is abnormally activated in most head and neck cancers (HNC).138–140 A STAT3 decoy (STAT3d) can be used to reduce STAT3 activation, inhibit cell proliferation and survival, and improve therapeutic resistance in HNC. However, its use in clinical treatment is limited because of a lack of stability and specific targeting. Recently, Zhang et al141 developed an innovative dual treatment strategy. In the study, they synthesized GNP-NUAP-STAT3d for the combined treatment of radiation-resistant HNC. Among them, GNPs served as a delivery vehicle for STAT3d and simultaneously as the radiosensitizers while the nucleolin aptamer (NUAP) is key to specifically target the nucleolin. They demonstrated that the construct was efficiently internalized in cells and that the combination of single-dose 4 Gy irradiation resulted in potent inhibition of A431, FADU cell proliferation, and greater radiosensitization when compared to cetuximab (an anti-EGFR humanized antibody).
Hypoxia and acidic pH, common features in the tumor microenvironment (TME), reduce the sensitivity of tumor cells to anticancer drugs and the reactivity to free radicals, causing hurdles in cancer therapy.142–146 Hence, several features of TME can be used to design active-targeting methods.143,144,147,148 For example, the acidic pH of TME effectively internalizes pH-sensitive nanocarriers into cancer cells and releases their therapeutic payload.149,150 Given the acidic pH of TME, Antosh et al151 modified 1.4 nm GNPs with pH-sensitive tumor-targeting agents (pH low-insertion peptide, pHLIP) to bind them to the plasma membrane lipid bilayer of cancer cells, and compared cell viability under different treatments (pHLIP-GNPs, GNPs alone, without GNPs). With 250kVP X-rays radiation, the results showed that actively targeted pHLIP-GNPs significantly reduced cell viability.
Oxygen is recognized as a radiosensitizer. However, the centers of solid tumors are often anoxic and the hypoxia-induced microenvironment of the tumor weakens the generation of ROS and oxygen-fixation reaction, resulting in poor response to radiotherapy.145,152–155 In addition, hypoxia-induced factor-1 is activated under hypoxia, leading to the increased expression of genes associated with angiogenesis, invasion, and metastasis of tumor cells, and promotes tumor chemo- and radio-resistance.156–158 Hypoxia-specific radiosensitizers or non-invasive imaging techniques for therapy of guided hypoxic regions have been used to overcome radiotherapy resistance in tumor hypoxic regions. GNPs combined with radiotherapy have been demonstrated to enhance the antitumor effect of radiotherapy to hypoxic tumors.159 Despite these advances, poor delivery to hypoxic regions remains an obstacle. Interestingly, the hypoxic regions may serve as an ideal habitat for many anaerobic bacteria. Recently, bacteria have been widely applied to cancer therapy because of the selective targeting of hypoxic or anoxic regions which activates the immune response.142,160,161
Inspired by these intrinsic advantages, Kefayat et al162 utilized attenuated strains of Salmonella Typhi Ty21a (a motile facultative anaerobic organism) as radiosensitizers and smart carriers to deliver GNPs to hypoxic tumor regions. In this study, flow cytometry and ICP-OES were used to investigate the uptake of 7 different modified GNPs (Citrate-GNPs, Gelatin-GNPs, BSA-GNPs, FA-GNPs, Glutamine-citrate-GNPs, Glut-BSA-GNPs, Glu-BSA-GNPs) by Salmonella typhimurium Ty21a. Out of these, FA-GNPs are the best choice for preparing the Golden Bacteria (GB). The GB can transmit more GNPs to the central anoxic areas of the tumor, which will ameliorate the radiation resistance deep inside the tumors. Furthermore, bacteria-mediated hypoxia-specific delivery of GNPs will benefit tumor radiotherapy and also contribute to the photothermal therapy (GNPs could also serve as photothermal sensitizing materials).142
In vivo Biodistribution of GNPs
The reticuloendothelial system (RES) is an indispensable barrier of the body, that includes the liver, spleen, lymph nodes, etc., in which the resident mononuclear macrophage system, through phagocytosis, can eliminate foreign bodies, bacteria, aging and mutated cells, as well as nanoparticles similar in size to bacteria and viruses. Evidence suggests that the biodistribution of GNPs plays an essential role in affecting the radiotherapy therapeutic response. Crucial factors such as the size, shape, charge, and surface functionalization of GNPs determine their circulation time in vivo and their accumulation in tumor tissues.
Prior studies indicate that surface functionalization of GNPs affects their blood circulation time and clearance rate in vivo. For instance, Cho et al163 investigated the biodistribution of PEG encapsulated GNPs in tumor-bearing mice. In their findings, nanoparticles (4 and 13 nm) were distributed in organs such as liver and spleen within 7 days after intravenous injection, and their concentration reached a peak in these RES organs at 7 days. While 100 nm PEG-GNPs were distributed to RES organs and peaked within 30 min after intravenous injection and maintained high levels for 6 months. Consistent with this finding, several studies confirmed that longer PEG chain lengths helped prolong the blood circulation time of NPs.49 To investigate the accumulation of nanoparticles in the tumor, Chou et al164 injected fluorescent-labeled PEG-GNPs into tumor-bearing mice and the tumor tissue was H&E stained 24h after injection. The results showed that nanoparticles accumulated more in areas with low cell density, open blood vessels and low level of extracellular matrix, and less in areas within dense extracellular matrix and collapsed blood vessels. Moreover, various types of targeting agents such as small molecules, peptides, antibodies and nucleic acids have been used to modify nanoparticles to achieve more tumor accumulation and increase the retention time of nanoparticles in tumors.
Apart from that, size and surface charge of GNPs also affect their in vivo distribution and thus radiosensitization.165 As discussed earlier, smaller size (< 6 nm) nanoparticles are usually eliminated within minutes from systemic circulation through renal excretion after intravenous administration, while larger nanoparticles accumulate in the RES, which leads to a decrease in tumor accumulation. Huang et al41 have showed that the tiopronin-coated GNPs with core sizes of 2 nm and 6 nm significantly increased perfusion throughout the tumor, while 15 nm nanoparticles tended to accumulate in the blood vessels around the tumor. Furthermore, studies have shown that positively charged GNPs have higher cellular uptake than negatively charged or neutral charged GNPs, which may be due to electrostatic interactions with negatively charged cell membranes.166
Therefore, nanoparticles should be designed to ensure higher tumor uptake, lower RES phagocytosis, longer blood circulation times as possible, so as to provide greater therapeutic effect within the range of acceptable biotoxicity.
The Potential Mechanisms of Radiosensitization
The use of radiosensitizers may increase the local dose and overcome the heterogeneity of response within hypoxia and rapid proliferative areas of tumors, thereby improving the contrast between tumors and normal tissues for additional therapeutic benefits.167,168 Regarding GNPs, experimental evidence from several in vitro and few in vivo investigations have supported the potential of GNPs as radiosensitizing agents. Radiosensitization by GNPs was initially identified as stem from initiating physical dose enhancement due to the strong photoelectric absorption of Au. However, many reports have indicated that this sensitization was also attributed to subsequent chemical and biological enhancement. These three parts are auxiliary to each other and together affect the interaction of GNPs with radiation. Although the precise mechanisms for GNPs-induced dose enhancement have not been fully elucidated, several bold conjectures have been proposed. These mechanisms will be discussed in different sections (from three different phases) to clarify the radiation effects on biological systems (Figure 4).
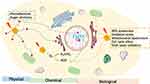 |
Figure 4 The potential mechanisms of GNP radiosensitization. |
Physical Dose Enhancement
The basic principle of GNPs as radiosensitizers is based on the difference of mass-energy absorption coefficients between them and soft tissues.169,170 The Compton effect or the photoelectric effect is the main mechanism by which photons lose energy. In the Compton effect, scattering occurs when the incident photon collides with weakly bound electrons; some energy transfers from the energetic photons to the electrons, thereby ejecting the electrons from the atom. Since these photons retain most of the energy after collisions, they tend to decelerate and have longer ranges in cases where the Compton effect dominates, which results in a very sparse distribution of ionization events. For the photoelectric effect, the incident photons are absorbed by bound electrons, and the energized electrons in the inner shell are excited to be ejected. The vacancies present in the K, L or M layers of the gold atom are usually filled by the Auger effect.4,169,171,172 When the outer shell layer electrons fall to fill the vacancy, the lower energy photons (fluorescence) and a cascade of secondary electrons (such as Auger electrons) are released. These low energy electrons have a range of several microns, which may lead to highly localized ionization events.171
The photoelectric effect is strongly dependent on the photon energy and the binding energy of the electrons in the atom. Considering the conservation of momentum, the absorption of photons in the photoelectric effect can only occur in the presence of the atomic nucleus. The X-ray cross section refers to the probability that the material interacts with radiation, depending on its atomic number. For the photoelectric effect, the X-ray cross section is approximately between Z3 and Z5.3,11,169,171 Consequently, by utilizing the difference in atomic number between Au (Z=79) and soft tissue (mainly composed of low atomic number organic materials), GNPs can provide more unit mass energy and increase the local radiation dose deposited in the target volume (tumor tissues), leading to radiosensitization.
Chemical Contributions
The “chemical mechanisms” of GNP radiosensitization are primarily involved in radical reactions and/or through inducing activation of “open” chromatin structures, which makes DNA more vulnerable to radiation-induced damage.3,169 Based on the subcellular localization of GNPs, two possible chemical mechanisms were proposed: 1) the chemical sensitization of DNA to radiation-induced damage; 2) the activation surface of GNPs increases the generation and catalysis of radicals. The former mechanism requires nuclear localization of GNPs to allow binding to DNA; however, in most studies, GNPs are usually limited to endo/lysosomal of the cytoplasm. Both low energy electrons (LEEs) with energies below the ionization threshold (<10eV) and secondary electrons play an important role in radiosensitization.169,173 Studies have shown that although the interaction between the LEEs and GNPs fails to produce secondary electrons, they may cause damage to a large number of DNA. This may be attributed to the formation of transient negative ions induced by LEEs, which weaken the hydrogen bond in DNA and produce chemical enhancement. In addition, this kind of chemical sensitivity also depends on the size and charge of the GNPs.3,173 Therefore, it is necessary to design GNPs that are localized to the nucleus and bind to DNA, making full use of this chemical sensitization mechanism.
Concerning the second chemical mechanism of radiosensitization, the electronically active surfaces of GNPs have been proved to catalyse various chemical reactions3,174,175 (particularly small GNPs <5nm with large surface areas exhibiting great catalytic activity) mediating the transfer of electrons from surface-bound donor groups to O2, thus generating free radicals. Such small-sized and high-curvature nanoparticles destroy the highly ordered crystal structure of bulk Au, and the alteration in the electron configuration of surface atoms leads to the production of free radicals on the reaction surface of GNPs.3 Alternately, the catalytic effect of GNPs also promotes the transfer of electrons and the production of ROS via the interaction between activated surface and molecular oxygen, and the catalytic performance would be further enhanced when combined with X-ray radiation. The increased ROS is closely related to the emission of photons and Auger electrons of GNPs, as well as the secondary radiolysis of water, resulting in indirect damage to DNA, proteins, and lipid membranes via oxidation, which initiates apoptosis/death.169,176–178
From these data, it can be concluded that GNPs enhance and fix radiation-induced cell damage by catalyzing radical reactions and increasing the production of ROS. In addition, the elevated intracellular ROS levels may also have biological consequences through oxidative stress; this will be described in the next section.
Biological Phase
Monte Carlo simulation, as a theoretical calculation method, is often used to evaluate the dose distribution and dose enhancement ratio (DER) of GNPs.179,180 However, based solely on physical dose enhancement, the radiosensitization of GNPs is significant at KV energy levels, but not at MV levels.179 Interestingly, the radiation dose enhancement ratio calculated using the biological model of MV radiation is significantly higher than the predicted theoretical Monte Carlo simulation values. The differences between theoretical predictions and experimental data reveal the existence of biological enhancement.181–184 The following three key biological pathways of radiosensitization are widely recognized: 1) ROS production, oxidative stress, and mitochondrial dysfunction; 2) cell-cycle effect; 3) DNA repair inhibition. Other possible biological mechanisms such as autophagy and endoplasmic reticulum (ER) stress have also been proposed.4
ROS Production, Oxidative Stress and Mitochondrial Dysfunction
One of the main mechanisms of radiation-induced cell death is the interaction of free radicals and ROS produced by water radiolysis with various cellular biomolecules, resulting in apoptosis/death.3,171,172,185 ROS, including superoxide anion radicals (O2−), hydrogen peroxide (H2O2), and hydroxyl radicals (OH), may cause cell damage directly by interacting with biomolecules including cellular DNA or cause apoptosis or necrosis of cells indirectly through the oxidation of lipids, proteins, and DNA, as well as mitochondrial dysfunction. As described above, GNPs with different sizes, shapes, and surface functions can induce ROS formation effectively; the subsequent oxidative stress can damage cells via the interaction between critical targets or reducing substance.
Additionally, Po2 in the tumor plays a crucial role in the efficacy of radiotherapy, and the hypoxic tumor microenvironment decreases the response of tumor cells to ionizing radiation.186 Mitochondria are considered as the “energy powerhouse of cells” and amplifiers for ROS production. It is one of the vital targets that cannot be ignored in the process of radiosensitization.187–189 Oxidative stress-induced mitochondrial dysfunction, including mitochondrial DNA (mtDNA) damage, mobilization of cytochrome C and other biological effects, may cause apoptosis/-death.190,191 In addition, it has been suggested that inhibition of proteins involved in maintaining cellular oxidative homeostasis (such as thioredoxin reductase TrxR1) may also lead to GNPs-triggered oxidative stress.192 The precise mechanism of GNPs-induced oxidative stress is not well understood and further studies are needed to explore it; currently, it is considered to occur through mitochondrial dysfunction as a result of the high intracellular ROS levels.
Cell-Cycle Effect
The cell cycle affects the sensitivity of cells to ionizing radiation, and ionizing radiation exposure can delay mammalian cell-cycle progression by inducing G1 or G2 phase arrest. It is well known that cells show different radiosensitivities at different stages of the cell cycle, cells in late S-phase have the strongest radio-resistance and those in the G2/M phase are most sensitive.3,4,193 Activation of cell-cycle checkpoints, as one of the pathways for DNA damage following ionizing radiation exposure, maintains genomic integrity via repair of radiation-induced cell damage or prevention of cell division.194 It has been reported that GNPs can also change cell-cycle distribution and increase the accumulation of G2-/-M phase cells, thus realizing radiosensitization.195 The influence of GNPs on cell cycle depends on the physicochemical properties of nanoparticles and the cell line. However, some studies have also pointed out that GNPs have no significant effect on cell-cycle distribution.186,196,197 Therefore, further studies are necessary to elucidate the effects of GNPs on the cell cycle.
DNA Repair Inhibition
Radiotherapy can elicit a variety of DNA damage, including single-strand breaks (SSBs), double-strand breaks (DSBs), DNA-protein cross-links, and DNA base modifications. Among them, DSBs are associated with clonogenic cell killing and are the primary lethal type of radiation-induced damage. If the failure of DNA DSBs repair affects genomic stability, it results in cell death in a variety of ways. DNA damage repair is evaluated by comet assays, Western blotting, and immunostaining. Phosphorylated histone variant γ-H2AX and p53-binding protein 1 (53BP1) are considered to be the earliest sensitive markers.4,198,199 Dynamic monitoring of γ-H2AX and 53BP1 foci impacts GNPs on DNA repair after radiation exposure; this was confirmed in many experiments. Therefore, DNA repair inhibition seems to be another momentous biological mechanism of GNP radiosensitization. However, other studies have suggested that GNPs have no influence on DNA repair kinetics.183 There is currently no consensus in the literature on the specific role of GNPs in the process of DNA damage repair. This requires further investigation to determine whether GNPs are involved in DNA repair inhibition.
Other Biological Mechanisms
In addition to the biological mechanisms described above, autophagy and ER stress are considered to be other possible biological mechanisms of radiation enhancement. Autophagy plays a major role in maintaining intracellular homeostasis, which promotes nutrient recycling through lysosome degradation of damaged and dysfunctional organelles, proteins, and other cellular components.200–203 The inhibition of autophagy in tumor cells induces anti-tumor effects;204 this autophagy response has positive and negative regulatory abilities to promote tumor development and is one of the radioprotective mechanisms of tumor cells.
ER stress derived from exogenous/endogenous damages resulting in impaired protein folding re-establishes ER homeostasis through activation of unfolded protein response (UPR). If ER stress continues to develop, cell functions often worsen, leading to cell death.205–208 Metal-based nanoparticles such as GNPs, AgNPs, and ZnO-NPs have been reported to cause accumulation and aggregation of misfolded proteins, inducing ER stress and activation of UPR.209–212 As a potential treatment, ER stress may become a new target in the radiosensitization process.
Furthermore, GNPs have also been shown to directly regulate cell activity, function and behavior, or react with thiols (such as GSH) to decrease cellular defences against oxidative stress and promote persistent cell damage, thus producing synergistic effects with ionizing radiation, finally leading to apoptosis, necrosis, mitotic catastrophe, senescence and other adverse outcomes.213
Preclinical Studies of GNPs
Overall, the unique physicochemical and biological properties of GNPs make them the new focus of cancer therapy. The diversity of design and function of GNPs can be developed in numerous ways aimed at enhancing radiotherapy through radiation dose enhancement. Although preclinical studies have demonstrated the possible applications of GNPs, the FDA has not yet approved their clinical use. To date, several GNPs-based nanomaterials have been analyzed in clinical research for cancer treatment:
- phase 0 and 1 trials have been conducted on pegylated recombinant human tumor necrosis factor (rhTNF)-coated GNPs (CYT-6091)214 for the treatment of primary, advanced and metastatic solid tumors (clinicaltrials.gov, NCT00436410, NCT00356980);
- pegylated silica core-Au shell nanoparticles (AuroShell®) have been used for photothermal therapy of the head and neck and lung cancers (clinicaltrials.gov, NCT00848042, NCT01679470);
- in an early Phase 1 trial, Bcl2-L12 targeted NU-0129 GNPs (the trial drug consisted of nucleic acids arranged on the surface of spherical gold nanoparticles) has been used in the treatment of patients with recurrent glioblastoma multiforme or gliosarcoma (clinicaltrials.gov, NCT03020017);
- gold nanoparticles-based Nano-Ayurvedic drugs: Nano Swarna Bhasma (NSB), approved by the Indian government regulatory authority of AYUSH (DNA_SPN_B001_17), has been used in female patients with pathologically confirmed invasive breast cancer stage IIIA or IIIB and showed acceptable safety and excellent efficacy.215
These studies provide strong evidence for the clinical potential of GNPs. However, as mentioned by the National Cancer Institute (NCI)-Radiation Therapy Oncology Group in translational program strategic guidelines for the early-stage development of radiosensitizers, the clinical transformation of any radiosensitizer is rarely a seamless transition directly from the bench to the bedside.216 Therefore, the clinical applications of GNPs require further exploration.
Since preclinical data and clinical trials in the field of GNPs-mediated radiation sensitization are currently sparse, we would like to summarize and discuss the current situation and obstacles of this treatment here. One of the key factors to be considered for the smooth conversion of GNPs is their design and selection. The physicochemical properties, biodistribution, pharmacokinetics and molecular mechanisms of GNPs should conform to the practical clinical considerations. Secondly, the safety and long-term toxicity associated with retention of GNPs entering the human body cannot be ignored, although gold is relatively non-toxic and biocompatible. As with other pharmaceutical radiosensitizers, all GNP formulations used for clinical evaluation need to be rigorously tested and combined with other drugs and/or treatments to ensure their safety in vivo. Since GNPs-mediated radiation sensitization has been shown to achieve better sensitization effects at keV energy, and radiation types commonly used in clinical settings are megavoltage radiation, charged particles, or brachytherapy, further optimization of radiotherapy techniques may be necessary to achieve optimal radiotherapy efficacy. Another challenge to the clinical translation of GNPs-mediated radiation sensitization is the choice of the target population. Most patients could benefit from pre-operative adjuvant radiotherapy and postoperative radiotherapy, however, due to the limitation of normal tissue availability, the local dose escalation is not feasible. At present, there are no exact criteria to screen the study population, and there is a lack of appropriate biomarkers or clinical predictors to evaluate whether the study population can benefit from GNPs-mediated radiation sensitization.49 Therefore, further preclinical studies are necessary to achieve optimum clinical translation.
Conclusion and Outlook
The main optimization of radiation therapy is to reduce the side effects of radiotherapy and increase the survival of healthy tissues. The use of radiosensitizers may help to achieve this goal. As a potential radiosensitizer, GNPs have been widely recognized in experimental and theoretical studies. From the basic knowledge of the photoelectric effect and other related effects, the combination of GNPs and radiotherapy may significantly improve treatment efficacy. In this review, we first briefly summarize the radiosensitization effects of GNPs with different surface modifications in cancer radiotherapy, describe the in vivo distribution of GNPs, and explain in detail their radiosensitization mechanisms. Then, we collected the data of pre-clinical and clinical studies on GNPs in recent years and discussed the difficulties of its clinical transformation. Overall, GNPs could act as radiosensitizers in cancer radiotherapy which may open new opportunities for the development and progress of cancer radiotherapy, and improve the clinical efficacy of radiotherapy in various cancers.
With regards to the transformation of GNPs-enhanced radiotherapy into commercial clinical practice, although many in vitro and in vivo studies have shown promising results, numerous challenges such as physicochemical properties, drug metabolism, and pharmacokinetic (DMPK) screening, safety concerns, in vivo efficacy, biocompatibility and stability, preparation costs and immunogenic issues remain. The mechanisms of action at the molecular and cellular levels including the potential impact on cellular signaling are yet to be fully studied, which would limit its clinical transformation. In order to solve these problems, it is necessary to further optimize GNPs and elucidate the precise mechanisms of radiosensitization to achieve better therapeutic effects, accelerating the translation of GNPs into clinical practice and reach the patients in need.
Abbreviations
GNPs, gold nanoparticles; Z, atomic number; EPR, enhanced permeation and retention; RES, reticuloendothelial system; REF, radiosensitization enhancement factor; MDR, multi-drug resistance; PEG, polyethylene glycol; DEF, dose enhancement factor; DOX, doxorubicin; BSA, bovine serum albumin; GBM, glioblastoma multiforme; HGNPs, hollow GNPs; FA, folic acid; HER2, human epidermis growth factor receptor 2; DSBs, double-strand breaks; EGF, epidermal growth factor; EGFR, epidermal growth factor receptor; GLUT, glucose transporter; ASGPR, asialoglycoprotein receptor; GAL, β-d-galactose; RGD, Arg-Gly-Asp; CPPs, cell-penetrating peptides; NLSs, nuclear localization sequences; NPC, nuclear pore complex; HNC, head and neck cancers; STAT3d, STAT3 decoy; NUAP, nucleolin aptamer; TME, tumor microenvironment; pHLIP, pH low-insertion peptide; GB, golden bacteria; LEEs, low energy electrons; ER, endoplasmic reticulum; mtDNA, mitochondrial DNA; SSBs, single-strand breaks; UPR, unfolded protein response; TNF-α, tumor necrosis factor-α; ROS, reactive oxygen species.
Acknowledgments
This work was supported by grants from the Union Project of Luzhou City and the Southwest Medical University (Nos. 14JC0144, 2013LZLY-J40). Figures were produced using Servier Medical Art (www.servier.com).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Song G, Cheng L, Chao Y, Yang K, Liu Z. Emerging nanotechnology and advanced materials for cancer radiation therapy. Adv Mater. 2017;29(32):1700996.
2. Haume K, Rosa S, Grellet S, et al. Gold nanoparticles for cancer radiotherapy: a review. Cancer Nanotechnol. 2016;7(1):8.
3. Her S, Jaffray DA, Allen C. Gold nanoparticles for applications in cancer radiotherapy: mechanisms and recent advancements. Adv Drug Deliv Rev. 2017;109:84–101. doi:10.1016/j.addr.2015.12.012
4. Liu Y, Zhang P, Li F, et al. Metal-based nanoenhancers for future radiotherapy: radiosensitizing and synergistic effects on tumor cells. Theranostics. 2018;8(7):1824–1849. doi:10.7150/thno.22172
5. Laprise-Pelletier M, Simão T, Fortin MA. Gold nanoparticles in radiotherapy and recent progress in nanobrachytherapy. Adv Healthc Mater. 2018;7(16):e1701460.
6. Brun E, Sanche L, Sicard-Roselli C. Parameters governing gold nanoparticle X-ray radiosensitization of DNA in solution. Colloids Surf B Biointerfaces. 2009;72(1):128–134. doi:10.1016/j.colsurfb.2009.03.025
7. Baumann M, Krause M, Overgaard J, et al. Radiation oncology in the era of precision medicine. Nat Rev Cancer. 2016;16(4):234–249.
8. Nagi NMS, Khair YAM, Abdalla AME. Capacity of gold nanoparticles in cancer radiotherapy. Jpn J Radiol. 2017;35(10):555–561.
9. Ngwa W, Kumar R, Sridhar S, et al. Targeted radiotherapy with gold nanoparticles: current status and future perspectives. Nanomedicine (Lond). 2014;9(7):1063–1082. doi:10.2217/nnm.14.55
10. Ishikura S. Optimal radiotherapy for non-small-cell lung cancer: current progress and future challenges. Gen Thorac Cardiovasc Surg. 2012;60(3):127–131. doi:10.1007/s11748-011-0832-y
11. Shrestha S, Cooper LN, Andreev OA, Reshetnyak YK, Antosh MP. Gold nanoparticles for radiation enhancement in vivo. Jacobs J Radiat Oncol. 2016;3(1):026.
12. Linam J, Yang LX. Recent developments in radiosensitization. Anticancer Res. 2015;35(5):2479–2485.
13. Mesbahi A. A review on gold nanoparticles radiosensitization effect in radiation therapy of cancer. Rep Pract Oncol Radiother. 2010;15(6):176–180. doi:10.1016/j.rpor.2010.09.001
14. Guo Z, Zhu S, Yong Y, et al. Synthesis of BSA-coated BiOI@Bi S semiconductor heterojunction nanoparticles and their applications for radio/photodynamic/photothermal synergistic therapy of tumor. Adv Mater. 2017;29(44):1704136. doi:10.1002/adma.201704136
15. Ma N, Jiang YW, Zhang X, et al. Enhanced radiosensitization of gold nanospikes via hyperthermia in combined cancer radiation and photothermal therapy. ACS Appl Mater Interfaces. 2016;8(42):28480–28494. doi:10.1021/acsami.6b10132
16. Zhang XD, Wu D, Shen X, et al. Size-dependent radiosensitization of PEG-coated gold nanoparticles for cancer radiation therapy. Biomaterials. 2012;33(27):6408–6419. doi:10.1016/j.biomaterials.2012.05.047
17. Kobayashi K, Usami N, Porcel E, Lacombe S, Le Sech C. Enhancement of radiation effect by heavy elements. Mutat Res. 2010;704(1–3):123–131. doi:10.1016/j.mrrev.2010.01.002
18. Yong Y, Cheng X, Bao T, et al. Tungsten sulfide quantum dots as multifunctional nanotheranostics for in vivo dual-modal image-guided photothermal/radiotherapy synergistic therapy. ACS Nano. 2015;9(12):12451–12463. doi:10.1021/acsnano.5b05825
19. Wen L, Chen L, Zheng S, et al. Ultrasmall biocompatible WO3- x nanodots for multi-modality imaging and combined therapy of cancers. Adv Mater. 2016;28(25):5072–5079. doi:10.1002/adma.201506428
20. Song G, Ji C, Liang C, et al. TaOx decorated perfluorocarbon nanodroplets as oxygen reservoirs to overcome tumor hypoxia and enhance cancer radiotherapy. Biomaterials. 2017;112:257–263. doi:10.1016/j.biomaterials.2016.10.020
21. Liu J, Yang Y, Zhu W, et al. Nanoscale metal-organic frameworks for combined photodynamic & radiation therapy in cancer treatment. Biomaterials. 2016;97:1–9. doi:10.1016/j.biomaterials.2016.04.034
22. Wu H, Lin J, Liu P, et al. Is the autophagy a friend or foe in the silver nanoparticles associated radiotherapy for glioma? Biomaterials. 2015;62:47–57. doi:10.1016/j.biomaterials.2015.05.033
23. Kim SR, Kim EH. Feasibility study on the use of gold nanoparticles in fractionated kilovoltage X-ray treatment of melanoma. Int J Radiat Biol. 2018;94(1):8–16. doi:10.1080/09553002.2018.1393579
24. Dou Y, Guo Y, Li X, et al. Size-tuning ionization to optimize gold nanoparticles for simultaneous enhanced CT imaging and radiotherapy. ACS Nano. 2016;10(2):2536–2548. doi:10.1021/acsnano.5b07473
25. Chang MY, Shiau AL, Chen YH, Chang CJ, Chen HH, Wu CL. Increased apoptotic potential and dose-enhancing effect of gold nanoparticles in combination with single-dose clinical electron beams on tumor-bearing mice. Cancer Sci. 2008;99(7):1479–1484. doi:10.1111/j.1349-7006.2008.00827.x
26. Singh P, Pandit S, Mokkapati VRSS, Garg A, Ravikumar V, Mijakovic I. Gold nanoparticles in diagnostics and therapeutics for human cancer. Int J Mol Sci. 2018;19(7):1979. doi:10.3390/ijms19071979
27. Yang YS, Carney RP, Stellacci F, Irvine DJ. Enhancing radiotherapy by lipid nanocapsule-mediated delivery of amphiphilic gold nanoparticles to intracellular membranes. ACS Nano. 2014;8(9):8992–9002. doi:10.1021/nn502146r
28. Kuncic Z, Lacombe S. Nanoparticle radio-enhancement: principles, progress and application to cancer treatment. Phys Med Biol. 2018;63(2):02TR01. doi:10.1088/1361-6560/aa99ce
29. Silva F, Paulo A, Pallier A, et al. Dual imaging gold nanoplatforms for targeted radiotheranostics. Materials (Basel). 2020;13(3):513. doi:10.3390/ma13030513
30. Siddique S, Chow JCL. Application of nanomaterials in biomedical imaging and cancer therapy. Nanomaterials (Basel). 2020;10(9):E1700.
31. Yeh YC, Creran B, Rotello VM. Gold nanoparticles: preparation, properties, and applications in bionanotechnology. Nanoscale. 2012;4(6):1871–1880. doi:10.1039/C1NR11188D
32. Alkilany AM, Thompson LB, Boulos SP, Sisco PN, Murphy CJ. Gold nanorods: their potential for photothermal therapeutics and drug delivery, tempered by the complexity of their biological interactions. Adv Drug Deliv Rev. 2012;64(2):190–199.
33. Mieszawska AJ, Mulder WJ, Fayad ZA, Cormode DP. Multifunctional gold nanoparticles for diagnosis and therapy of disease. Mol Pharm. 2013;10(3):831–847. doi:10.1021/mp3005885
34. Rana S, Bajaj A, Mout R, Rotello VM. Monolayer coated gold nanoparticles for delivery applications. Adv Drug Deliv Rev. 2012;64(2):200–216. doi:10.1016/j.addr.2011.08.006
35. Arvizo R, Bhattacharya R, Mukherjee P. Gold nanoparticles: opportunities and challenges in nanomedicine. Expert Opin Drug Deliv. 2010;7(6):753–763. doi:10.1517/17425241003777010
36. Zheng T, Bott S, Huo Q. Techniques for accurate sizing of gold nanoparticles using dynamic light scattering with particular application to chemical and biological sensing based on aggregate formation. ACS Appl Mater Interfaces. 2016;8(33):21585–21594. doi:10.1021/acsami.6b06903
37. Yu Y, Yang T, Sun T. New insights into the synthesis, toxicity and applications of gold nanoparticles in CT imaging and treatment of cancer. Nanomedicine (Lond). 2020;15(11):1127–1145. doi:10.2217/nnm-2019-0395
38. Guo J, Rahme K, He Y, Li LL, Holmes JD, O’Driscoll CM. Gold nanoparticles enlighten the future of cancer theranostics. Int J Nanomedicine. 2017;12:6131–6152. doi:10.2147/IJN.S140772
39. Siddique S, Chow JCL. Gold nanoparticles for drug delivery and cancer therapy. Appl Sci. 2020;10(11):3824. doi:10.3390/app10113824
40. Babaei M, Ganjalikhani M. A systematic review of gold nanoparticles as novel cancer therapeutics. Nanomed J. 2014;1(4):211–219.
41. Huang K, Ma H, Liu J, et al. Size-dependent localization and penetration of ultrasmall gold nanoparticles in cancer cells, multicellular spheroids, and tumors in vivo. ACS Nano. 2012;6(5):4483–4493. doi:10.1021/nn301282m
42. Ma N, Wu FG, Zhang X, et al. Shape-dependent radiosensitization effect of gold nanostructures in cancer radiotherapy: comparison of gold nanoparticles, nanospikes, and nanorods. ACS Appl Mater Interfaces. 2017;9(15):13037–13048.
43. Li J, Li JJ, Zhang J, Wang X, Kawazoe N, Chen G. Gold nanoparticle size and shape influence on osteogenesis of mesenchymal stem cells. Nanoscale. 2016;8(15):7992–8007. doi:10.1039/C5NR08808A
44. Kumar R, Korideck H, Ngwa W, Berbeco RI, Makrigiorgos GM, Sridhar S. Third generation gold nanoplatform optimized for radiation therapy. Transl Cancer Res. 2013;2(4). doi:10.3978/j.issn.2218-676X.2013.07.02
45. Caldorera-Moore M, Guimard N, Shi L, Roy K. Designer nanoparticles: incorporating size, shape and triggered release into nanoscale drug carriers. Expert Opin Drug Deliv. 2010;7(4):479–495. doi:10.1517/17425240903579971
46. Morozov KV, Kolyvanova MA, Kartseva ME, et al. Radiosensitization by gold nanoparticles: impact of the size, dose rate, and photon energy. Nanomaterials (Basel). 2020;10(5):952. doi:10.3390/nano10050952
47. Jain S, Coulter JA, Butterworth KT, et al. Gold nanoparticle cellular uptake, toxicity and radiosensitisation in hypoxic conditions. Radiother Oncol. 2014;110(2):342–347. doi:10.1016/j.radonc.2013.12.013
48. Gilles M, Brun E, Sicard-Roselli C. Gold nanoparticles functionalization notably decreases radiosensitization through hydroxyl radical production under ionizing radiation. Colloids Surf B Biointerfaces. 2014;123:770–777. doi:10.1016/j.colsurfb.2014.10.028
49. Schuemann J, Berbeco R, Chithrani DB, et al. Roadmap to clinical use of gold nanoparticles for radiation sensitization. Int J Radiat Oncol Biol Phys. 2016;94(1):189–205. doi:10.1016/j.ijrobp.2015.09.032
50. Coulter JA, Jain S, Butterworth KT, et al. Cell type-dependent uptake, localization, and cytotoxicity of 1.9 nm gold nanoparticles. Int J Nanomed. 2012;7:2673–2685. doi:10.2147/IJN.S31751
51. Hainfeld JF, Slatkin DN, Smilowitz HM. The use of gold nanoparticles to enhance radiotherapy in mice. Phys Med Biol. 2004;49(18):N309–N315. doi:10.1088/0031-9155/49/18/N03
52. Hainfeld JF, Dilmanian FA, Zhong Z, Slatkin DN, Kalef-Ezra JA, Smilowitz HM. Gold nanoparticles enhance the radiation therapy of a murine squamous cell carcinoma. Phys Med Biol. 2010;55(11):3045–3059.
53. Hainfeld JF, Smilowitz HM, O’Connor MJ, Dilmanian FA, Slatkin DN. Gold nanoparticle imaging and radiotherapy of brain tumors in mice. Nanomedicine (Lond). 2013;8(10):1601–1609. doi:10.2217/nnm.12.165
54. Joh DY, Sun L, Stangl M, et al. Selective targeting of brain tumors with gold nanoparticle-induced radiosensitization. PLoS One. 2013;8(4):e62425. doi:10.1371/journal.pone.0062425
55. Cifter G, Chin J, Cifter F, et al. Targeted radiotherapy enhancement during electronic brachytherapy of accelerated partial breast irradiation (APBI) using controlled release of gold nanoparticles. Phys Med. 2015;31(8):1070–1074.
56. Mousavie Anijdan SH, Mahdavi SR, Shirazi A, Zarrinfard MA, Hajati J. Megavoltage X-ray dose enhancement with gold nanoparticles in tumor bearing mice. Int J Mol Cell Med. 2013;2(3):118–123.
57. Hau H, Khanal D, Rogers L, et al. Dose enhancement and cytotoxicity of gold nanoparticles in colon cancer cells when irradiated with kilo- and mega-voltage radiation. Bioeng Transl Med. 2016;1(1):94–102. doi:10.1002/btm2.10007
58. Abbasian M, Baharlouei A, Arab-Bafrani Z, Lightfoot DA. Combination of gold nanoparticles with low-LET irradiation: an approach to enhance DNA DSB induction in HT29 colorectal cancer stem-like cells. J Cancer Res Clin Oncol. 2019;145(1):97–107. doi:10.1007/s00432-018-2769-3
59. Liu Y, Liu X, Jin X, et al. The dependence of radiation enhancement effect on the concentration of gold nanoparticles exposed to low- and high-LET radiations. Phys Med. 2015;31(3):210–218. doi:10.1016/j.ejmp.2015.01.006
60. Kanavi MR, Asadi S, Balagholi S, Alikarami F, Nosrati H, Ahmadieh H. Gamma irradiation of ocular melanoma and lymphoma cells in the presence of gold nanoparticles: in vitro study. J Appl Clin Med Phys. 2018;19(3):268–275. doi:10.1002/acm2.12336
61. Jain S, Hirst DG, O’Sullivan JM. Gold nanoparticles as novel agents for cancer therapy. Br J Radiol. 2012;85(1010):101–113. doi:10.1259/bjr/59448833
62. Perrault SD, Walkey C, Jennings T, Fischer HC, Chan WC. Mediating tumor targeting efficiency of nanoparticles through design. Nano Lett. 2009;9(5):1909–1915. doi:10.1021/nl900031y
63. Lechtman E, Chattopadhyay N, Cai Z, Mashouf S, Reilly R, Pignol JP. Implications on clinical scenario of gold nanoparticle radiosensitization in regards to photon energy, nanoparticle size, concentration and location. Phys Med Biol. 2011;56(15):4631–4647. doi:10.1088/0031-9155/56/15/001
64. Chithrani DB, Jelveh S, Jalali F, et al. Gold nanoparticles as radiation sensitizers in cancer therapy. Radiat Res. 2010;173(6):719–728.
65. Chithrani DB. Nanoparticles for improved therapeutics and imaging in cancer therapy. Recent Pat Nanotechnol. 2010;4(3):171–180. doi:10.2174/187221010792483726
66. Wong C, Stylianopoulos T, Cui J, et al. Multistage nanoparticle delivery system for deep penetration into tumor tissue. Proc Natl Acad Sci U S A. 2011;108(6):2426–2431. doi:10.1073/pnas.1018382108
67. Cedervall T, Lynch I, Lindman S, et al. Understanding the nanoparticle-protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proc Natl Acad Sci U S A. 2007;104(7):2050–2055. doi:10.1073/pnas.0608582104
68. Choi CH, Alabi CA, Webster P, Davis ME. Mechanism of active targeting in solid tumors with transferrin-containing gold nanoparticles. Proc Natl Acad Sci U S A. 2010;107(3):1235–1240.
69. Yasui H, Takeuchi R, Nagane M, et al. Radiosensitization of tumor cells through endoplasmic reticulum stress induced by PEGylated nanogel containing gold nanoparticles. Cancer Lett. 2014;347(1):151–158.
70. Alalaiwe A, Roberts G, Carpinone P, Munson J, Roberts S. Influence of PEG coating on the oral bioavailability of gold nanoparticles in rats. Drug Deliv. 2017;24(1):591–598. doi:10.1080/10717544.2017.1282554
71. Hinkley GK, Carpinone P, Munson JW, Powers KW, Roberts SM. Oral absorption of PEG-coated versus uncoated gold nanospheres: does agglomeration matter? Part Fibre Toxicol. 2015;12:9. doi:10.1186/s12989-015-0085-5
72. Cao-Milán R, Liz-Marzán LM. Gold nanoparticle conjugates: recent advances toward clinical applications. Expert Opin Drug Deliv. 2014;11(5):741–752.
73. Charbgoo F, Nejabat M, Abnous K, et al. Gold nanoparticle should understand protein corona for being a clinical nanomaterial. J Control Release. 2018;272:39–53. doi:10.1016/j.jconrel.2018.01.002
74. Jokerst JV, Lobovkina T, Zare RN, Gambhir SS. Nanoparticle PEGylation for imaging and therapy. Nanomedicine (Lond). 2011;6(4):715–728. doi:10.2217/nnm.11.19
75. Liu S, Lämmerhofer M. Functionalized gold nanoparticles for sample preparation: a review. Electrophoresis. 2019;40:2438–2461.
76. Mousavi M, Nedaei HA, Khoei S, et al. Enhancement of radiosensitivity of melanoma cells by pegylated gold nanoparticles under irradiation of megavoltage electrons. Int J Radiat Biol. 2017;93(2):214–221. doi:10.1080/09553002.2017.1231944
77. Muxika A, Etxabide A, Uranga J, Guerrero P, de la Caba K. Chitosan as a bioactive polymer: processing, properties and applications. Int J Biol Macromol. 2017;105:1358–1368. doi:10.1016/j.ijbiomac.2017.07.087
78. Younes I, Rinaudo M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar Drugs. 2015;13(3):1133–1174. doi:10.3390/md13031133
79. Fathy MM, Mohamed FS, Elbialy N, Elshemey WM. Multifunctional chitosan-capped gold nanoparticles for enhanced cancer chemo-radiotherapy: an invitro study. Phys Med. 2018;48:76–83. doi:10.1016/j.ejmp.2018.04.002
80. Cheung RC, Ng TB, Wong JH, Chan WY. Chitosan: an update on potential biomedical and pharmaceutical applications. Mar Drugs. 2015;13(8):5156–5186. doi:10.3390/md13085156
81. Eblan MJ, Wang AZ. Improving chemoradiotherapy with nanoparticle therapeutics. Transl Cancer Res. 2013;2(4):320–329.
82. Pearcey R, Brundage M, Drouin P, et al. Phase III trial comparing radical radiotherapy with and without cisplatin chemotherapy in patients with advanced squamous cell cancer of the cervix. J Clin Oncol. 2002;20(4):966–972. doi:10.1200/JCO.2002.20.4.966
83. Chemoradiotherapy for Cervical Cancer Meta-Analysis Collaboration. Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: a systematic review and meta-analysis of individual patient data from 18 randomized trials. J Clin Oncol. 2008;26(35):5802–5812. doi:10.1200/JCO.2008.16.4368
84. Dong L, Li M, Zhang S, et al. Cytotoxicity of BSA-stabilized gold nanoclusters: in vitro and in vivo study. Small. 2015;11(21):2571–2581. doi:10.1002/smll.201403481
85. Al-Jawad SMH, Taha AA, Al-Halbosiy MMF, Al-Barram LFA. Synthesis and characterization of small-sized gold nanoparticles coated by bovine serum albumin (BSA) for cancer photothermal therapy. Photodiagnosis Photodyn Ther. 2018;21:201–210. doi:10.1016/j.pdpdt.2017.12.004
86. Murawala P, Tirmale A, Shiras A, Prasad BL. In situ synthesized BSA capped gold nanoparticles: effective carrier of anticancer drug methotrexate to MCF-7 breast cancer cells. Mater Sci Eng C Mater Biol Appl. 2014;34:158–167. doi:10.1016/j.msec.2013.09.004
87. Kefayat A, Ghahremani F, Motaghi H, Mehrgardi MA. Investigation of different targeting decorations effect on the radiosensitizing efficacy of albumin-stabilized gold nanoparticles for breast cancer radiation therapy. Eur J Pharm Sci. 2019;130:225–233. doi:10.1016/j.ejps.2019.01.037
88. Zhou B, Song J, Wang M, et al. BSA-bioinspired gold nanorods loaded with immunoadjuvant for the treatment of melanoma by combined photothermal therapy and immunotherapy. Nanoscale. 2018;10(46):21640–21647.
89. Liu S, Piao J, Liu Y, et al. Radiosensitizing effects of different size bovine serum albumin-templated gold nanoparticles on H22 hepatoma-bearing mice. Nanomedicine (Lond). 2018;13(11):1371–1383. doi:10.2217/nnm-2018-0059
90. Koutcher L, Sherman E, Fury M, et al. Concurrent cisplatin and radiation versus cetuximab and radiation for locally advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2011;81(4):915–922. doi:10.1016/j.ijrobp.2010.07.008
91. Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharm. 2014;740:364–378. doi:10.1016/j.ejphar.2014.07.025
92. Setua S, Ouberai M, Piccirillo SG, Watts C, Welland M. Cisplatin-tethered gold nanospheres for multimodal chemo-radiotherapy of glioblastoma. Nanoscale. 2014;6(18):10865–10873. doi:10.1039/C4NR03693J
93. Davidi ES, Dreifuss T, Motiei M, et al. Cisplatin-conjugated gold nanoparticles as a theranostic agent for head and neck cancer. Head Neck. 2018;40(1):70–78. doi:10.1002/hed.24935
94. Cui L, Her S, Dunne M, et al. Significant radiation enhancement effects by gold nanoparticles in combination with cisplatin in triple negative breast cancer cells and tumor xenografts. Radiat Res. 2017;187(2):147–160. doi:10.1667/RR14578.1
95. Park J, Park J, Ju EJ, et al. Multifunctional hollow gold nanoparticles designed for triple combination therapy and CT imaging. J Control Release. 2015;207:77–85. doi:10.1016/j.jconrel.2015.04.007
96. Rosenblum D, Joshi N, Tao W, Karp JM, Peer D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat Commun. 2018;9(1):1410. doi:10.1038/s41467-018-03705-y
97. Sinha R, Kim GJ, Nie S, Shin DM. Nanotechnology in cancer therapeutics: bioconjugated nanoparticles for drug delivery. Mol Cancer Ther. 2006;5(8):1909–1917. doi:10.1158/1535-7163.MCT-06-0141
98. Khoshgard K, Hashemi B, Arbabi A, Rasaee MJ, Soleimani M. Radiosensitization effect of folate-conjugated gold nanoparticles on HeLa cancer cells under orthovoltage superficial radiotherapy techniques. Phys Med Biol. 2014;59(9):2249–2263. doi:10.1088/0031-9155/59/9/2249
99. Samadian H, Hosseini-Nami S, Kamrava SK, Ghaznavi H, Shakeri-Zadeh A. Folate-conjugated gold nanoparticle as a new nanoplatform for targeted cancer therapy. J Cancer Res Clin Oncol. 2016;142(11):2217–2229. doi:10.1007/s00432-016-2179-3
100. Kefayat A, Ghahremani F, Motaghi H, Amouheidari A. Ultra-small but ultra-effective: folic acid-targeted gold nanoclusters for enhancement of intracranial glioma tumors’ radiation therapy efficacy. Nanomedicine. 2019;16:173–184. doi:10.1016/j.nano.2018.12.007
101. Cruz E, Kayser V. Synthesis and enhanced cellular uptake in vitro of anti-HER2 multifunctional gold nanoparticles. Cancers. 2019;11(6):870. doi:10.3390/cancers11060870
102. Liu M, Yang YJ, Zheng H, et al. Membrane-bound complement regulatory proteins are prognostic factors of operable breast cancer treated with adjuvant trastuzumab: a retrospective study. Oncol Rep. 2014;32(6):2619–2627. doi:10.3892/or.2014.3496
103. Cai Z, Yook S, Lu Y, et al. Local radiation treatment of HER2-positive breast cancer using trastuzumab-modified gold nanoparticles labeled with Lu. Pharm Res. 2017;34(3):579–590. doi:10.1007/s11095-016-2082-2
104. Yook S, Cai Z, Lu Y, Winnik MA, Pignol JP, Reilly RM. Radiation nanomedicine for EGFR-positive breast cancer: panitumumab-modified gold nanoparticles complexed to the β-particle-emitter, (177)Lu. Mol Pharm. 2015;12(11):3963–3972. doi:10.1021/acs.molpharmaceut.5b00425
105. Yook S, Cai Z, Lu Y, Winnik MA, Pignol JP, Reilly RM. Intratumorally injected 177Lu-labeled gold nanoparticles: gold nanoseed brachytherapy with application for neoadjuvant treatment of locally advanced breast cancer. J Nucl Med. 2016;57(6):936–942. doi:10.2967/jnumed.115.168906
106. Hatoyama K, Kitamura N, Takano-Kasuya M, et al. Quantitative analyses of amount and localization of radiosensitizer gold nanoparticles interacting with cancer cells to optimize radiation therapy. Biochem Biophys Res Commun. 2019;508(4):1093–1100. doi:10.1016/j.bbrc.2018.12.016
107. Popovtzer A, Mizrachi A, Motiei M, et al. Actively targeted gold nanoparticles as novel radiosensitizer agents: an in vivo head and neck cancer model. Nanoscale. 2016;8(5):2678–2685. doi:10.1039/C5NR07496G
108. Jóźwiak P, Lipińska A. The role of glucose transporter 1 (GLUT1) in the diagnosis and therapy of tumors. Postepy Hig Med Dosw. 2012;66:165–174. (Article in Polish).
109. Song L, Falzone N, Vallis KA. EGF-coated gold nanoparticles provide an efficient nano-scale delivery system for the molecular radiotherapy of EGFR-positive cancer. Int J Radiat Biol. 2016;92(11):716–723. doi:10.3109/09553002.2016.1145360
110. Barron CC, Bilan PJ, Tsakiridis T, Tsiani E. Facilitative glucose transporters: implications for cancer detection, prognosis and treatment. Metabolism. 2016;65(2):124–139. doi:10.1016/j.metabol.2015.10.007
111. Calvo MB, Figueroa A, Pulido EG, Campelo RG, Aparicio LA. Potential role of sugar transporters in cancer and their relationship with anticancer therapy. Int J Endocrinol. 2010;2010:205357. doi:10.1155/2010/205357
112. Kong T, Zeng J, Wang X, et al. Enhancement of radiation cytotoxicity in breast-cancer cells by localized attachment of gold nanoparticles. Small. 2008;4(9):1537–1543. doi:10.1002/smll.200700794
113. Roa W, Zhang X, Guo L, et al. Gold nanoparticle sensitize radiotherapy of prostate cancer cells by regulation of the cell cycle. Nanotechnology. 2009;20(37):375101. doi:10.1088/0957-4484/20/37/375101
114. Wang C, Jiang Y, Li X, Hu L. Thioglucose-bound gold nanoparticles increase the radiosensitivity of a triple-negative breast cancer cell line (MDA-MB-231). Breast Cancer. 2015;22(4):413–420. doi:10.1007/s12282-013-0496-9
115. Soleymanifard S, Rostami A, Aledavood SA, Matin MM, Sazgarnia A. Increased radiotoxicity in two cancerous cell lines irradiated by low and high energy photons in the presence of thio-glucose bound gold nanoparticles. Int J Radiat Biol. 2017;93(4):407–415. doi:10.1080/09553002.2017.1268282
116. Hu C, Niestroj M, Yuan D, Chang S, Chen J. Treating cancer stem cells and cancer metastasis using glucose-coated gold nanoparticles. Int J Nanomed. 2015;10:2065–2077.
117. Park EI, Manzella SM, Baenziger JU. Rapid clearance of sialylated glycoproteins by the asialoglycoprotein receptor. J Biol Chem. 2003;278(7):4597–4602. doi:10.1074/jbc.M210612200
118. Zhu CD, Zheng Q, Wang LX, et al. Synthesis of novel galactose functionalized gold nanoparticles and its radiosensitizing mechanism. J Nanobiotechnol. 2015;13:67. doi:10.1186/s12951-015-0129-x
119. Kunjachan S, Pola R, Gremse F, et al. Passive versus active tumor targeting using RGD- and NGR-modified polymeric nanomedicines. Nano Lett. 2014;14(2):972–981. doi:10.1021/nl404391r
120. Ruoslahti E, Pierschbacher MD. New perspectives in cell adhesion: RGD and integrins. Science. 1987;238(4826):491–497. doi:10.1126/science.2821619
121. Wu PH, Onodera Y, Ichikawa Y, et al. Targeting integrins with RGD-conjugated gold nanoparticles in radiotherapy decreases the invasive activity of breast cancer cells. Int J Nanomed. 2017;12:5069–5085. doi:10.2147/IJN.S137833
122. Özcelik S, Pratx G. Nuclear-targeted gold nanoparticles enhance cancer cell radiosensitization. Nanotechnology. 2020;31(41):415102. doi:10.1088/1361-6528/aba02b
123. Kunjachan S, Detappe A, Kumar R, et al. Nanoparticle mediated tumor vascular disruption: a novel strategy in radiation therapy. Nano Lett. 2015;15(11):7488–7496. doi:10.1021/acs.nanolett.5b03073
124. Banerjee D, Harfouche R, Sengupta S. Nanotechnology-mediated targeting of tumor angiogenesis. Vasc Cell. 2011;3(1):3. doi:10.1186/2045-824X-3-3
125. Yang CJ, Chithrani DB. Nuclear targeting of gold nanoparticles for improved therapeutics. Curr Top Med Chem. 2016;16(3):271–280. doi:10.2174/1568026615666150701115012
126. Farkhani SM, Valizadeh A, Karami H, Mohammadi S, Sohrabi N, Badrzadeh F. Cell penetrating peptides: efficient vectors for delivery of nanoparticles, nanocarriers, therapeutic and diagnostic molecules. Peptides. 2014;57:78–94. doi:10.1016/j.peptides.2014.04.015
127. Kalmouni M, Al-Hosani S, Magzoub M. Cancer targeting peptides. Cell Mol Life Sci. 2019;76(11):2171–2183.
128. Ramsey JD, Flynn NH. Cell-penetrating peptides transport therapeutics into cells. Pharmacol Ther. 2015;154:78–86. doi:10.1016/j.pharmthera.2015.07.003
129. Wang F, Wang Y, Zhang X, Zhang W, Guo S, Jin F. Recent progress of cell-penetrating peptides as new carriers for intracellular cargo delivery. J Control Release. 2014;174:126–136. doi:10.1016/j.jconrel.2013.11.020
130. Zhang X, Wang H, Coulter JA, Yang R. Octaarginine-modified gold nanoparticles enhance the radiosensitivity of human colorectal cancer cell line LS180 to megavoltage radiation. Int J Nanomed. 2018;13:3541–3552. doi:10.2147/IJN.S161157
131. Yang C, Neshatian M, van Prooijen M. Cancer nanotechnology: enhanced therapeutic response using peptide-modified gold nanoparticles. J Nanosci Nanotechnol. 2014;14(7):4813–4819. doi:10.1166/jnn.2014.9280
132. Yang C, Uertz J, Yohan D, Chithrani BD. Peptide modified gold nanoparticles for improved cellular uptake, nuclear transport, and intracellular retention. Nanoscale. 2014;6(20):12026–12033. doi:10.1039/C4NR02535K
133. Mackey MA, El-Sayed MA. Chemosensitization of cancer cells via gold nanoparticle-induced cell cycle regulation. Photochem Photobiol. 2014;90(2):306–312. doi:10.1111/php.12226
134. Bates PJ, Laber DA, Miller DM, Thomas SD, Trent JO. Discovery and development of the G-rich oligonucleotide AS1411 as a novel treatment for cancer. Exp Mol Pathol. 2009;86(3):151–164. doi:10.1016/j.yexmp.2009.01.004
135. Zhu G, Chen X. Aptamer-based targeted therapy. Adv Drug Deliv Rev. 2018;134:65–78. doi:10.1016/j.addr.2018.08.005
136. Morita Y, Leslie M, Kameyama H, Volk DE, Tanaka T. Aptamer therapeutics in cancer: current and future. Cancers (Basel). 2018;10(3):80. doi:10.3390/cancers10030080
137. Soundararajan S, Chen W, Spicer EK, Courtenay-Luck N, Fernandes DJ. The nucleolin targeting aptamer AS1411 destabilizes Bcl-2 messenger RNA in human breast cancer cells. Cancer Res. 2008;68(7):2358–2365. doi:10.1158/0008-5472.CAN-07-5723
138. Li X, Wang H, Lu X, Di, B. Silencing STAT3 with short hairpin RNA enhances radiosensitivity of human laryngeal squamous cell carcinoma xenografts in vivo. Exp Ther Med. 2010;1(6):947–953.
139. Geiger JL, Grandis JR, Bauman JE. The STAT3 pathway as a therapeutic target in head and neck cancer: barriers and innovations. Oral Oncol. 2016;56:84–92. doi:10.1016/j.oraloncology.2015.11.022
140. Oweida AJ, Darragh L, Phan A, et al. STAT3 modulation of regulatory T cells in response to radiation therapy in head and neck cancer. J Natl Cancer Inst. 2019;111(12):1339–1349. doi:10.1093/jnci/djz036
141. Zhang S, Gupta S, Fitzgerald TJ, Bogdanov AA. Dual radiosensitization and anti-STAT3 anti-proliferative strategy based on delivery of gold nanoparticle - oligonucleotide nanoconstructs to head and neck cancer cells. Nanotheranostics. 2018;2(1):1–11. doi:10.7150/ntno.22335
142. Luo CH, Huang CT, Su CH, Yeh CS. Bacteria-mediated hypoxia-specific delivery of nanoparticles for tumors imaging and therapy. Nano Lett. 2016;16(6):3493–3499. doi:10.1021/acs.nanolett.6b00262
143. Roma-Rodrigues C, Pombo I, Raposo L, Pedrosa P, Fernandes AR, Baptista PV. Nanotheranostics targeting the tumor microenvironment. Front Bioeng Biotechnol. 2019;7:197.
144. Zhao Z, Xu H, Li S, et al. Hypoxic radiosensitizer-lipid coated gold nanoparticles enhance the effects of radiation therapy on tumor growth. J Biomed Nanotechnol. 2019;15(9):1982–1993. doi:10.1166/jbn.2019.2830
145. Wang Y, Shang W, Niu M, Tian J, Xu K. Hypoxia-active nanoparticles used in tumor theranostic. Int J Nanomed. 2019;14:3705–3722. doi:10.2147/IJN.S196959
146. Chen Q, Feng L, Liu J, et al. Intelligent albumin-MnO2 nanoparticles as pH-/H2O2 -responsive dissociable nanocarriers to modulate tumor hypoxia for effective combination therapy. Adv Mater. 2016;28(33):7129–7136. doi:10.1002/adma.201601902
147. Ding Y, Sun Z, Tong Z, et al. Tumor microenvironment-responsive multifunctional peptide coated ultrasmall gold nanoparticles and their application in cancer radiotherapy. Theranostics. 2020;10(12):5195–5208. doi:10.7150/thno.45017
148. Zhang Y, Yang L, Yang C, Liu J. Recent advances of smart acid-responsive gold nanoparticles in tumor therapy. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2020;12(4):e1619. doi:10.1002/wnan.1619
149. Yao L, Daniels J, Moshnikova A, et al. pHLIP peptide targets nanogold particles to tumors. Proc Natl Acad Sci U S A. 2013;110(2):465–470. doi:10.1073/pnas.1219665110
150. Daniels JL, Crawford TM, Andreev OA, Reshetnyak YK. Synthesis and characterization of pHLIP coated gold nanoparticles. Biochem Biophys Rep. 2017;10:62–69.
151. Antosh MP, Wijesinghe DD, Shrestha S, et al. Enhancement of radiation effect on cancer cells by gold-pHLIP. Proc Natl Acad Sci U S A. 2015;112(17):5372–5376. doi:10.1073/pnas.1501628112
152. Horsman MR, Overgaard J. The impact of hypoxia and its modification of the outcome of radiotherapy. J Radiat Res. 2016;Suppl 1:i90–i98. doi:10.1093/jrr/rrw007
153. Martin JD, Fukumura D, Duda DG, Boucher Y, Jain RK. Reengineering the tumor microenvironment to alleviate hypoxia and overcome cancer heterogeneity. Cold Spring Harb Perspect Med. 2016;6(12):a027094. doi:10.1101/cshperspect.a027094
154. Barker HE, Paget JT, Khan AA, Harrington KJ. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat Rev Cancer. 2015;15(7):409–425. doi:10.1038/nrc3958
155. Xie J, Gong L, Zhu S, Yong Y, Gu Z, Zhao Y. Emerging strategies of nanomaterial-mediated tumor radiosensitization. Adv Mater. 2019;31(3):e1802244. doi:10.1002/adma.201802244
156. Semenza GL. Hypoxia-inducible factors: mediators of cancer progression and targets for cancer therapy. Trends Pharmacol Sci. 2012;33(4):207–214. doi:10.1016/j.tips.2012.01.005
157. Schito L, Semenza GL. Hypoxia-inducible factors: master regulators of cancer progression. Trends Cancer. 2016;2(12):758–770. doi:10.1016/j.trecan.2016.10.016
158. Patiar S, Harris AL. Role of hypoxia-inducible factor-1alpha as a cancer therapy target. Endocr Relat Cancer. 2006;Suppl 1:S61–S75. doi:10.1677/erc.1.01290
159. Kim MS, Lee EJ, Kim JW, et al. Gold nanoparticles enhance anti-tumor effect of radiotherapy to hypoxic tumor. Radiat Oncol J. 2016;34(3):230–238. doi:10.3857/roj.2016.01788
160. Gong F, Yang N, Wang X, et al. Tumor microenvironment-responsive intelligent nanoplatforms for cancer theranostics. Nano Today. 2020;32:100851. doi:10.1016/j.nantod.2020.100851
161. Li J, Shang W, Li Y, Fu S, Tian J, Lu L. Advanced nanomaterials targeting hypoxia to enhance radiotherapy. Int J Nanomed. 2018;13:5925. doi:10.2147/IJN.S173914
162. Kefayat A, Ghahremani F, Motaghi H, Rostami S, Mehrgardi MA. Alive attenuated Salmonella as a cargo shuttle for smart carrying of gold nanoparticles to tumour hypoxic regions. J Drug Target. 2019;27(3):315–324. doi:10.1080/1061186X.2018.1523417
163. Cho WS, Cho M, Jeong J, et al. Size-dependent tissue kinetics of PEG-coated gold nanoparticles. Toxicol Appl Pharmacol. 2010;245(1):116–123. doi:10.1016/j.taap.2010.02.013
164. Chou LY, Chan WC. Fluorescence‐tagged gold nanoparticles for rapidly characterizing the size‐dependent biodistribution in tumor models. Adv Healthc Mater. 2012;1(6):714–721.
165. Li B, Lane LA. Probing the biological obstacles of nanomedicine with gold nanoparticles. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2019;11(3):e1542.
166. Bai X, Liu F, Liu Y, et al. Toward a systematic exploration of nano-bio interactions. Toxicol Appl Pharmacol. 2017;323:66–73. doi:10.1016/j.taap.2017.03.011
167. Abdulle A, Chow JCL. Contrast enhancement for portal imaging in nanoparticle-enhanced radiotherapy: a monte carlo phantom evaluation using flattening-filter-free photon beams. Nanomaterials (Basel). 2019;9(7):920. doi:10.3390/nano9070920
168. Albayedh F, Chow JCL. Monte carlo simulation on the imaging contrast enhancement in nanoparticle-enhanced radiotherapy. J Med Phys. 2018;43(3):195–199.
169. Dimitriou NM, Tsekenis G, Balanikas EC, et al. Gold nanoparticles, radiations and the immune system: current insights into the physical mechanisms and the biological interactions of this new alliance towards cancer therapy. Pharmacol Ther. 2017;178:1–17. doi:10.1016/j.pharmthera.2017.03.006
170. Batooei S, Khajeali A, Khodadadi R, Islamian JP. Metal-based nanoparticles as radio-sensitizer in gastric cancer therapy. J Drug Deliv Sci Technol. 2020;56:101576. doi:10.1016/j.jddst.2020.101576
171. Butterworth KT, McMahon SJ, Currell FJ, Prise KM. Physical basis and biological mechanisms of gold nanoparticle radiosensitization. Nanoscale. 2012;4(16):4830–4838. doi:10.1039/c2nr31227a
172. Rosa S, Connolly C, Schettino G, Butterworth KT, Prise KM. Biological mechanisms of gold nanoparticle radiosensitization. Cancer Nanotechnol. 2017;8(1):2. doi:10.1186/s12645-017-0026-0
173. Retif P, Pinel S, Toussaint M, et al. Nanoparticles for radiation therapy enhancement: the key parameters. Theranostics. 2015;5(9):1030–1044. doi:10.7150/thno.11642
174. Yao X, Huang C, Chen X, Yi Z, Sanche L. Chemical radiosensitivity of DNA induced by gold nanoparticles. J Biomed Nanotechnol. 2015;11(3):478–485. doi:10.1166/jbn.2015.1922
175. Liu K, Han L, Zhuang J, Yang DP. Protein-directed gold nanoparticles with excellent catalytic activity for 4-nitrophenol reduction. Mater Sci Eng C Mater Biol Appl. 2017;78:429–434. doi:10.1016/j.msec.2017.04.052
176. Paunesku T, Gutiontov S, Brown K, Woloschak GE. Radiosensitization and nanoparticles. Cancer Treat Res. 2015;166:151–171.
177. Cui L, Her S, Borst GR, Bristow RG, Jaffray DA, Allen C. Radiosensitization by gold nanoparticles: will they ever make it to the clinic? Radiother Oncol. 2017;124(3):344–356. doi:10.1016/j.radonc.2017.07.007
178. Pateras IS, Havaki S, Nikitopoulou X, et al. The DNA damage response and immune signaling alliance: is it good or bad? Nature decides when and where. Pharmacol Ther. 2015;154:36–56. doi:10.1016/j.pharmthera.2015.06.011
179. Martelli S, Chow JCL. Dose enhancement for the flattening-filter-free and flattening-filter photon beams in nanoparticle-enhanced radiotherapy: a Monte Carlo phantom Study. Nanomaterials (Basel). 2020;10(4):637. doi:10.3390/nano10040637
180. Dewmini Mututantri-Bastiyange JC, Chow L. Imaging dose of cone-beam computed tomography in nanoparticle-enhanced image-guided radiotherapy: a Monte Carlo phantom study. AIMS Bioeng. 2020;7(1):1–11. doi:10.3934/bioeng.2020001
181. Cho SH. Estimation of tumour dose enhancement due to gold nanoparticles during typical radiation treatments: a preliminary Monte Carlo study. Phys Med Biol. 2005;50(15):N163–N173. doi:10.1088/0031-9155/50/15/N01
182. Chow JC, Leung MK, Jaffray DA. Monte Carlo simulation on a gold nanoparticle irradiated by electron beams. Phys Med Biol. 2012;57(11):3323–3331. doi:10.1088/0031-9155/57/11/3323
183. Jain S, Coulter JA, Hounsell AR, et al. Cell-specific radiosensitization by gold nanoparticles at megavoltage radiation energies. Int J Radiat Oncol Biol Phys. 2011;79(2):531–539. doi:10.1016/j.ijrobp.2010.08.044
184. Lin Y, McMahon SJ, Scarpelli M, Paganetti H, Schuemann J. Comparing gold nano-particle enhanced radiotherapy with protons, megavoltage photons and kilovoltage photons: a Monte Carlo simulation. Phys Med Biol. 2014;59(24):7675–7689. doi:10.1088/0031-9155/59/24/7675
185. Jeynes JC, Merchant MJ, Spindler A, Wera AC, Kirkby KJ. Investigation of gold nanoparticle radiosensitization mechanisms using a free radical scavenger and protons of different energies. Phys Med Biol. 2014;59(21):6431–6443. doi:10.1088/0031-9155/59/21/6431
186. Cui L, Tse K, Zahedi P, et al. Hypoxia and cellular localization influence the radiosensitizing effect of gold nanoparticles (AuNPs) in breast cancer cells. Radiat Res. 2014;182(5):475–488. doi:10.1667/RR13642.1
187. Mkandawire MM, Lakatos M, Springer A, et al. Induction of apoptosis in human cancer cells by targeting mitochondria with gold nanoparticles. Nanoscale. 2015;7(24):10634–10640. doi:10.1039/C5NR01483B
188. Yang Y, Ren L, Wang H. Strategies in the design of gold nanoparticles for intracellular targeting: opportunities and challenges. Ther Deliv. 2017;8(10):879–897. doi:10.4155/tde-2017-0049
189. Pathak RK, Kolishetti N, Dhar S. Targeted nanoparticles in mitochondrial medicine. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015;7(3):315–329. doi:10.1002/wnan.1305
190. Mateo D, Morales P, Ávalos A, Haza AI. Oxidative stress contributes to gold nanoparticle-induced cytotoxicity in human tumor cells. Toxicol Mech Methods. 2014;24(3):161–172. doi:10.3109/15376516.2013.869783
191. Decrock E, Hoorelbeke D, Ramadan R, et al. Calcium, oxidative stress and connexin channels, a harmonious orchestra directing the response to radiotherapy treatment? Biochim Biophys Acta Mol Cell Res. 2017;1864(6):1099–1120. doi:10.1016/j.bbamcr.2017.02.007
192. Liu R, Wang Y, Yuan Q, An D, Li J, Gao X. The Au clusters induce tumor cell apoptosis via specifically targeting thioredoxin reductase 1 (TrxR1) and suppressing its activity. Chem Commun (Camb). 2014;50(73):10687–10690. doi:10.1039/C4CC03320E
193. Pawlik TM, Keyomarsi K. Role of cell cycle in mediating sensitivity to radiotherapy. Int J Radiat Oncol Biol Phys. 2004;59(4):928–942. doi:10.1016/j.ijrobp.2004.03.005
194. Kastan MB, Bartek J. Cell-cycle checkpoints and cancer. Nature. 2004;432(7015):316–323. doi:10.1038/nature03097
195. Mackey MA, Saira F, Mahmoud MA, El-Sayed MA. Inducing cancer cell death by targeting its nucleus: solid gold nanospheres versus hollow gold nanocages. Bioconjug Chem. 2013;24(6):897–906. doi:10.1021/bc300592d
196. Pan Y, Leifert A, Ruau D, et al. Gold nanoparticles of diameter 1.4 nm trigger necrosis by oxidative stress and mitochondrial damage. Small. 2009;5(18):2067–2076. doi:10.1002/smll.200900466
197. Butterworth KT, Coulter JA, Jain S, et al. Evaluation of cytotoxicity and radiation enhancement using 1.9 nm gold particles: potential application for cancer therapy. Nanotechnology. 2010;21(29):295101. doi:10.1088/0957-4484/21/29/295101
198. Djuzenova CS, Elsner I, Katzer A, et al. Radiosensitivity in breast cancer assessed by the histone γ-H2AX and 53BP1 foci. Radiat Oncol. 2013;8:98. doi:10.1186/1748-717X-8-98
199. Vignard J, Mirey G, Salles B. Ionizing-radiation induced DNA double-strand breaks: a direct and indirect lighting up. Radiother Oncol. 2013;108(3):362–369. doi:10.1016/j.radonc.2013.06.013
200. Galluzzi L, Pietrocola F, Bravo-San Pedro JM, et al. Autophagy in malignant transformation and cancer progression. EMBO J. 2015;34(7):856–880. doi:10.15252/embj.201490784
201. Yang A, Rajeshkumar NV, Wang X, et al. Autophagy is critical for pancreatic tumor growth and progression in tumors with p53 alterations. Cancer Discov. 2014;4(8):905–913. doi:10.1158/2159-8290.CD-14-0362
202. Xu DW, Zhang GQ, Wang ZW, Xu XY, Liu TX. Autophagy in tumorigenesis and cancer treatment. Asian Pac J Cancer Prev. 2015;16(6):2167–2175. doi:10.7314/APJCP.2015.16.6.2167
203. Towers CG, Thorburn A. Therapeutic targeting of autophagy. EBioMedicine. 2016;14:15–23. doi:10.1016/j.ebiom.2016.10.034
204. Ma X, Wu Y, Jin S, et al. Gold nanoparticles induce autophagosome accumulation through size-dependent nanoparticle uptake and lysosome impairment. ACS Nano. 2011;5(11):8629–8639. doi:10.1021/nn202155y
205. Verfaillie T, Garg AD, Agostinis P. Targeting ER stress induced apoptosis and inflammation in cancer. Cancer Lett. 2013;332(2):249–264. doi:10.1016/j.canlet.2010.07.016
206. Bhat TA, Chaudhary AK, Kumar S, et al. Endoplasmic reticulum-mediated unfolded protein response and mitochondrial apoptosis in cancer. Biochim Biophys Acta Rev Cancer. 2017;1867(1):58–66. doi:10.1016/j.bbcan.2016.12.002
207. Cubillos-Ruiz JR, Bettigole SE, Glimcher LH. Tumorigenic and Immunosuppressive effects of endoplasmic reticulum stress in cancer. Cell. 2017;168(4):692–706. doi:10.1016/j.cell.2016.12.004
208. Mohamed E, Cao Y, Rodriguez PC. Endoplasmic reticulum stress regulates tumor growth and anti-tumor immunity: a promising opportunity for cancer immunotherapy. Cancer Immunol Immunother. 2017;66(8):1069–1078.
209. Tsai YY, Huang YH, Chao YL, et al. Identification of the nanogold particle-induced endoplasmic reticulum stress by omic techniques and systems biology analysis. ACS Nano. 2011;5(12):9354–9369. doi:10.1021/nn2027775
210. Simard JC, Durocher I, Girard D. Silver nanoparticles induce irremediable endoplasmic reticulum stress leading to unfolded protein response dependent apoptosis in breast cancer cells. Apoptosis. 2016;21(11):1279–1290. doi:10.1007/s10495-016-1285-7
211. Chen R, Huo L, Shi X, et al. Endoplasmic reticulum stress induced by zinc oxide nanoparticles is an earlier biomarker for nanotoxicological evaluation. ACS Nano. 2014;8(3):2562–2574. doi:10.1021/nn406184r
212. Yang X, Shao H, Liu W, et al. Endoplasmic reticulum stress and oxidative stress are involved in ZnO nanoparticle-induced hepatotoxicity. Toxicol Lett. 2015;234(1):40–49. doi:10.1016/j.toxlet.2015.02.004
213. Le Sech C, Kobayashi K, Usami N, Furusawa Y, Porcel E, Lacombe S. Comment on therapeutic application of metallic nanoparticles combined with particle-induced x-ray emission effect. Nanotechnology. 2012;23(7):078001. doi:10.1088/0957-4484/23/7/078001
214. Koonce NA, Quick CM, Hardee ME, et al. Combination of gold nanoparticle-conjugated tumor necrosis factor-α and radiation therapy results in a synergistic antitumor response in murine carcinoma models. Int J Radiat Oncol Biol Phys. 2015;93(3):588–596. doi:10.1016/j.ijrobp.2015.07.2275
215. Khoobchandani M, Katti KK, Karikachery AR, et al. New approaches in breast cancer therapy through green nanotechnology and nano-ayurvedic medicine - pre-clinical and pilot human clinical investigations. Int J Nanomed. 2020;15:181–197. doi:10.2147/IJN.S219042
216. Lawrence YR, Vikram B, Dignam JJ, et al. NCI-RTOG translational program strategic guidelines for the early-stage development of radiosensitizers. J Natl Cancer Inst. 2013;105(1):11–24. doi:10.1093/jnci/djs472
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
